Alumina-Supported Manganese Catalysts for Soot Combustion Prepared by Thermal Decomposition of KMnO4
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Results
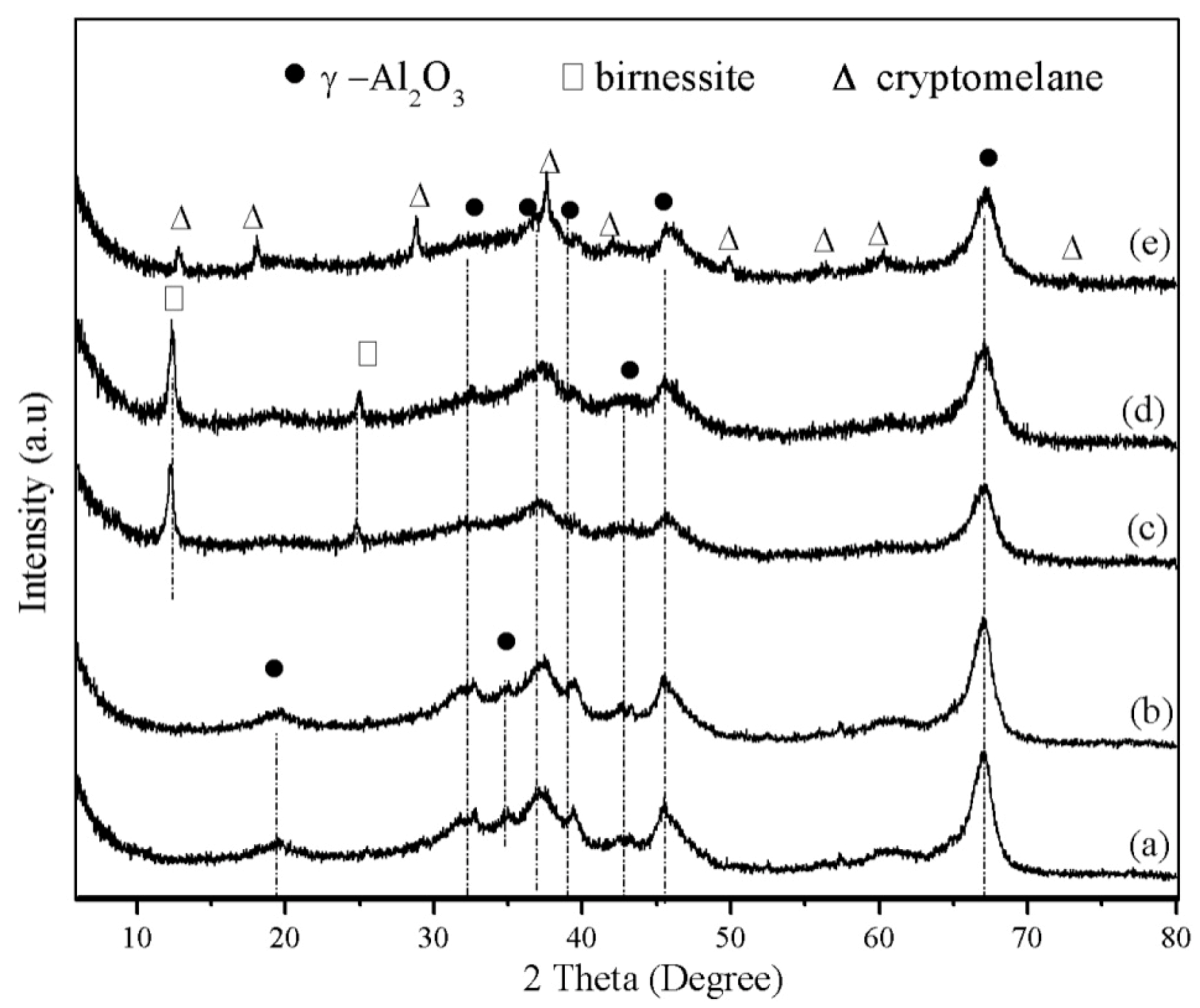
| Sample | Al (wt%) | Mn (wt%) | K (wt%) | K/Mn (Molar ratio) |
