Catalytic Carboxylation of Terminal Alkynes with CO2: An Overview
Abstract
1. Introduction
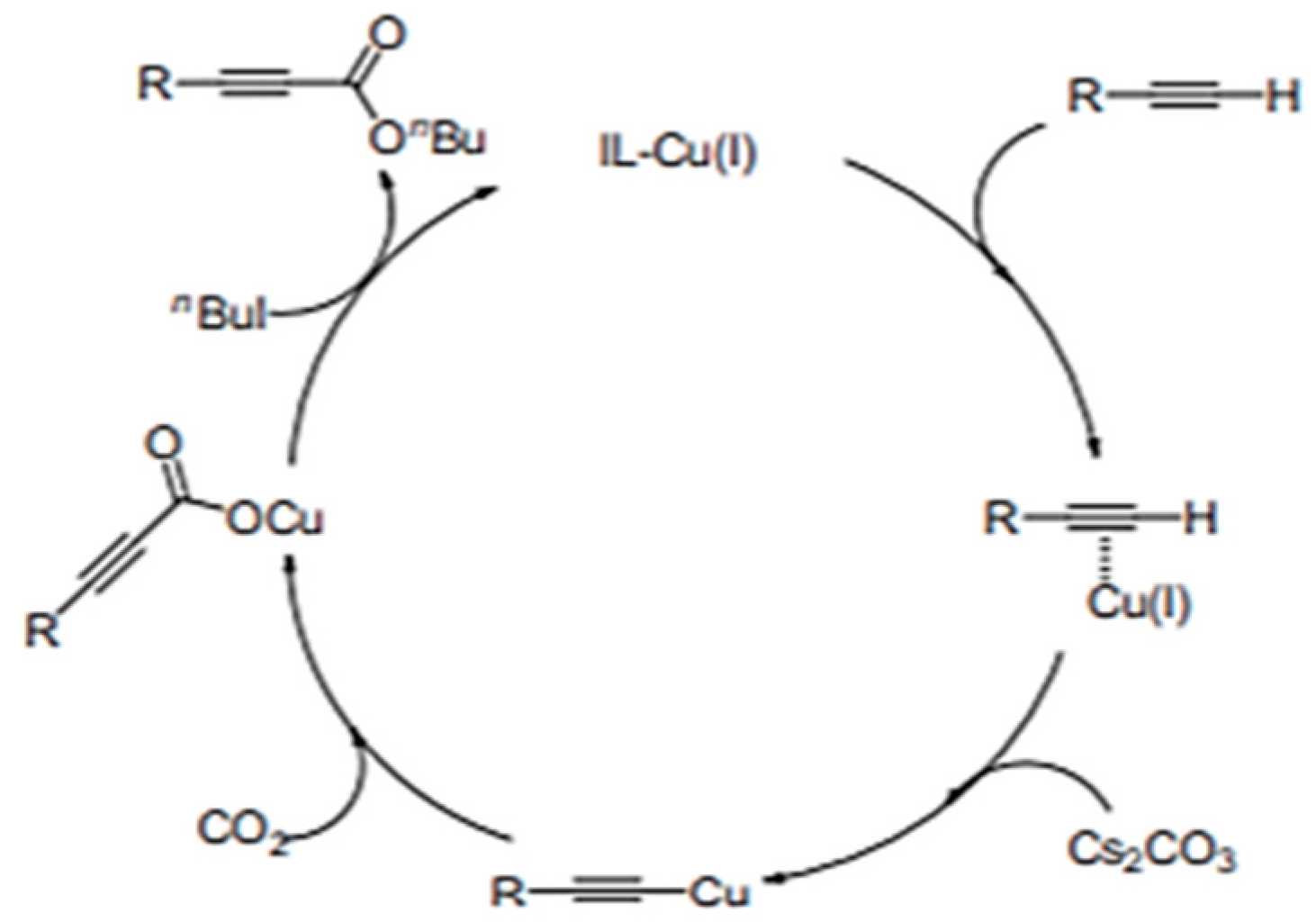
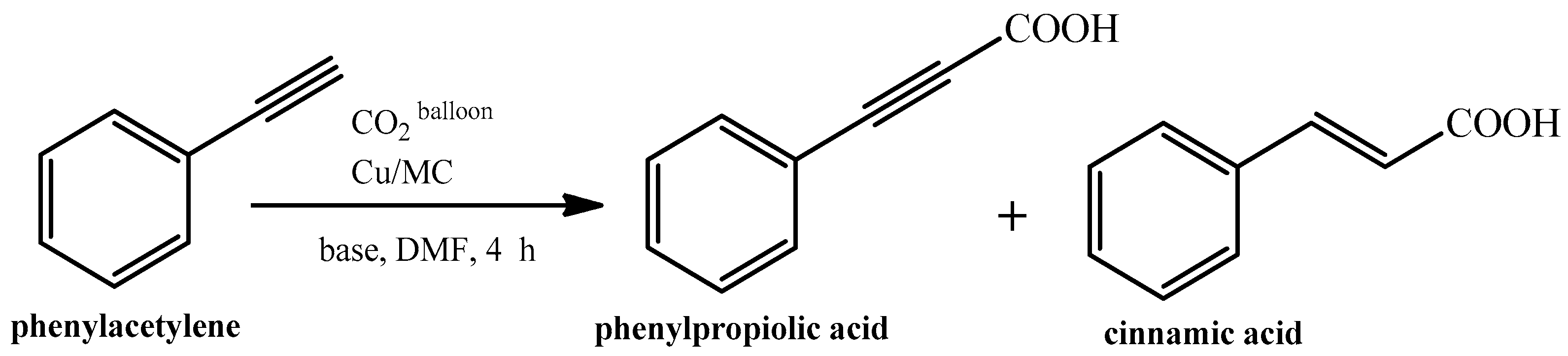
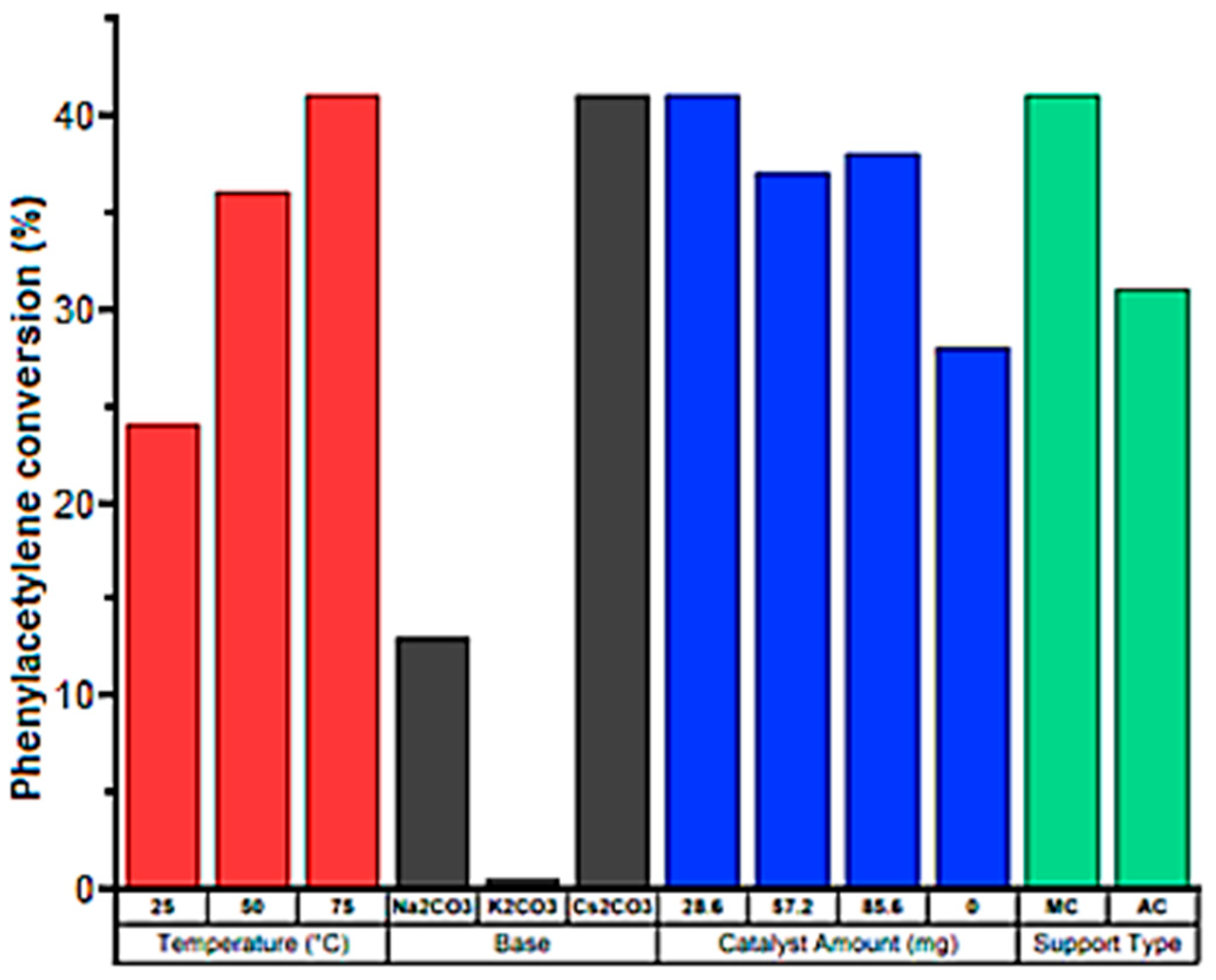
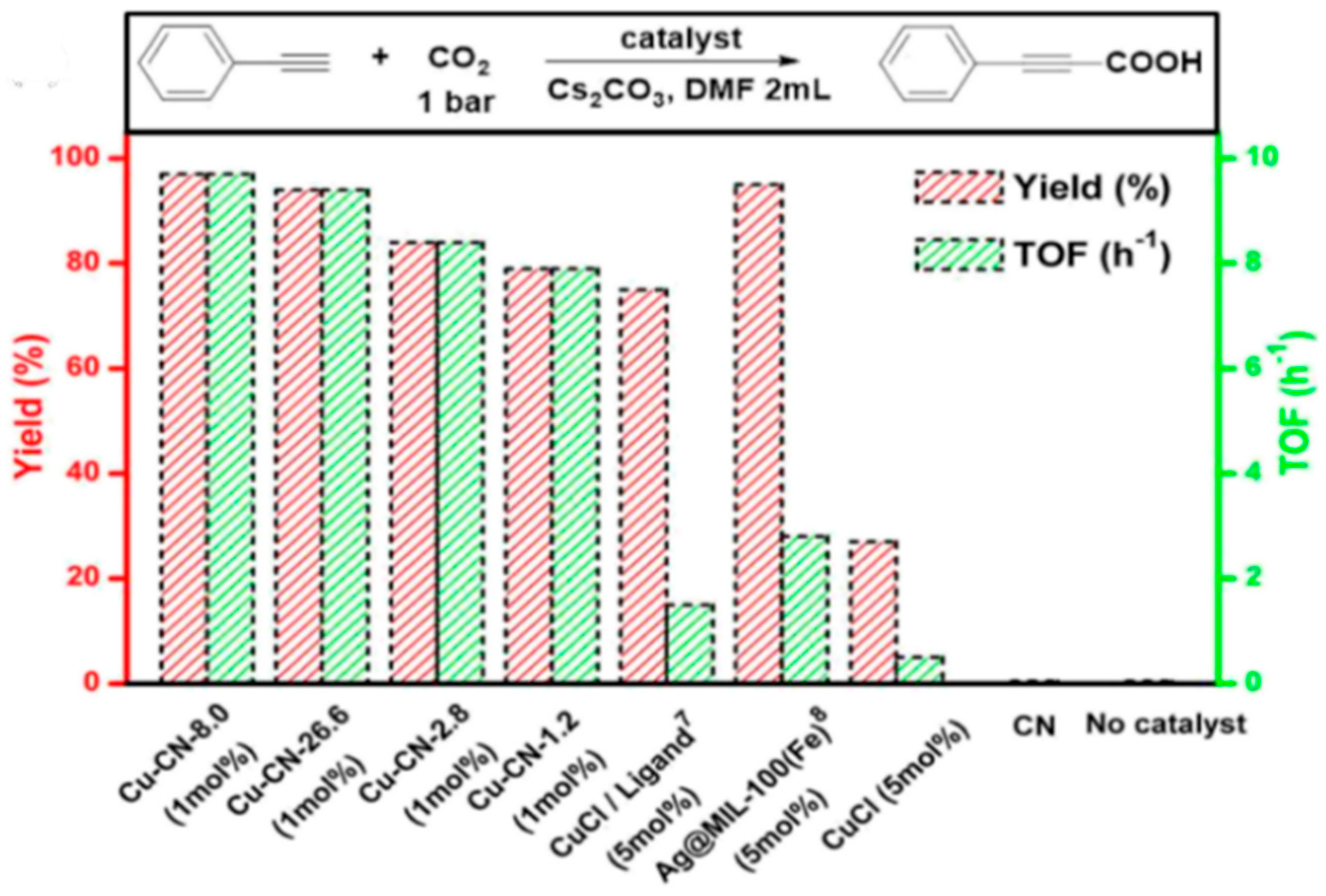
2. Copper-Containing Catalysts for Carboxylation Reaction of Aromatic Alkynes with Carbon Dioxide
3. Silver-Containing Catalysts for Carboxylation Reaction of Aromatic Alkynes with Carbon Dioxide
| Catalyst Base | Solvent | Substrate | Reaction Conditions | Yield of Product, % | Ref. | ||
|---|---|---|---|---|---|---|---|
| T (°C) | P, atm | Cat. Loading | |||||
| AgNO3 Cs2CO3 | DMSO (20 mL) | phenylacetylene | 50 | 60 | 0.4 mmol 6 mmol | phenylpropiolic acid—89.3 | [71] |
| 0.5% Ag/F-Al2O3 Cs2CO3 | DMSO (20 mL) | phenylacetylene | 50 | 60 | 0.47 mmol 13.7 mmol | phenylpropiolic acid—62.1 | [71] |
| 0.5 Ag@ZIF-8 Cs2CO3 | DMF (20 mL) | tret-butylacetylene | 40 | 1 | 50 mg 7.2 mmol | tret-butylpropiolic acid—91.0 | [72] |
| 4.16% Ag@MIL-101 Cs2CO3 | DMF (5 mL) | phenylacetylene | 50 | 1 | 70 mg 1.5 mmol | phenylpropiolic acid—96.5 | [73] |
| 0.10 Ag/IRFC Cs2CO3 | DMSO (20 mL) | phenylacetylene | 70 | 1 | 50 mg 10 mmol | phenylpropiolic acid—99.0 | [83] |
| Au12Ag32(SR)30@ZIF-8 K2CO3 | DMSO | phenylacetylene | 50 | 1 | - | phenylpropiolic acid—100.0 | [84] |
| Ag/tert-GO Cs2CO3 | DMF (4 mL) | 4-chlorophenylacetylene | 50 | 1 | 10 mg 0.75 mmol | 4-chlorophenylpropiolic acid—96.5 | [85] |
| AgNPs/rGO Na2CO3 | DMF | phenylacetylene | 50 | - | - | phenylpropiolic acid—99.5 | [89] |
| AgNPs/Co-MOF Cs2CO3 | DMF (5 mL) | phenylacetylene | 40 | 1 | 50 mg 1.5 mmol | 28.0 | [90] |
| AgNPs/Co-MOF Cs2CO3 | DMF (5 mL) | phenylacetylene | 80 | 1 | 50 mg 1.5 mmol | 98.0 | [90] |
| AgNPs/Co-MOF Cs2CO3 | DMF (5 mL) | phenylacetylene | 90 | 1 | 50 mg 1.5 mmol | 81.0 | [90] |
| 1% Ag-NHC Cs2CO3 | DMF (10 mL) | phenylacetylene | 40 | 1 | 1.5 mmol | 83 | [91] |
| 5% Ag-NHC Cs2CO3 | DMF (10 mL) | phenylacetylene | 40 | 1 | 1.5 mmol | 68 | [91] |
| 2.5% Ag2WO4 Cs2CO3 | DMF (3 mL) | phenylacetylene | 25 | - | 0.0511 g 1.2 mmol | 25 | [92] |
| 2.5% Ag2WO4 Cs2CO3 | MeCN (3 mL) | phenylacetylene | 25 | - | 0.0511 g 1.2 mmol | >99 | [92] |
4. The Effect of Solvent and Base on the Carboxylation Reaction of Terminal Alkynes
4.1. Selecting an Optimal Base for Carboxylation of Terminal Alkynes
4.2. Selecting an Optimal Solvent for Carboxylation of Terminal Alkynes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rana, A.; Andino, J.M. A Review of Materials for Carbon Dioxide Capture. Catalysts 2025, 15, 273. [Google Scholar] [CrossRef]
- Saini, S.; Prajapati, P.K.; Jain, S.L. Transition Metal-Catalyzed Carboxylation of Olefins with Carbon Dioxide: A Comprehensive Review. Cat. Rev. 2020, 64, 631–677. [Google Scholar] [CrossRef]
- Iglesias, D.; Tinajero, C.; Marchetti, S.; Roppolo, I.; Zanatta, M.; Sans, V. Multi-Step Oxidative Carboxylation of Olefins with Carbon Dioxide by Combining Electrochemical and 3D-Printed Flow Reactors. Green Chem. 2023, 25, 9934–9940. [Google Scholar] [CrossRef]
- Maeda, C.; Miyazaki, Y.; Ema, T. Recent Progress in Catalytic Conversions of Carbon Dioxide. Catal. Sci. Technol. 2014, 4, 1482–1497. [Google Scholar] [CrossRef]
- Alsaiari, R.; Perrott, L.T.; Nowicka, E.; Engel, R.V.; Miedziak, P.J.; Kondrat, S.A.; Hutchings, G.J. The Effect of Ring Size on the Selective Carboxylation of Cycloalkene Oxides. Catal. Sci. Technol. 2017, 7, 1433–1439. [Google Scholar] [CrossRef]
- Global Monitoring Laboratory. Trends in Atmospheric Carbon Dioxide (CO2). Available online: https://gml.noaa.gov/ccgg/trends/global.html (accessed on 5 June 2025). (In English)
- Pimparkar, S.; Dalvi, A.K.; Koodan, A.; Maiti, S.; Al-Thabaiti, S.A.; Mokhtar, M.; Dutta, A.; Lee, Y.R.; Maiti, D. Recent Advances in the Incorporation of CO2 for C–H and C–C Bond Functionalization. Green Chem. 2021, 23, 9283–9317. [Google Scholar] [CrossRef]
- Lan, J.; Lu, X.; Ren, B.; Duo, F.; Niu, X.; Si, J. Visible-Light-Driven Photocatalytic Carboxylation to Aromatic Carboxylic Acids with CO2. Org. Biomol. Chem. 2024, 22, 682–693. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary advancements in carbon dioxide valorization via metal-organic framework-based strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Kuznetsov, N.Y.; Maximov, A.L.; Beletskaya, I.P. Novel Technological Paradigm of the Application of Carbon Dioxide as a C1 Synthon in Organic Chemistry: I. Synthesis of Hydroxybenzoic Acids, Methanol, and Formic Acid. Russ. J. Org. Chem. 2022, 58, 1681–1711. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Batkin, A.M.; Garifullina, C.; Zalyatdinov, A.A.; Yang, D.; Dai, Y.; Yang, Y.; Kustov, L.M. Catalytic systems for hydrogenation of CO2 to methanol. Mol. Catal. 2024, 566, 114403. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. The Role of Alkali and Alkaline Earth Metals in the CO2 Methanation Reaction and the Combined Capture and Methanation of CO2. Catalysts 2020, 10, 812. [Google Scholar] [CrossRef]
- Latsiou, A.I.; Charisiou, N.D.; Frontistis, Z.; Bansode, A.; Goula, M.A. CO2 hydrogenation for the production of higher alcohols: Trends in catalyst developments, challenges and opportunities. Catal. Today 2023, 420, 114179. [Google Scholar] [CrossRef]
- Mafokoane, M.A.; Ou, X.; Musyoka, N.M. Chang. Carbon Dioxide Activation and Hydrogenation into Value-Added C1 Chemicals over Metal Hydride Catalysts. Catalysts 2025, 15, 424. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Q.; Yao, Y.; Gu, P.; Sun, J.; Sun, S. Visible-Light-Promoted Cascade Carboxylation/Arylation of Unactivated Alkenes with CO2 for the Synthesis of Carboxylated Indole-Fused Heterocycles. Org. Lett. 2024, 26, 6341–6346. [Google Scholar] [CrossRef]
- Xiao, H.-Z.; Yu, B.; Yan, S.-S.; Zhang, W.; Li, X.-X.; Bao, Y.; Luo, S.-P.; Ye, J.-H.; Yu, D.-G. Photocatalytic 1,3-Dicarboxylation of Unactivated Alkenes with CO2. Chin. J. Catal. 2023, 50, 222–228. [Google Scholar] [CrossRef]
- Du, F.-M.; Yan, L.-Y.; Fu, M.-C. Metal-Free Photoinduced Defluorinative Carboxylation of Trifluoromethylalkenes with Formate. Eur. J. Org. Chem. 2023, 26, e202300883. [Google Scholar] [CrossRef]
- Sathe, A.A.; Nambiar, A.M.K.; Rioux, R.M. Synthesis of Cyclic Organic Carbonates via Catalytic Oxidative Carboxylation of Olefins in Flow Reactors. Catal. Sci. Technol. 2017, 7, 84–89. [Google Scholar] [CrossRef]
- He, X.; Qiu, L.-Q.; Wang, W.-J.; Chen, K.-H.; He, L.-N. Photocarboxylation with CO2: An Appealing and Sustainable Strategy for CO2 Fixation. Green Chem. 2020, 22, 7301–7320. [Google Scholar] [CrossRef]
- Hou, J.; Li, J.-S.; Wu, J. Recent Development of Light-Mediated Carboxylation Using CO2 as the Feedstock. Asian J. Org. Chem. 2018, 7, 1439–1447. [Google Scholar] [CrossRef]
- Shi, J.-B.; Bu, Q.; Liu, B.-Y.; Dai, B.; Liu, N. Organocatalytic Strategy for the Fixation of CO2 via Carboxylation of Terminal Alkynes. J. Org. Chem. 2021, 86, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Feng, X.; Sui, K.; Fang, D.; Bao, M. Transition Metal-Free Carboxylation of Terminal Alkynes with Carbon Dioxide Through Dual Activation: Synthesis of Propiolic Acids. J. CO2 Util. 2019, 32, 140–145. [Google Scholar] [CrossRef]
- Bhatt, S.; Malik, A.; Soni, A.; Abraham, B.M.; Sen, A.; Jain, S.L. Photocatalytic Reductive Carboxylation of Terminal Alkynes with CO2 Using Heterostructured ZIF-7/BiOBr Under Visible-Light Illumination. J. CO2 Util. 2023, 67, 102334. [Google Scholar] [CrossRef]
- Zhang, L.; Bu, R.; Liu, X.-Y.; Mu, P.-F.; Gao, E.-Q. Schiff-Base Molecules and COFs as Metal-Free Catalysts or Silver Supports for Carboxylation of Alkynes with CO2. Green Chem. 2021, 23, 7620–7629. [Google Scholar] [CrossRef]
- Shi, X.-L.; Sun, B.; Hu, Q.; Liu, K.; Li, P.; Liu, B. A Novel Fiber-Supported Cooperative Catalyst for the Carboxylation of Terminal Alkynes Through CO2 Utilization. Chem. Eng. J. 2020, 395, 125084. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Tamboli, A.H.; Kim, H. CuCl2@Poly-IL Catalyzed Carboxylation of Terminal Alkynes Through CO2 Utilization. Chem. Eng. J. 2017, 326, 1009–1019. [Google Scholar] [CrossRef]
- Shi, N.; Wang, H.-B.; Gao, L.; Zhang, J.-Y.; Guo, J.-F.; Fang, W.; Ebadi, A. Sila-, Bora-, Thio-, and Phosphono-Carboxylation of Unsaturated Compounds with Carbon Dioxide: An Overview. J. CO2 Util. 2021, 48, 101522. [Google Scholar] [CrossRef]