|---|---|---|---|---|
| MnAlT400 | 30.3 | 5.8 | 3.6 | 0.87 |
| MnAlT400W | 38.2 | 5.8 | 1.8 | 0.44 |
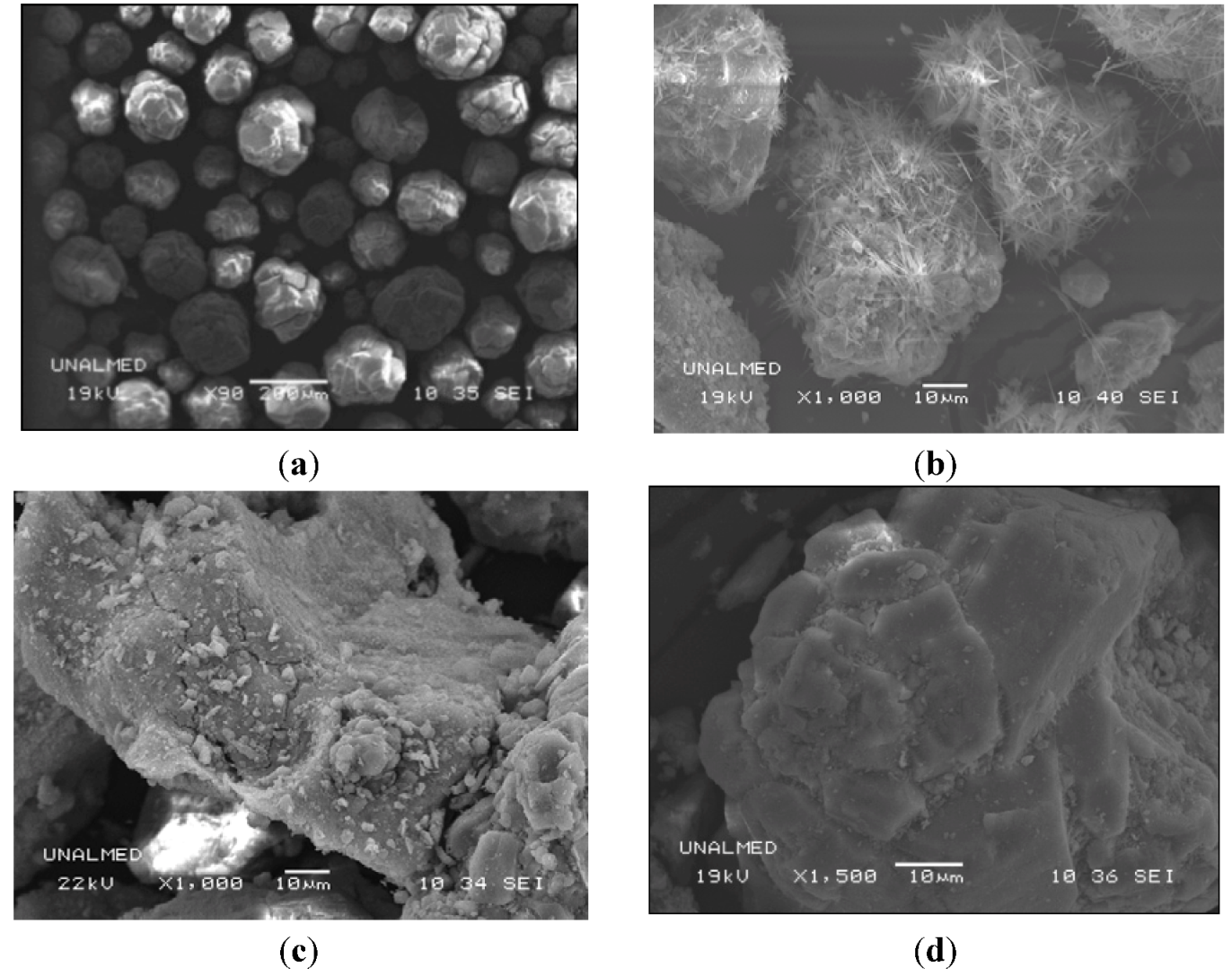
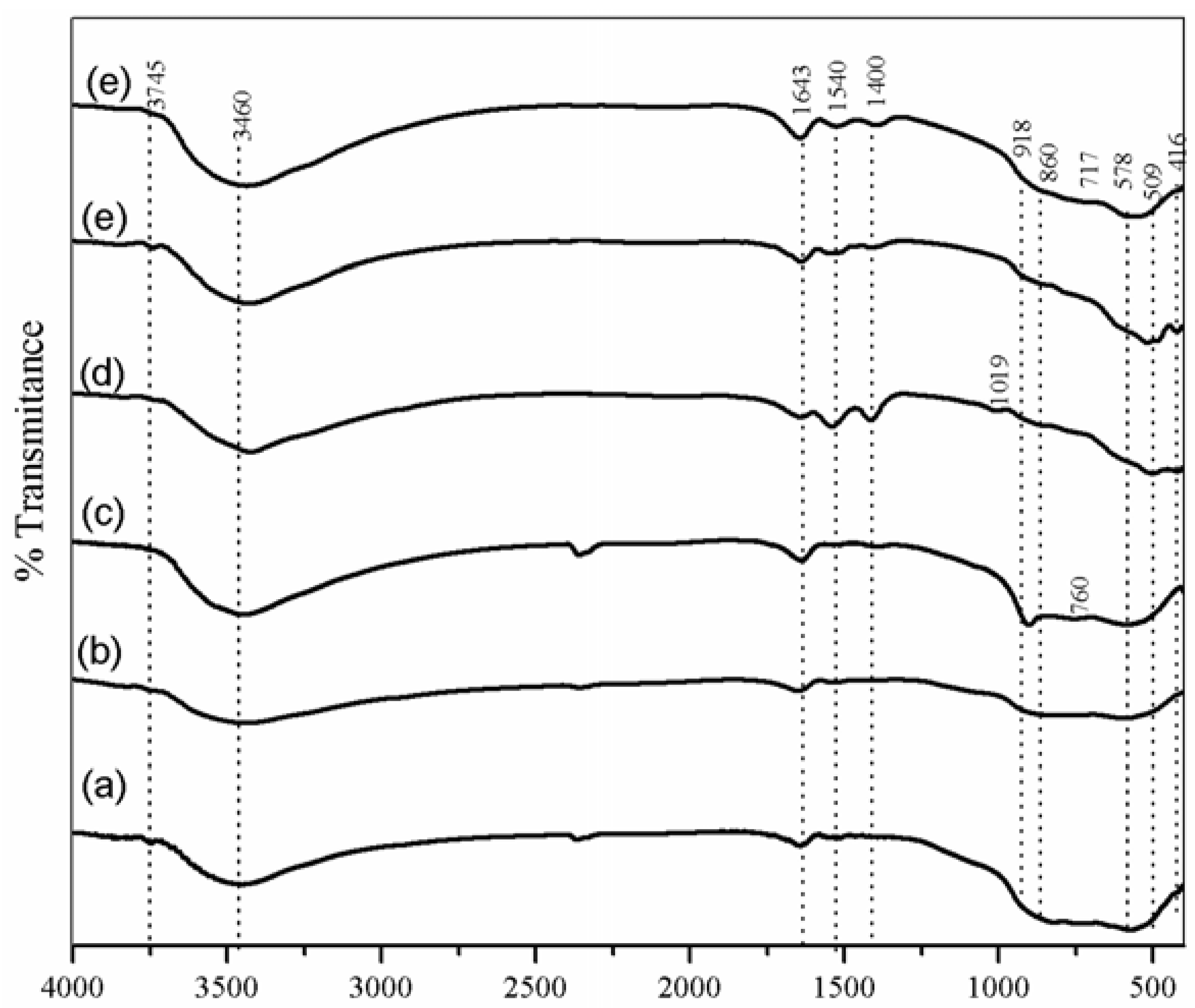
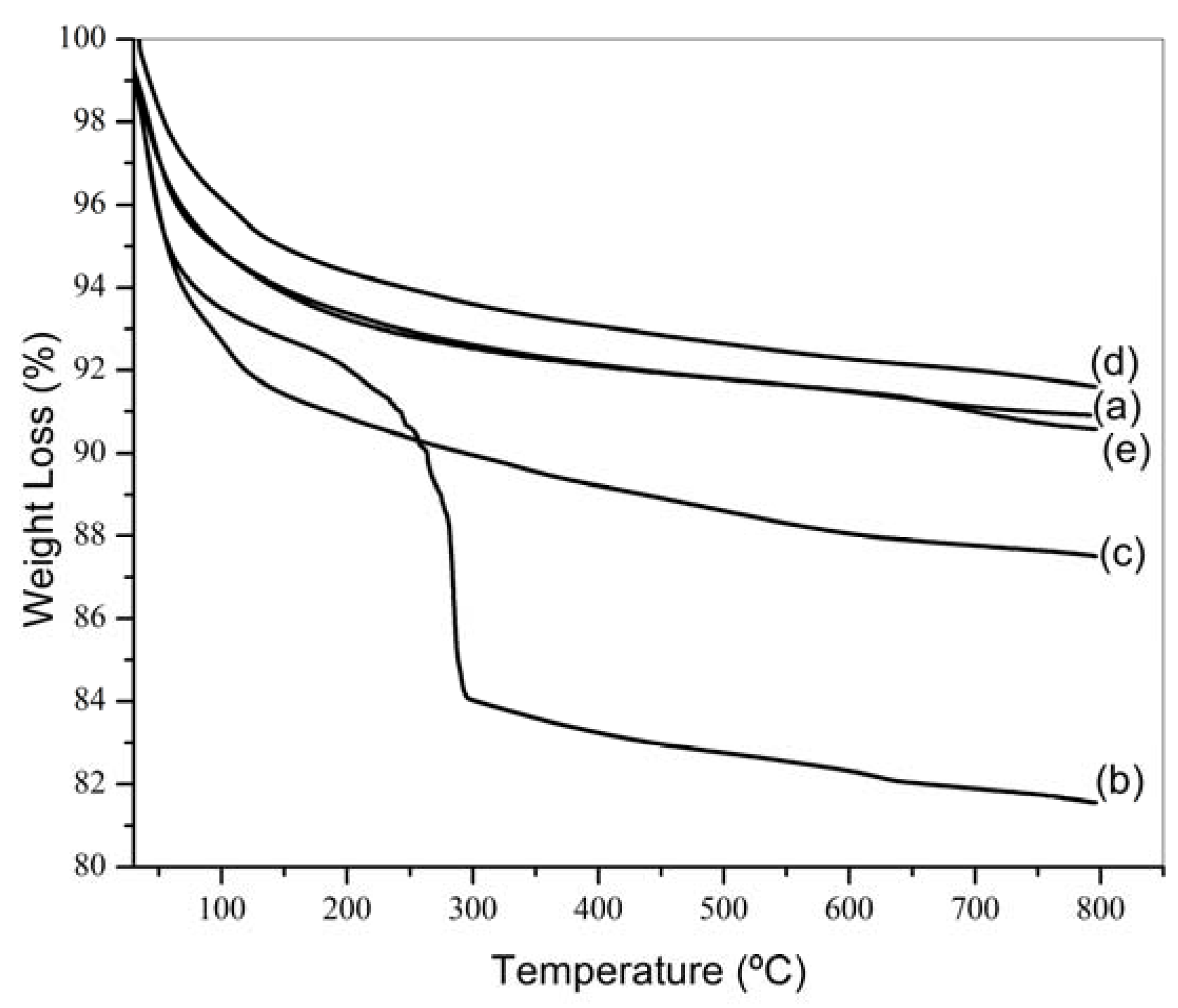
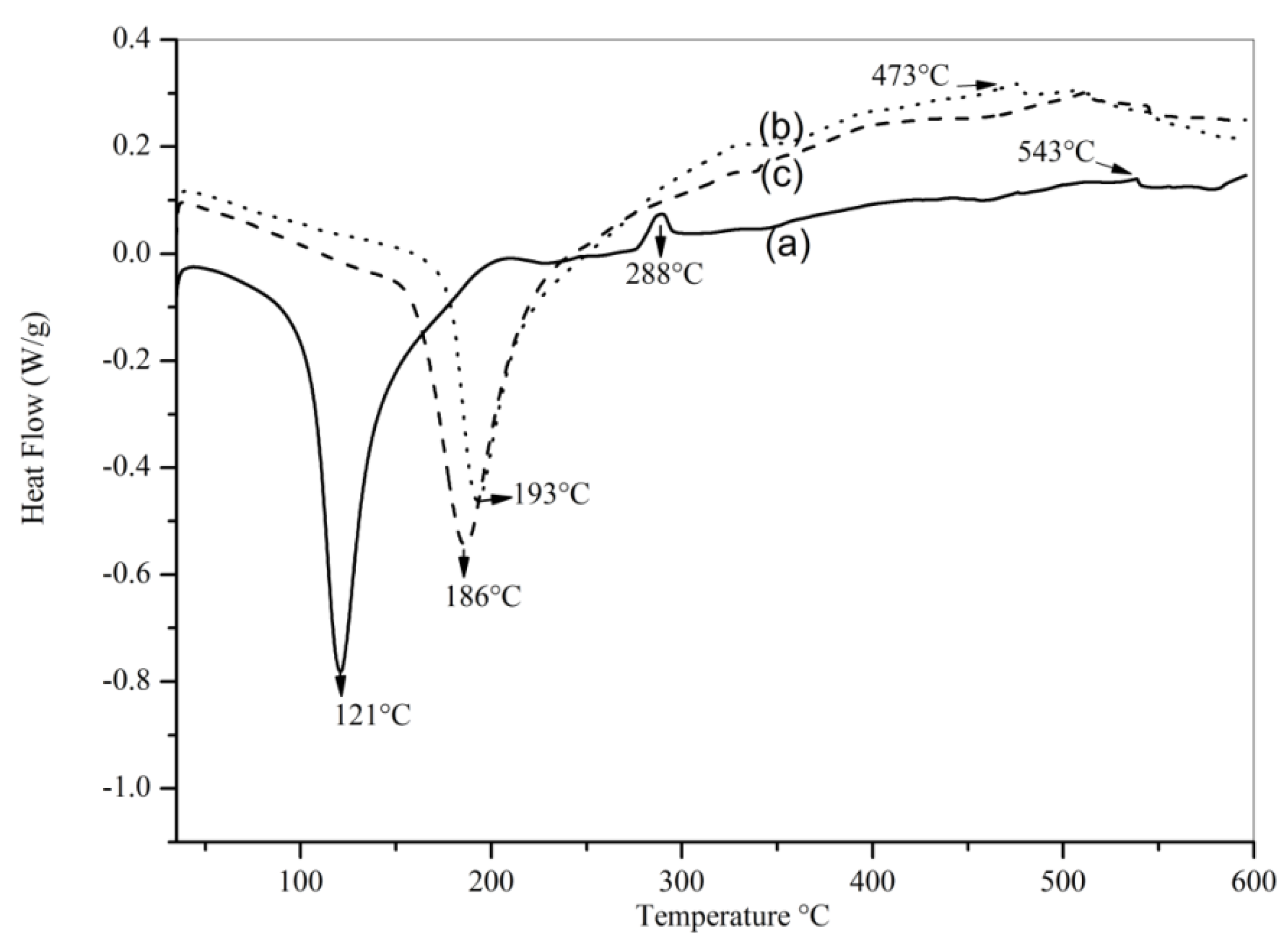
| Sample | Until 250 °C (wt%) | 250 to 500 °C (wt%) | Above 500 °C (wt%) | Total (wt%) |
|---|---|---|---|---|
| γ-Al2O3 | 7.2 | 1.0 | 0.9 | 9.1 |
| KMnO4 impregnated on γ-Al2O3 (non-calcined) | 9.5 | 7.8 | 1.2 | 18.5 |