- Julián, A.; Polo, V.; Fernández-Alvarez, F.J.; Oro, L.A. Iridium–NSiN Catalyzed Formation of Silylphosphinecarboxylates from the Reaction of CO2 with P(SiMe3)R2 (R = Ph, Cy). Catal. Sci. Technol. 2017, 7, 1372–1378. [Google Scholar] [CrossRef]
- Lv, X.; Wu, Y.-B.; Lu, G. Computational Exploration of Ligand Effects in Copper-Catalyzed Boracarboxylation of Styrene with CO2. Catal. Sci. Technol. 2017, 7, 5049–5054. [Google Scholar] [CrossRef]
- Mannisto, J.K.; Pavlovic, L.; Tiainen, T.; Nieger, M.; Sahari, A.; Hopmann, K.H.; Repo, T. Mechanistic Insights Into Carbamate Formation from CO2 And Amines: The Role of Guanidine–CO2 Adducts. Catal. Sci. Technol. 2021, 11, 6877–6886. [Google Scholar] [CrossRef]
- Katagiri, T.; Amao, Y. Recent Advances in Light-Driven C-H Bond Activation and Building C-C Bonds with CO2 as a Feedstock for Carbon Capture and Utilization Technology. Green Chem. 2020, 22, 6682–6713. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Lai, L.; Zhou, L.; Ye, K.; Chen, F.-E. Carbon Dioxide Cycle via Electrocatalysis: Electrochemical Carboxylation of CO2 And Decarboxylative Functionalization of Carboxylic Acids. Green Synth. Catal. 2021, 2, 19–26. [Google Scholar] [CrossRef]
- Roca, R.A.; Sczancoski, J.C.; Nogueira, I.C.; Fabbro, M.T.; Alves, H.C.; Gracia, L.; Cavalcante, L.S. Facet-Dependent Photocatalytic and Antibacterial Properties of α-Ag2WO4 Crystals: Combining Experimental Data and Theoretical Insights. Catal. Sci. Technol. 2015, 5, 4091–4107. [Google Scholar] [CrossRef]
- Cui, W.G.; Zhang, G.Y.; Hu, T.L.; Bu, X.H. Metal-organic framework-based heterogeneous catalysts for the conversion of C1 chemistry: CO, CO2 and CH4. Coord. Chem. Rev. 2019, 387, 79–120. [Google Scholar] [CrossRef]
- Gulati, S.; Vijayan, S.; Mansi; Kumar, S.; Harikumar, B.; Trivedi, M.; Varma, R.S. Recent advances in the application of metal-organic frameworks (MOFs)-based nanocatalysts for direct conversion of carbon dioxide (CO2) to value-added chemicals. Coord. Chem. Rev. 2023, 474, 214853. [Google Scholar] [CrossRef]
- Qiao, C.; Cao, Y.; He, L.-N. Transition Metal-Catalyzed Carboxylation of Terminal Alkynes with CO2. Mini-Rev. Org. Chem. 2018, 15, 283–290. [Google Scholar] [CrossRef]
- Jover, J.; Maseras, F. Computational characterization of the mechanism for coinage-metal-catalysed carboxylation of terminal alkynes. J. Org. Chem. 2014, 79, 11981–11987. [Google Scholar] [CrossRef]
- Xia, J.; Yu, B.; Guo, C.; He, L. Copper(I)/Phosphine-Catalyzed Tandem Carboxylation/Annulation of Terminal Alkynes with Ambient Pressure of CO2: One-Pot Access to 3a-Hydroxyisoxazolo[3,2-α]Isoindol-8(3aH)-Ones. Green Chem. 2015, 17, 4061–4067. [Google Scholar] [CrossRef]
- Hong, J.; Li, M.; Zhang, J.; Sun, B.; Mo, F. C-H Bond Carboxylation with Carbon Dioxide. ChemSusChem 2019, 12, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A Comprehensive Review on Different Approaches for CO2 Utilization and Conversion Pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Dutta, A. Energy Issues in the Utilization of CO2 in the Synthesis of Chemicals: The Case of the Direct Carboxylation of Alcohols to Dialkyl-Carbonates. Catal. Today 2017, 281, 345–351. [Google Scholar] [CrossRef]
- Liu, X.-F.; Zhang, K.; Tao, L.; Lu, X.-B.; Zhang, W.-Z. Recent advances in electrochemical carboxylation reactions using carbon dioxide. Green Chem. Eng. 2022, 3, 125–137. [Google Scholar] [CrossRef]
- Yu, B.; Xie, J.-N.; Zhong, C.-L.; Li, W.; He, L.-N. Copper(I)@Carbon-Catalyzed Carboxylation of Terminal Alkynes with CO2 at Atmospheric Pressure. ACS Catal. 2015, 5, 3940–3944. [Google Scholar] [CrossRef]
- Shi, G.; Xu, W.; Wang, J.; Yuan, Y.; Chaemchuen, S.; Verpoort, F. A Cu-Based MOF for the Effective Carboxylation of Terminal Alkynes with CO2 Under Mild Conditions. J. CO2 Util. 2020, 39, 101177. [Google Scholar] [CrossRef]
- Tan, K.; Nijem, N.; Canepa, P.; Gong, Q.; Li, J.; Thonhauser, T.; Chabal, Y.J. Stability and Hydrolyzation of Metal Organic Frameworks with Paddle-Wheel SBUs upon Hydration. Chem. Mater. 2012, 24, 3153–3167. [Google Scholar] [CrossRef]
- Jasuja, H.; Burtch, N.C.; Huang, Y.-G.; Cai, Y.; Walton, K.S. Kinetic Water Stability of an Isostructural Family of Zinc-Based Pillared Metal-Organic Frameworks. Langmuir 2013, 29, 633–642. [Google Scholar] [CrossRef]