| MnAlT400 | 9.7 | 1.7 | 1.1 | 12.5 |
| MnAl T400W | 6.5 | 0.9 | 1.0 | 8.4 |
| MnAlW600 | 7.2 | 1.0 | 1.2 | 9.4 |
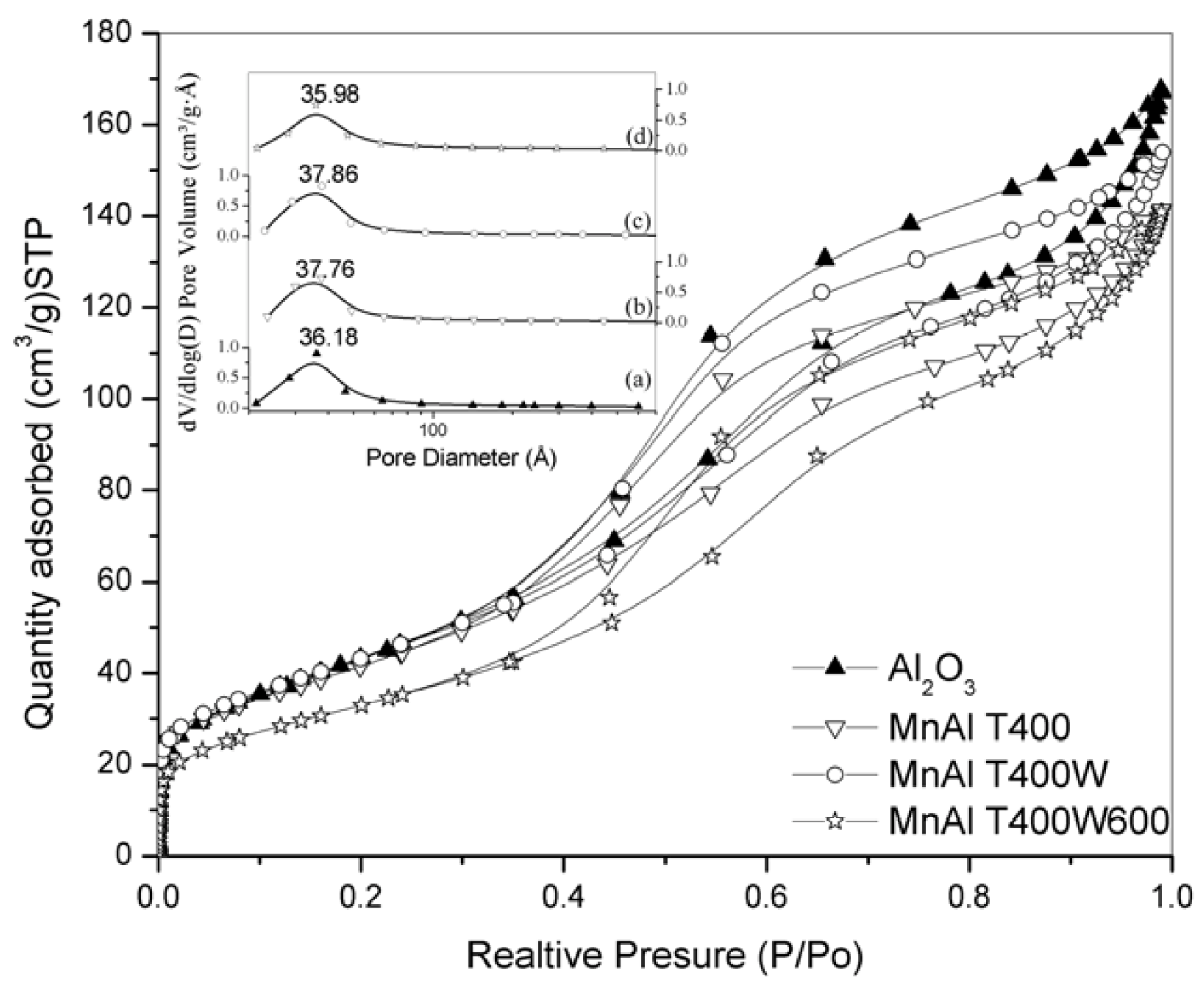
| Material | SBET (m2/g) | Vpore (cm3/g) | DBJH (Å) |
|---|---|---|---|
| γ-Al2O3 | 165 | 0.26 | 41.2 |
| KMnO4 impregnated on γ-Al2O3 (non-calcined) | 148 | 0.20 | 36.8 |
| MnAlT400 | 151 | 0.22 | 41.2 |
| MnAlT400W | 158 | 0.25 | 41.4 |
| MnAlT400W600 | 121 | 0.22 | 44.7 |

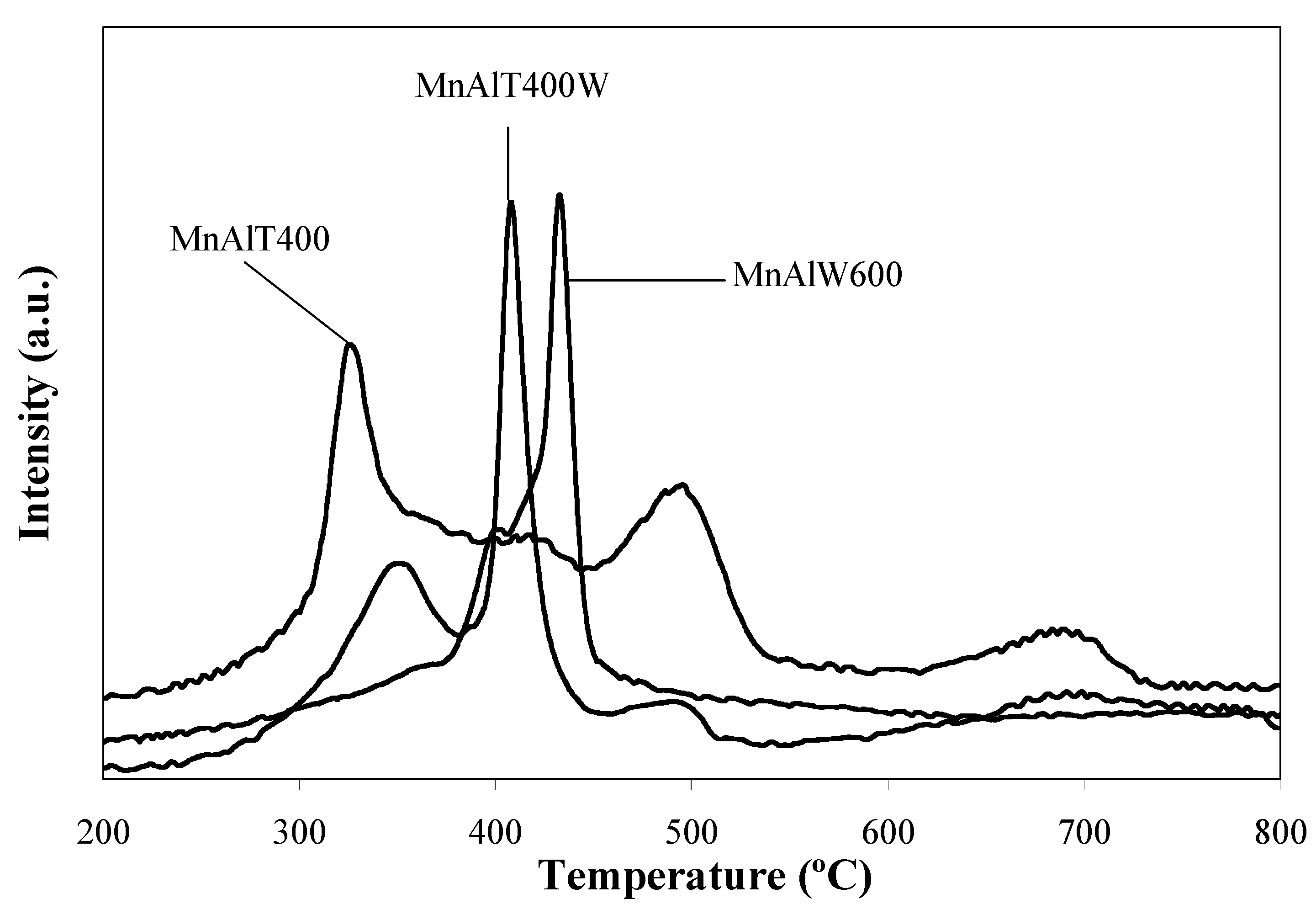

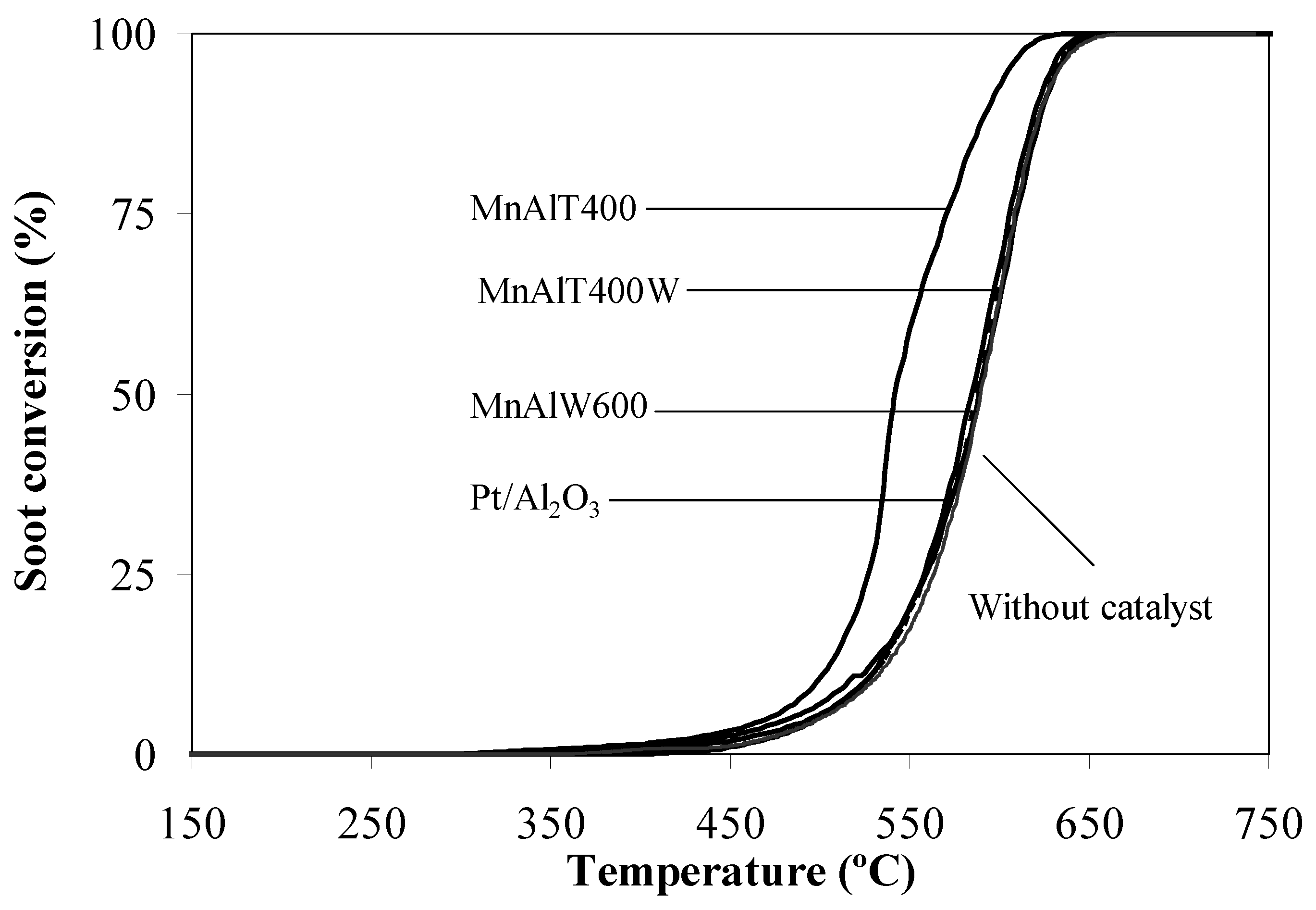