- Sun, Z.-H.; Wang, X.-Y.; Huang, K.-L.; He, M.-Y.; Chen, S.-C. Heterogeneous Catalytic Carboxylation of Terminal Alkynes with CO2 Over a Copper(II)-Based Metal-Organic Framework Catalyst. Catal. Commun. 2022, 169, 106472. [Google Scholar] [CrossRef]
- Kojčinović, A.; Likozar, B.; Grilc, M. Heterogeneous Catalytic Materials for Carboxylation Reactions with CO2 as Reactant. J. CO2 Util. 2022, 66, 102250. [Google Scholar] [CrossRef]
- Brill, M.; Lazreg, F.; Cazin, C.S.J.; Nolan, S.P. Transition Metal-Catalyzed Carboxylation of Organic Substrates with Carbon Dioxide. In Carbon Dioxide and Organometallics; Topics in Organometallic Chemistry; Springer: Cham, Switzerland, 2015; Volume 53, pp. 225–278. [Google Scholar]
- Bondarenko, G.N.; Dvurechenskaya, E.G.; Magommedov, E.S.; Beletskaya, I.P. Copper(0) Nanoparticles Supported on Al2O3 as Catalyst for Carboxylation of Terminal Alkynes. Catal. Lett. 2017, 147, 2570–2580. [Google Scholar] [CrossRef]
- Xie, J.-N.; Yu, B.; Zhou, Z.-H.; Fu, H.-C.; Wang, N.; He, L.-N. Copper(I)-Based Ionic Liquid-Catalyzed Carboxylation of Terminal Alkynes with CO2 at Atmospheric Pressure. Tetrahedron Lett. 2015, 56, 7059–7062. [Google Scholar] [CrossRef]
- Li, F.-W.; Suo, Q.-L.; Hong, H.-L.; Zhu, N.; Wang, Y.-Q.; Han, L.-M. DBU and Copper(I) Mediated Carboxylation of Terminal Alkynes Using Supercritical CO2 as a Reactant and Solvent. Tetrahedron Lett. 2014, 55, 3878–3880. [Google Scholar] [CrossRef]
- Thakur, K.G.; Sekar, G. Copper (I)-Catalyzed Caryl-Calkynyl Bond Formation of Aryl Iodides with Terminal Alkynes. Synthesis 2009, 16, 2785–2789. [Google Scholar] [CrossRef]
- Wei, W.; Hu, X.-Y.; Yan, X.-W.; Zhang, Q.; Cheng, M.; Ji, J.-X. Direct Use of Dioxygen as an Oxygen Source: Catalytic Oxidative Synthesis of Amides. Chem. Commun. 2012, 48, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Huang, H.; Zhou, Y.; Ye, D.; Jiang, H.; Liu, H. Copper(I)-Catalyzed One-Pot Synthesis of 2H-1,4-Benzoxazin-3-(4H)-Ones from o-Halophenols and 2-Chloroacetamides. J. Org. Chem. 2009, 74, 2846–2849. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, Y. Copper- and Copper—N-Heterocyclic Carbene-Catalyzed C—H Activating Carboxylation of Terminal Alkynes with CO2 at Ambient Conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 20184–20189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, J.; Ohishi, T.; Hou, Z. Copper-Catalyzed Direct Carboxylation of CH Bonds with Carbon Dioxide. Angew. Chem. Int. Ed. 2010, 49, 8670–8673. [Google Scholar] [CrossRef]
- Wang, X.; Lim, Y.N.; Lee, C.; Jang, H.-Y.; Lee, B.Y. 1,5,7-Triazabicyclo[4.4.0]dec-1-ene-Mediated Acetylene Dicarboxylation and Alkyne Carboxylation Using Carbon Dioxide. Eur. J. Org. Chem. 2013, 10, 1867–1871. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Q.; Wen, H. Theoretical Discussion on the Mechanism of Binding CO2 by DBU and Alcohol. Mol. Simulat. 2013, 39, 822–827. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Yonker, C.R.; Jessop, P.G.; Phan, L. Organic Liquid CO2 Capture Agents with High Gravimetric CO2 Capacity. Energy Environ. Sci. 2008, 1, 487–493. [Google Scholar] [CrossRef]
- Yu, B.; Diao, Z.-F.; Guo, C.-X.; Zhong, C.-L.; He, L.-N.; Zhao, Y.-N.; Song, Q.-W.; Liu, A.-H.; Wang, J.-Q. Carboxylation of Terminal Alkynes at Ambient CO2 Pressure in Ethylene Carbonate. Green Chem. 2013, 15, 2401–2407. [Google Scholar] [CrossRef]
- Dang, L.; Lin, Z.; Marder, T.B. DFT Studies on the Carboxylation of Arylboronate Esters with CO2 Catalyzed by Copper(I) Complexes. Organometallics 2010, 29, 917–927. [Google Scholar] [CrossRef]
- Amalia, P.N.; Abdullah, I.; Rahayu, D.U.C.; Krisnandi, Y.K. Synthesis and Characterization of Copper Impregnated Mesoporous Carbon as Heterogeneous Catalyst for Phenylacetylene Carboxylation with Carbon Dioxide. Indian J. Chem. 2021, 21, 77–87. [Google Scholar] [CrossRef]
- Yuan, Z. Applications of Bases in Transition Metal Catalyzed Reactions. Postdoc J. 2014, 2, 17–28. [Google Scholar] [CrossRef]
- Yang, P.; Zuo, S.W.; Zhang, F.T.; Yu, B.; Guo, S.E.; Yu, X.X.; Zhao, Y.F.; Zhang, J.; Liu, Z.M. Carbon Nitride-Based Single-Atom Cu Catalysts for Highly Efficient Carboxylation of Alkynes with Atmospheric CO2. Ind. Eng. Chem. Res. 2020, 59, 7327–7335. [Google Scholar] [CrossRef]