2.2. Catalytic Tests
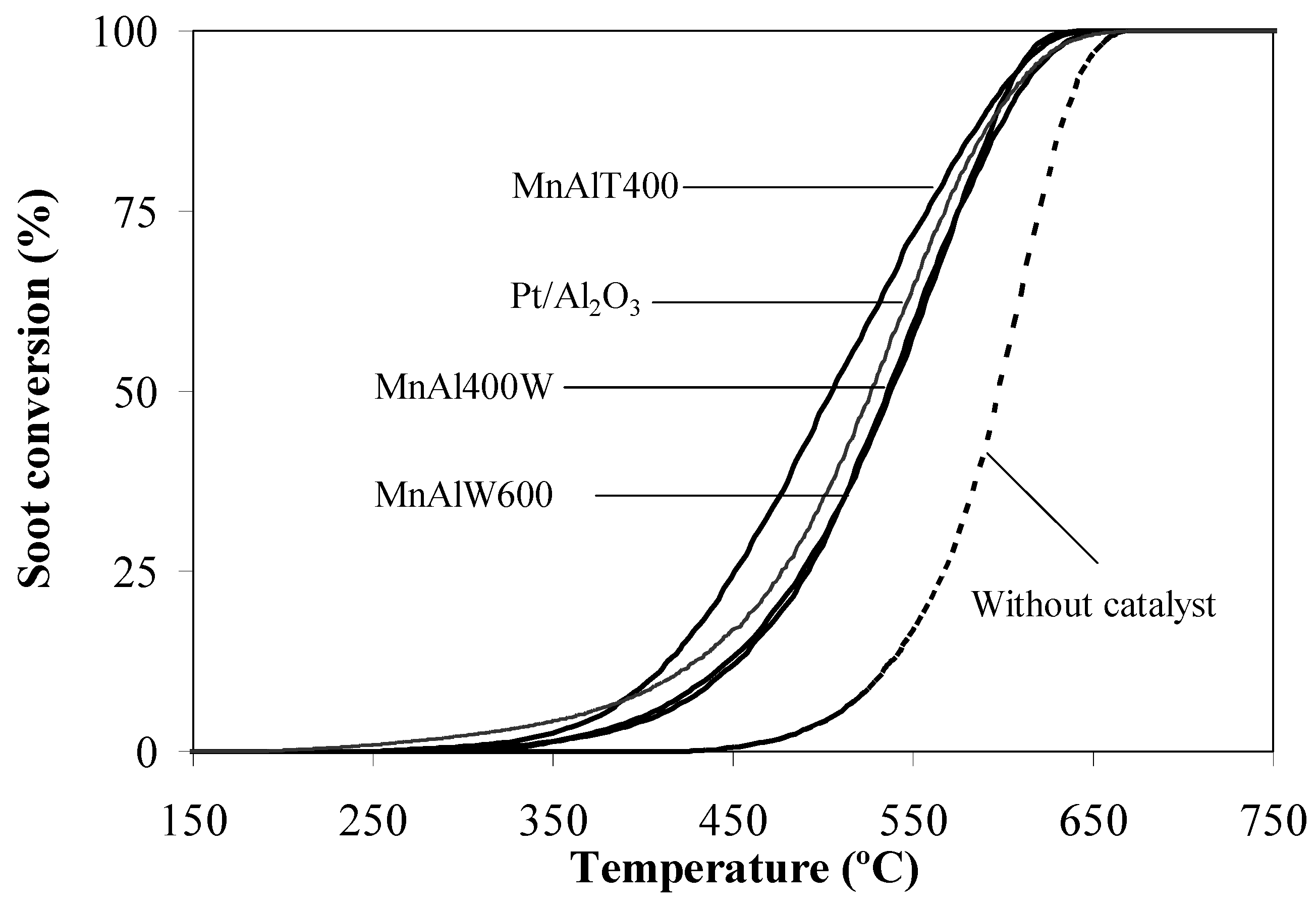

3. Experimental Section
3.1. Preparation of Sample
- - MnAlT400: After KMnO4 impregnation on γ-Al2O3, the sample is calcined in air at 400 °C for 6 h (heating rate 10°C/min).
- - MnAlT400W: The previously prepared sample MnAlT400 is washed with distilled and deionized water to remove soluble salts (potassium manganate), and after washing, the sample is dried at 60°C for 24 h.
- - MnAlW600: The previously prepared sample MnAlT400W is calcined at 600°C for 6 h (heating rate 10°C/min).
3.2. Characterization of Samples
3.3. Catalytic Tests
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and technology of catalytic diesel particulate filters. Catal. Rev. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Bueno-López, A.; Soriano-Mora, J.M.; García-García, A. Study of the temperature window for the selective reduction of NOx in O2-rich gas mixtures by metal-loaded carbon. Catal. Commun. 2006, 7, 678–684. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J. Cu/Al2O3 catalysts for soot oxidation: Copper loading effect. Appl. Catal. 2008, 84, 651–658. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Ura, B.; Trawczynski, J. Potassium stability in soot combustion perovskite catalysts. Top. Catal. 2009, 55, 2097–2100. [Google Scholar]
- Atribak, I.; Bueno-Lopez, A.; García-García, A.; Navarro, P.; Frías, D.; Montes, M. Catalytic activity for soot combustion of birnessite and cryptomelane. Appl. Catal. 2010, 93, 267–273. [Google Scholar] [CrossRef]
- Becerra, M.E.; Arias, N.P.; Giraldo, O.H.; López Suárez, F.E.; Illán Gómez, M.J.; Bueno López, A. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4. Appl. Catal. 2011, 102, 260–266. [Google Scholar] [CrossRef]
- Ciambelli, P.; Palma, V.; Russo, P.; Vaccaro, S.J. Redox properties of a TiO2 supported Cu-V-K-Cl catalyst in low temperature soot oxidation. J. Mol. Catal. 2003, 204–205, 673–681. [Google Scholar] [CrossRef]
- Harrison, P.G.; Ball, I.K.; Daniell, W.; Lukinskas, P.; Caspedes, M.E.; Mirò, E.; Ulla, M.A. Cobalt catalysts for the oxidation of diesel soot particulate. Chem. Eng. J. 2003, 95, 47–55. [Google Scholar] [CrossRef]
- Russo, N.; Furfori, S.; Fino, D.; Saracco, G.; Specchia, V. Lanthanum cobaltite catalysts for diesel soot combustion. Appl. Catal. 2008, 83, 85–95. [Google Scholar] [CrossRef]
- Peralta, M.A.; Gross, M.S.; Ulla, M.A.; Querini, C.A. Catalyst formulation to avoid reaction runaway during diesel soot combustion. Appl. Catal. 2009, 367, 59–69. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; de Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Soot combustion over silver-supported catalysts. Appl. Catal. 2009, 91, 489–498. [Google Scholar] [CrossRef]
- Wu, X.; Lin, F.; Xu, H.; Weng, D. Effects of adsorbed and gaseous NOx species on catalytic oxidation of diesel soot with MnOx-CeO2 mixed oxides. Appl. Catal. 2010, 96, 101–109. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Duan, A.; Jiang, G. Simultaneous Removal of Soot and NOx over the (La1.7Rb0.3CuO4)x/nm CeO2 Nanocomposite Catalysts. Ind. Eng. Chem. Res. 2010, 49, 3112–3119. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Q.; Huang, A.; Suib, S.L. Total oxidation of volatile organic compounds with hydrophobic cryptomelane-type octahedral molecular sieves. Microporous Mesoporous Mater. 2000, 35, 209–216. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zerger, R.P.; DeGuzman, R.N.; Suib, S.L.; McCurdy, L.; Potter, D.I. Octahedral molecular sieves: Synthesis, characterization and applications. Science 1993, 260, 511–515. [Google Scholar]
- Ching, S.; Landrigan, J.A.; Jorgensen, M.L.; Duan, N.G.; Suib, S.L.; O’Young, C.L. Sol-Gel synthesis of birnessite from KMnO4 and simple sugars. Chem. Mater. 1995, 7, 1604–1606. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD), Powder Diffraction Database PDF-2; International Centre for Diffraction Data: Newton Square, PA, 2003; Set 53.