- Zhu, N.-N.; Liu, X.-H.; Li, T.; Ma, J.-G.; Cheng, P.; Yang, G.-M. Composite System of Ag Nanoparticles and Metal−Organic Frameworks for the Capture and Conversion of Carbon Dioxide under Mild Conditions. Inorg. Chem. 2017, 56, 3414–3420. [Google Scholar] [CrossRef] [PubMed]
- Whang, H.S.; Lim, J.; Choi, M.S.; Lee, J.; Lee, H. Heterogeneous Catalysts for Catalytic CO2 Conversion into Value-Added Chemicals. BMC Chem. 2019, 1, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.Z.; Ren, X.; Zhang, L.L.; Lu, X.B. Ligand-Free Ag(I)-Catalyzed Carboxylation of Terminal Alkynes with CO2. Org. Lett. 2011, 13, 2402–2405. [Google Scholar] [CrossRef]
- Yu, D.; Tan, M.X.; Zhang, Y. Carboxylation of Terminal Alkynes with Carbon Dioxide Catalyzed by Poly(N-Heterocyclic Carbene)-Supported Silver Nanoparticles. Adv. Synth. Catal. 2012, 354, 969–974. [Google Scholar] [CrossRef]
- Inamoto, K.; Asano, N.; Kobayashi, K.; Yonemoto, M.; Kondo, Y. A Copper-Based Catalytic System for Carboxylation of Terminal Alkynes: Synthesis of Alkyl 2-Alkynoates. Org. Biomol. Chem. 2012, 10, 1514–1516. [Google Scholar] [CrossRef]
- Finashina, E.D.; Kustov, L.M.; Tkachenko, O.P. Carbon Dioxide on Heterogeneous Ag-Containing Catalysts. Russ. Chem. Bull. 2014, 63, 2652–2656. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Sun, N.; Hu, D.; Shen, Q.; Mao, F.; Wei, W. Facile and Rapid Preparation of Ag@ZIF-8 for Carboxylation of Terminal Alkynes with CO2 in Mild Conditions. ACS Appl. Mater. Interfaces 2019, 11, 28858–28867. [Google Scholar] [CrossRef]
- Liu, X.-H.; Ma, J.-G.; Niu, Z.; Yang, G.-M.; Cheng, P. An Efficient Nanoscale Heterogeneous Catalyst for the Capture and Conversion of Carbon Dioxide at Ambient Pressure. Angew. Chem. Int. Ed. 2014, 54, 988–991. [Google Scholar] [CrossRef]
- Xiong, G.; Yu, B.; Dong, J.; Shi, Y.; Zhao, B.; He, L.N. Cluster-ased MOFs with Accelerating Chemical Conversion of CO2 Through C-C Bond Formation. Chem. Commun. 2017, 53, 6013–6016. [Google Scholar] [CrossRef]
- Fan, Y.R.; Li, X.; Gao, K.; Liu, Y.; Meng, X.R.; Wu, J.; Hou, H.W. Co(II)-Cluster-Based Metal-Organic Frameworks as Efficient Heterogeneous Catalysts for Selective Oxidation of Arylalkanes. CrystEngComm 2019, 21, 1666–1673. [Google Scholar] [CrossRef]
- Gao, W.-Y.; Wojtas, L.; Ma, S. A Porous Metal–Metalloporphyrin Framework Featuring High-Density Active Sites for Chemical Fixation Of CO2 Under Ambient Conditions. Chem. Commun. 2014, 50, 5316–5318. [Google Scholar] [CrossRef] [PubMed]
- Parmar, B.; Patel, P.; Pillai, R.S.; Tak, R.K.; Kureshy, R.I.; Khan, N.-U.H.; Suresh, E. Efficient Catalytic Conversion of Terminal/Internal Epoxides to Cyclic Carbonates by Porous Co(II) MOF at Ambient Conditions: Structure Property Correlation and Computational Studies. Inorg. Chem. 2019, 58, 10084–10096. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Qin, J.S.; Lollar, C.T.; Zhou, H.C. Stable Metal−Organic Frameworks with Group 4 Metals: Current Status and Trends. ACS Cent. Sci. 2018, 4, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xie, Y.-Q.; Wang, X.-S.; Wang, Q.; Liu, T.-T.; Huang, Y.-B.; Cao, R. An Imidazolium-Functionalized Mesoporous Cationic Metal–Organic Framework for Cooperative CO2 Fixation into Cyclic Carbonate. Chem. Commun. 2018, 54, 342–345. [Google Scholar] [CrossRef]
- Kitagawa, S. Future Porous Materials. Acc. Chem. Res. 2017, 50, 514–516. [Google Scholar] [CrossRef]
- Trickett, C.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The Chemistry of Metal–Organic Frameworks for CO2 Capture, Regeneration and Conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Qin, J.S.; Yuan, S.; Lollar, C.; Pang, J.; Alsalme, A.; Zhou, H.C. Stable Metal–Organic Frameworks as a Host Platform for Catalysis and Biomimetics. Chem. Commun. 2018, 54, 4231–4249. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, J.; Zhu, T.; Zhang, L.; Wei, W. Nitrogen-Doped Mesoporous Carbon Single Crystal-Based Ag Nanoparticles for Boosting Mild CO2 Conversion with Terminal Alkynes. J. Colloid Interface Sci. 2022, 627, 81–89. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, E.-Q. Catalytic C(sp)-H Carboxylation with CO2. Coord. Chem. Rev. 2023, 486, 215138. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Shi, Y.; Han, J.; Yang, Z.; Zhang, Y.; Long, C.; Guo, J.; Zhu, Y.; Qiu, X.; et al. Boosting CO2 Conversion with Terminal Alkynes by Molecular Architecture of Graphene Oxide-Supported Ag Nanoparticles. Matter 2020, 3, 558–570. [Google Scholar] [CrossRef]