- Klose, F.; Wolff, T.; Lorenz, H.; Seidel-Morgenstern, A.; Suchorski, Y.; Piórkowska, M.; Weiss, H. Active species on γ-alumina-supported vanadia catalysts: Nature and reducibility. J. Catal. 2007, 247, 176–193. [Google Scholar] [CrossRef]
- Drits, V.A.; Silvester, E.; Gorshkov, A.I.; Manceau, A. The structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite. I. Results from X-ray diffraction and selected area electron diffraction. Am. Mineral. 1997, 82, 946–961. [Google Scholar]
- Tang, Q.; Huang, X.; Wu, C.; Zhao, P.; Chen, Y.; Yang, Y. Structure and catalytic properties of K-doped manganese oxide supported on alumina. J. Mol. Catal. Chem. 2009, 306, 48–53. [Google Scholar] [CrossRef]
- Stoilova, D.G.; Nickolov, R.N.; Cheshkova, K.T. Surface and texture characterization of alumina-supported copper and mixed copper–manganese oxide catalysts and their formate precursors. J. Colloid. Interface Sci. 2000, 228, 24–31. [Google Scholar] [CrossRef]
- Tejedor-Tejedor, M.I.; Anderson, M.A. The protonation of phosphate on the surface of goethite as studied by CIR-FTIR and electrophoretic mobility. Langmuir 1990, 6, 602–611. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry, 5th ed; John Wiley and Sons: New York, NY, USA, 1997; p. 199. [Google Scholar]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochim. Acta Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Q.; Suib, S.L. Mechanistic and kinetic studies of crystallization of birnessite. Inorg. Chem. 2000, 39, 741–747. [Google Scholar] [CrossRef]
- Ali, A.A.; Al-Sagheer, F.A.; Zaki, M.I. Surface texture, morphology and chemical composition of hydrothermally synthesized tunnel-structured manganese (IV) oxide. Int. J. Inorg. Mater. 2001, 3, 427–435. [Google Scholar] [CrossRef]
- Gao, T.; Glerup, M.; Krumeich, F.; Nesper, R.; Fjellvåg, H.; Norby, P. Microstructures and spectroscopic properties of cryptomelane-type manganese dioxide nanofibers. J. Phys. Chem. 2008, 112, 13134–13140. [Google Scholar]
- Boxun, H.; Hu, Y.C.; Samuel, J.; Frueh, L.J.; Raymond, J.; Steven, L.S. Removal of aqueous phenol by adsorption and oxidation with doped hydrophobic cryptomelane-type manganese oxide (K-OMS-2) nanofibers. J. Phys. Chem. 2010, 114, 21. [Google Scholar]
- Kappenstein, C.; Pirault-Roy, L.; Guérin, M.; Wahdan, T.; Ali, A.A.; Al-Sagheer, F.A.; Zaki, M.I. Monopropellant decomposition catalysts: V. Thermal decomposition and reduction of permanganates as models for the preparation of supported MnOx catalysts. Appl. Catal. Gen. 2002, 234, 145–153. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Steven, L.; Suib, S.L. Porous manganese oxide octahedral molecular sieves and octahedral layered materials. Acc. Chem. Res. 2008, 41, 479–487. [Google Scholar] [CrossRef]
- De Boer, J.H. The Structure and Properties of Porous Materials; Everett, D.H., Stone, F.S., Eds.; Colston Papers: London, UK, 1958; pp. 10–95. [Google Scholar]
- Gauden, P.A.; Terzyk, A.P.; Jaroniec, M.; Kowalczyk, P. Bimodal pore size distributions for carbons: Experimental results and computational studies. Colloid. Interface Sci. 2007, 310, 205–216. [Google Scholar] [CrossRef]
- Aguero, F.N.; Barbero, P.; Cadus, L.E. Combustion of volatile organic compounds over supported manganese oxide: Influence of the support, the precursor and the manganese loading. Catal. Today 2008, 133, 493–501. [Google Scholar] [CrossRef]
- Álvarez-Galván, M.C.; Pawelec, B.; de la Peña O’Shea, V.A.; Fierro, J.L.G.; Arias, P.L. Formaldehyde/methanol combustion on alumina-supported manganese-palladium oxide catalyst. Appl. Catal. 2004, 51, 83–91. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Gandia, L.M.; Vicente, M.A.; Gil, A. Preparation and characterization of manganese oxide catalysts supported on alumina and zirconia-pillared clays. Appl. Catal. 2000, 196, 281–292. [Google Scholar] [CrossRef]
- Perego, C.; Villa, P. Catalyst preparation methods. Catal. Today 1997, 34, 281–305. [Google Scholar] [CrossRef]
- Analytical Methods for Atomic Absorption Spectroscopy; The Perkin Elmer Corporation: Norwalk, CT, USA, 1994.
- Neeft, J.P.A.; van Pruissen, O.P.; Makkee, M.; Moulijn, J.A. Catalysts for the oxidation of soot from diesel exhaust gases II. Contact between soot and catalyst under practical conditions. Appl. Catal. 1997, 12, 21–31. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Becerra, M.-E.; Arias, N.-P.; Giraldo, O.-H.; López-Suárez, F.-E.; Illán-Gómez, M.-J.; Bueno-López, A. Alumina-Supported Manganese Catalysts for Soot Combustion Prepared by Thermal Decomposition of KMnO4. Catalysts 2012, 2, 352-367. https://doi.org/10.3390/catal2030352
Becerra M-E, Arias N-P, Giraldo O-H, López-Suárez F-E, Illán-Gómez M-J, Bueno-López A. Alumina-Supported Manganese Catalysts for Soot Combustion Prepared by Thermal Decomposition of KMnO4. Catalysts. 2012; 2(3):352-367. https://doi.org/10.3390/catal2030352
Chicago/Turabian StyleBecerra, Maria-Eugenia, Nayda-Patricia Arias, Oscar-Hernan Giraldo, Franz-Edwin López-Suárez, Maria-Jose Illán-Gómez, and Agustin Bueno-López. 2012. "Alumina-Supported Manganese Catalysts for Soot Combustion Prepared by Thermal Decomposition of KMnO4" Catalysts 2, no. 3: 352-367. https://doi.org/10.3390/catal2030352
APA StyleBecerra, M.-E., Arias, N.-P., Giraldo, O.-H., López-Suárez, F.-E., Illán-Gómez, M.-J., & Bueno-López, A. (2012). Alumina-Supported Manganese Catalysts for Soot Combustion Prepared by Thermal Decomposition of KMnO4. Catalysts, 2(3), 352-367. https://doi.org/10.3390/catal2030352