- Amirov, R.R.; Shayimova, J.; Nasirova, Z.; Solodov, A.; Dimiev, A.M. Analysis of Competitive Binding of Several Metal Cations by Graphene Oxide Reveals the Quantity and Spatial Distribution of Carboxyl Groups on Its Surface. Phys. Chem. Chem. Phys. 2018, 20, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Grote, F.; Gruber, C.; Borrnert, F.; Kaiser, U.; Eigler, S. Thermal Disproportionation of Oxo-Functionalized Graphene. Angew. Chem. Int. Ed. Engl. 2017, 56, 9222–9225. [Google Scholar] [CrossRef]
- Erickson, B.K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, I.; Suryani, R.A.; Ristiana, D.D.; Maristya, A.H.; Krisnandi, Y.K.; Handayani, M. Nanosilver-Decorated Reduced Graphene Oxide for Catalytic Carboxylation of Phenylacetylene with CO2. Mater. Chem. Phys. 2024, 314, 128852. [Google Scholar] [CrossRef]
- Molla, R.A.; Ghosh, K.; Banerjee, B.; Iqubal, M.A.; Kundu, S.K.; Islam, S.M.; Bhaumik, A. Silver Nanoparticles Embedded Over Porous MOF for CO2 Fixation via Carboxylation of Terminal Alkynes at Ambient Pressure. J. Colloid Interface Sci. 2016, 477, 220–229. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Zhang, Z.; Xie, R.; Fang, X.; Zhou, M. Carboxylation of Terminal Alkynes with CO2 Catalyzed by Novel Silver N-Heterocyclic Carbene Complexes. Dalton Trans. 2016, 45, 10577–10584. [Google Scholar] [CrossRef]
- Guo, C.-X.; Yu, B.; Xie, J.-N.; He, L.-N. Silver Tungstate: A Single-Component Bifunctional Catalyst for Carboxylation of Terminal Alkynes with CO2 in Ambient Conditions. Green Chem. 2015, 17, 474–479. [Google Scholar] [CrossRef]
- Faba, L.; Ordóñez, S. Carboxylation Reactions for the Sustainable Manufacture of Chemicals and Monomers. RSC Sustain. 2024, 2, 3167–3182. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Kayal, U.; Chowdhury, I.H.; Ghosh, S.; Islam, S.M. Nanoporous ZnO Supported Cubr (Cubr/Zno): An Efficient Catalyst for CO2 Fixation Reactions. ChemSel 2019, 4, 1069–1077. [Google Scholar] [CrossRef]
- Liu, C.; Luo, Y.; Zhang, W.; Qu, J.; Lu, X. DFT Studies on the Silver-Catalyzed Carboxylation of Terminal Alkynes with CO2: An Insight into the Catalytically Active Species. Organometallics 2014, 33, 2984–2989. [Google Scholar] [CrossRef]
- Fang, G.; Bi, X. Silver-Catalysed Reactions of Alkynes: Recent Advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef]
- Bu, R.; Zhang, L.; Gao, L.-L.; Sun, W.-J.; Yang, S.-L.; Gao, E.-Q. Copper(I)-Modified Covalent Organic Framework for CO2 Insertion to Terminal Alkynes. Mol. Catal. 2021, 499, 111319. [Google Scholar] [CrossRef]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-Organic Frameworks for CO2 Chemical Transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, C.; Zeng, C.; Mousavi, B.; Chaemchuen, S.; Verpoort, F. Carboxylation of Terminal Alkynes with Carbon Dioxide Catalyzed by an in-situ Ag2O/N-Heterocyclic Carbene Precursor System. ChemCatChem 2017, 9, 882–887. [Google Scholar] [CrossRef]
- Salam, N.; Paul, P.; Ghosh, S.; Mandi, U.; Khan, A.; Alam, S.M.; Das, D.; Islam, S.M. AgNPs Encapsulated by an Amine-Functionalized Polymer Nanocatalyst for CO2 Fixation as a Carboxylic Acid and the Oxidation of Cyclohexane Under Ambient Conditions. New J. Chem. 2020, 44, 5448–5456. [Google Scholar] [CrossRef]
- Trivedi, M.; Bhaskaran, B.; Kumar, A.; Singh, G.; Kumar, A.; Rath, N.P. Metal-Organic Framework MIL-101 Supported Bimetallic Pd-Cu Nanocrystals as Efficient Catalysts for Chromium Reduction and Conversion of Carbon Dioxide at Room Temperature. New J. Chem. 2016, 40, 3109–3118. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Jing, M.; Liu, J.; Zhao, Z.; Xu, G.; Song, W.; Wei, Y.; Sun, Y. Ordered Mesoporous CeO2-Supported Ag as an Effective Catalyst for Carboxylative Coupling Reaction Using CO2. ChemCatChem 2019, 11, 2089–2098. [Google Scholar] [CrossRef]
- Yun, Y.; Sheng, H.; Bao, K.; Xu, L.; Zhang, Y.; Astruc, D.; Zhu, M. Design and Remarkable Efficiency of the Robust Sandwich Cluster Composite Nanocatalysts ZIF-8@Au25@ZIF-67. J. Am. Chem. Soc. 2020, 142, 4126–4130. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Jiang, H.-Q.; Xu, H.; Wang, S.; Jiang, H.-X.; Zhu, S.-L.; Yin, L.; Guo, D.; Zhu, X. Photocatalytic Deuterocarboxylation of Alkynes with Oxalate. Chem. Sci. 2024, 15, 13041–13048. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-W.; Suo, Q.-L.; Hong, H.-L.; Zhu, N.; Wang, Y.-Q.; Han, L.-M. A Study on C—H Carboxylation Reaction of Terminal Alkynes with CO2 in Supercritical CO2. Chin. J. Org. Chem. 2015, 34, 2172–2177. [Google Scholar] [CrossRef]
- Dutta, G.; Jana, A.K.; Singh, D.K.; Eswaramoorthy, M.; Natarajan, S. Encapsulation of Silver Nanoparticles in an Amine-Functionalized Porphyrin, Met. Framew. Its Use a Heterog. Catal. CO2 Fixat. Atmos. Press. Chem.—Asian J. 2018, 13, 2677–2684. [Google Scholar] [CrossRef]
- Gulati, U.; Rajesh, U.C.; Rawat, D.S.; Zaleski, J.M. Development of Magnesium Oxide-Silver Hybrid Nanocatalysts for Synergistic Carbon Dioxide Activation to Afford Esters and Heterocycles at Ambient Pressure. Green Chem. 2020, 22, 3170–3177. [Google Scholar] [CrossRef]
- Rajesh, U.C.; Losovyj, Y.; Chen, C.H.; Zaleski, J.M. Designing Synergistic Nanocatalysts for Multiple Substrate Activation: Interlattice Ag-Fe3O4 Hybrid Materials for CO2-Inserted Lactones. ACS Catal. 2020, 10, 3349–3359. [Google Scholar] [CrossRef]

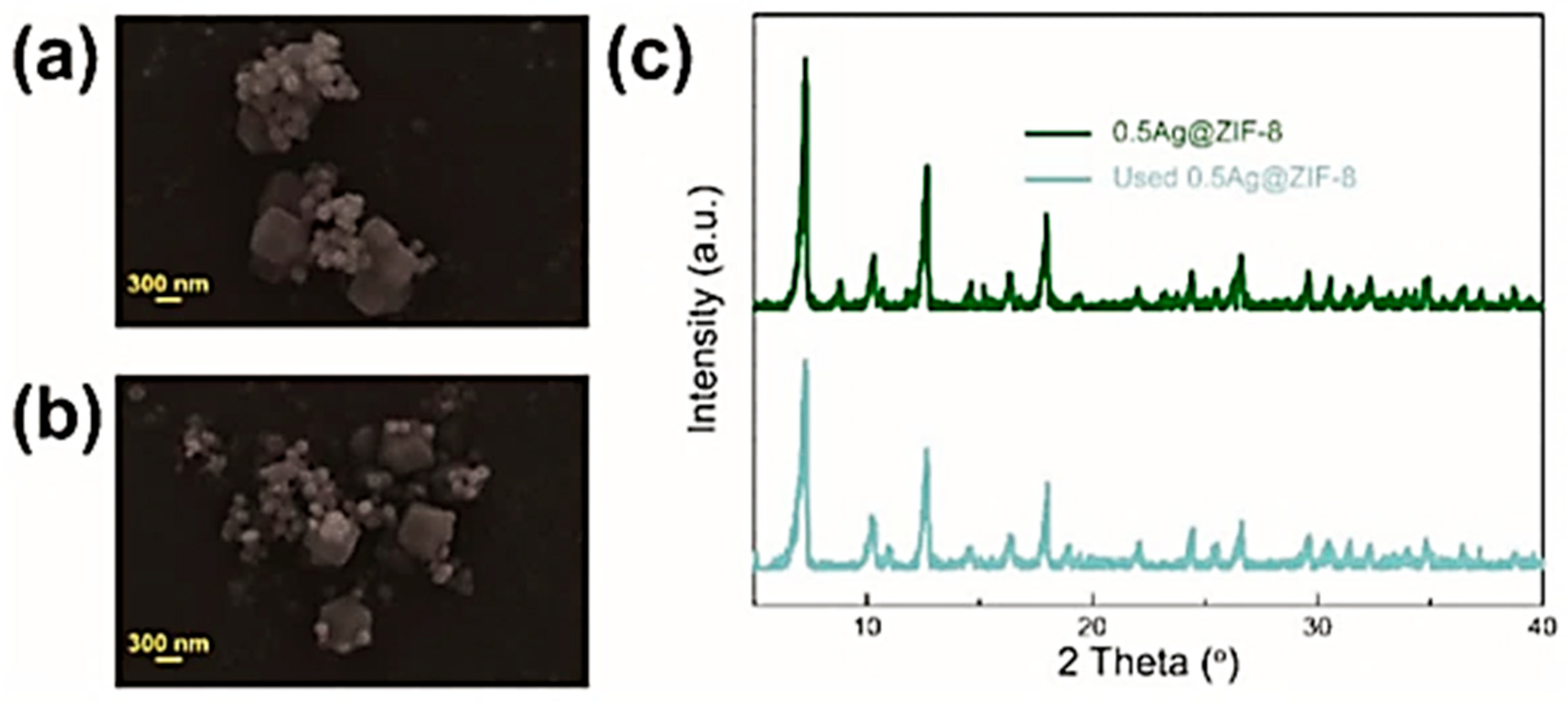


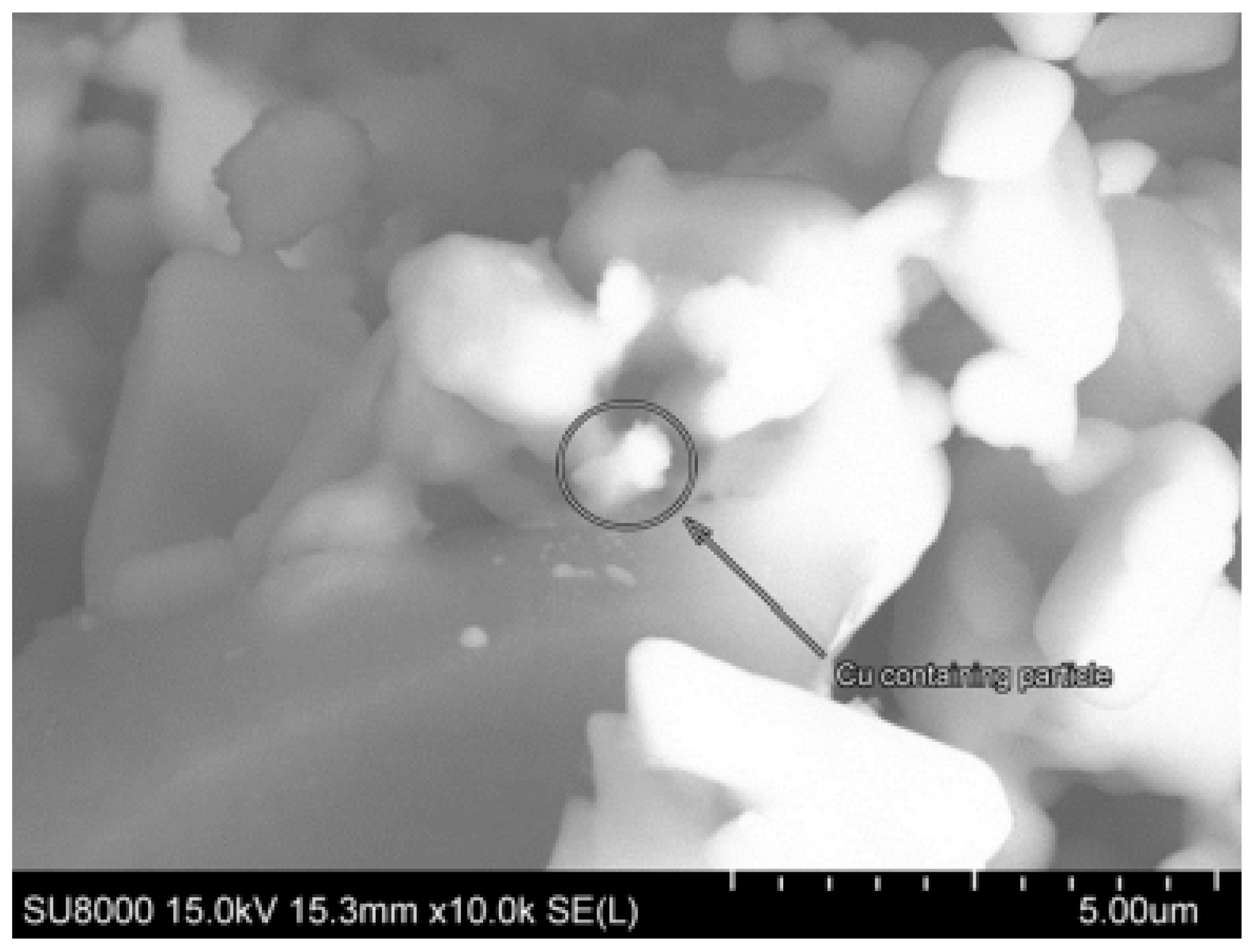
| Catalyst | Solvent | Substrate | Reaction Conditions | Yield, % | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Product | T (°C) | Pressure, MPa | Cat. Loading | |||||
| 5% CuBr@C Cs2CO3 | EC (3 mL) | phenylacetylene | phenylpropiolic acid | 80 | - | 196 mg, 0.6 mmol | 90 | [43] |
| 3-chlorophenylacetylene | 3-chlorophenylpropiolic acid | 77 | ||||||
| Cu(IN)-MOF Cs2CO3 | DMF (3 mL) | 4-ethylphenylacetylene | 4-ethylphenylpropiolic acid | 80 | 0.1 | 30 mg 1.5 mmol | 80 | [44] |
| 1% CZU-7 Cs2CO3 | DMF (20 mL) | 4-fluorophenylacetylene | 4-fluorophenylpropiolic acid | 100 | 0.3 | 4 mmol 6 mmol | 78 | [47] |
| 30 Cu(IN)-MOF Cs2CO3 | DMF (3 mL) | phenylacetylene | phenylpropiolic acid | 80 | 0.1 | 30 mg 1.5 mmol | 80 | [48] |
| 1% CuNO3/phenanthroline Cs2CO3 | DMF (3 mL) | phenylacetylene | phenylpropiolic acid | 50 | 0.5 | - | 98 | [49] |
| 5% CuNPs/Al2O3 Cs2CO3 | DMF | 2,4,6-trimethylphenylacetylene | butyl 2,4,6-trimethylphenylpropiolate | 60 | 0.2 | 2 mmol | 90 | [50] |
| [Cu(Im12)2][CuBr2] Cs2CO3 | DMF (2.5 mL) | cyclopropylacetylene | butyl cyclopropylpropiolate | 25 | - | 0.019 g 0.6 mmol | 97 | [51] |
| 5% CuCl Cs2CO3 | - | phenylacetylene | phenylpropiolic acid | 50 | 8 | - | 25 | [52] |
| 2% CuI DBU | - | phenylacetylene | - | 50 | 8 | - | 92 | [52] |
 | |||||
|---|---|---|---|---|---|
| Entry | Time (h) | Catalyst Loading (mg (Cu % mmol) a) | Temperature (°C) | Solvent b (3 mL) | Yield c (%) |
| 1 | 1 | 30 (0.47) | 80 | DMF | 55 |
| 2 | 2 | 30 (0.47) | 80 | DMF | 63 |
| 3 | 4 | 30 (0.47) | 80 | DMF | 80 |
| 4 | 12 | 30 (0.47) | 80 | DMF | 74 |
| 5 | 4 | 10 (0.16) | 80 | DMF | 72 |
| 6 | 4 | 50 (0.78) | 80 | DMF | 69 |
| 7 | 4 | 30 (0.47) | 60 | DMF | 33 |
| 8 | 4 | 30 (0.47) | 100 | DMF | 64 |
| 9 | 4 | 30 (0.47) | 80 | DMSO | 62 |
| 10 | 4 | 30 (0.47) | 80 | CH3CN | 52 |
| 11 | 4 | 30 (0.47) | 80 | DMI | 45 |
| 12 | 4 | 30 (0.47) | 80 | MeOH | - |
| 13 | 4 | 30 (0.47) | 80 | EC | 19 |
| 14 d | 4 | 30 (0.47) | 80 | DMF | 12 |
| 15 e | 4 | 30 (0.47) | 80 | DMF | 31 |
| 16 f | 4 | 28 (0.47) | 80 | DMF | 45 |
| 17 g | 4 | 17.5 (0) | 80 | DMF | - |
| 18 h | 4 | 30 (0.47) | 80 | DMF | - |
| 19 i | 4 | 30 (0.47) | 80 | DMF | 83 |
| 20 j | 4 | 30 (0.47) | 80 | DMF | 84 |
| 21 k | 4 | 30 (0.47) | 80 | DMF | 84 |
| 22 l | 4 | 30 (0.47) | 80 | DMF | 83 |
| 23 m | 4 | 30 (0.47) | 80 | DMF | 79 |
| 24 n | 4 | 30 (0.47) | 80 | DMF | 68 |
 | ||||
|---|---|---|---|---|
| Entry | Pressure (MPa) | T (°C) | Time (h) | Yield b (%) |
| 1 | 8 | 60 | 24 | 64 |
| 2 | 8 | 70 | 24 | 78 |
| 3 | 8 | 80 | 24 | 84 |
| 4 | 8 | 90 | 24 | 71 |
| 5 | 6 | 80 | 24 | 73 |
| 6 | 12 | 80 | 24 | 86 |
| 7 | 12 | 80 | 16 | 90 |
| 8 c | 12 | 80 | 16 | 72 |
 | ||
|---|---|---|
| Entry | Solvent | Yield b (%) |
| 1 | DMF | 70 |
| 2 | DMC | 15 |
| 3 | DEC | 16 |
| 4 | PC | 73 |
| 5 | EC | 88 |
| Entry | Temperature (°C) | Time (h) | Conversion (%) b |
|---|---|---|---|
| 1 | 40 | 14 | 28 |
| 2 | 50 | 14 | 51 |
| 3 | 60 | 14 | 69 |
| 4 | 70 | 14 | 73 |
| 5 | 80 | 14 | 98 |
| 6 | 90 | 14 | 81 |
| 7 | 80 | 8 | 56 |
| 8 | 80 | 10 | 68 |
| 9 | 80 | 12 | 81 |
| 10 | 80 | 16 | 98 |
| 11 c | 80 | 14 | 23 |
| 12 d | 80 | 14 | 46 |
| Entry | Base | Amount of Base (mmol) | Yield (%) b |
|---|---|---|---|
| 1 | Cs2CO3 | 0.8 | 45 |
| 2 | Cs2CO3 | 1 | 67 |
| 3 | Cs2CO3 | 1.2 | 80 |
| 4 | Cs2CO3 | 1.5 | 98 |
| 5 | Cs2CO3 | 1.8 | 94 |
| 6 | tBuOK | 1.5 | 67 |
| 7 | Na2CO3 | 1.5 | 42 |
| 8 | K2CO3 | 1.5 | 35 |
| 9 c | - | - | Trace |
| 10 d | Cs2CO3 | 1.5 | Trace |
| 11 e | Cs2CO3 | 1.5 | 25 |
| 12 f | Cs2CO3 | 1.5 | <5 |
| 13 g | Cs2CO3 | 1.5 | <5 |
| Entry | Cat. (mol%) | Base | Solvent b | Temp. (°C) | Conv. b (%) | Select. b (%) |
|---|---|---|---|---|---|---|
| 1 | CZU-7 (0.4) | Cs2CO3 | DMF | 100 | 41 | 100 |
| 2 | CZU-7 (0.6) | Cs2CO3 | DMF | 100 | 53 | 100 |
| 3 | CZU-7 (0.8) | Cs2CO3 | DMF | 100 | 72 | 100 |
| 4 | CZU-7 (1.0) | Cs2CO3 | DMF | 100 | 87 | 100 |
| 5 c | CZU-7 (1.0) | Cs2CO3 | DMF | 100 | 82 | 100 |
| 6 | CZU-7 (1.0) | Na2CO3 | DMF | 100 | 17 | 100 |
| 7 | CZU-7 (1.0) | K2CO3 | DMF | 100 | 29 | 100 |
| 8 | CZU-7 (1.0) | CsF | DMF | 100 | 3 | 100 |
| 9 | CZU-7 (1.0) | CsOAc | DMF | 100 | 2 | 100 |
| Entry | Base | Yield b (%) |
|---|---|---|
| 1 c | Cs2CO3 | 76 |
| 2 | K2CO3 | 5 |
| 3 | KOH | <1 |
| 4 | CsF | <1 |
| 5 | tBuOLi | 2 |
| 6 | CsOAc | <1 |
| 7 | TBD | 21.2 |
| 8 | NaNH2 | <1 |
| Entry | Catalyst | Base | Solvent | Time (h) | Yield(%) b |
|---|---|---|---|---|---|
| 1 | TpBpy-Cu-14 | Cs2CO3 | DMSO | 6 | 62 |
| 2 | TpBpy-Cu-14 | Cs2CO3 | DMF | 6 | 56 |
| 3 | TpBpy-Cu-14 | Cs2CO3 | PC | 6 | 11 |
| Entry | Base | Solvent | Catalyst Loading (mol%) b | Yield |
|---|---|---|---|---|
| 1 | Cs2CO3 | DMF | 0.25 | 98 |
| 2 | Cs2CO3 | DMSO | 0.25 | 92 |
| 3 | Cs2CO3 | CH3CN | 1.00 | 11 |
| 4 | Cs2CO3 | H2O | 0.50 | - |
| 5 | Cs2CO3 | MeOH | 0.50 | - |
| 6 | K2CO3 c | DMF | 0.25 | 41 |
| 7 | KOtBu | DMF | 0.25 | 47 |
| 8 d | Cs2CO3 | DMF | 0.25 | 48 |
| 9 e | Cs2CO3 | DMF | 0.25 | 80 |
| 10 | Cs2CO3 | DMF | 0.10 | 95 |
| 11 | Cs2CO3 | DMF | 0.075 | 87 |
| S. No. | Catalyst | Solvent | Light Irradiation | Conv. b | Yield | Ratio (A:B) | |
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| 1 | CZU-7 (0.4) | DMF | Yes | 30 | 18 | 12 | 1.5:1 |
| 2 | CZU-7 (0.6) | DMF | Yes | 20 | 11 | 9 | 1.1:0.9 |
| 3 | ZBr-10 | DMF | Yes | 84 | 44 | 40 | 1.1:1 |
| 4 | ZBr-10 | DMF | No | - | - | - | - |
| 5 | - | DMF | Yes | - | - | - | - |
| 6 | ZBr-10 | DMA | Yes | 65 | 36 | 29 | 1.2:1 |
| 7 | ZBr-10 | THF | Yes | 22 | 12 | 10 | 1.2:1 |
| 8 | ZBr-10 | DMSO | Yes | 30 | 17 | 13 | 1.3:1 |
| 9 | ZBr-10 | Water | Yes | 25 | 12 | 13 | 0.92:1 |
| 10 | ZBr-10 | ACN | Yes | 45 | 24 | 21 | 1.14:1 |
| 11 | ZBr-5 | DMF | Yes | 65 | 34 | 31 | 1.1:1 |
| 12 | ZBr-15 | DMF | Yes | 83 | 42 | 40 | 1.1:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myakota, V.; Strekalova, A.; Shesterkina, A.; Kirichenko, O.; Kustov, A.; Kustov, L. Catalytic Carboxylation of Terminal Alkynes with CO2: An Overview. Catalysts 2025, 15, 870. https://doi.org/10.3390/catal15090870
Myakota V, Strekalova A, Shesterkina A, Kirichenko O, Kustov A, Kustov L. Catalytic Carboxylation of Terminal Alkynes with CO2: An Overview. Catalysts. 2025; 15(9):870. https://doi.org/10.3390/catal15090870
Chicago/Turabian StyleMyakota, Valeria, Anna Strekalova, Anastasiya Shesterkina, Olga Kirichenko, Alexander Kustov, and Leonid Kustov. 2025. "Catalytic Carboxylation of Terminal Alkynes with CO2: An Overview" Catalysts 15, no. 9: 870. https://doi.org/10.3390/catal15090870
APA StyleMyakota, V., Strekalova, A., Shesterkina, A., Kirichenko, O., Kustov, A., & Kustov, L. (2025). Catalytic Carboxylation of Terminal Alkynes with CO2: An Overview. Catalysts, 15(9), 870. https://doi.org/10.3390/catal15090870










