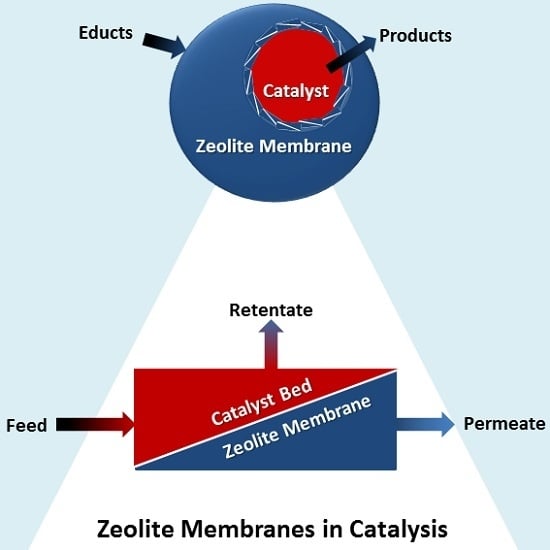
Zeolite Membranes in Catalysis—From Separate Units to Particle Coatings
Abstract
:1. Introduction
2. Separation by Zeolite Membranes
2.1. Synthesis of Zeolite Membranes

2.2. Permeation in Zeolite Membranes
2.3. Zeolite Membrane Separation Spatially Decoupled from the Catalytic Unit

3. Membrane Reactor Concepts

3.1. Extractor Type Zeolite Membrane Reactors
| Reaction | Reactor Type | Feed | Operating Conditions | Catalyst | Membrane | XCR (%) SCR (%) YCR (%) | XMR (%) SMR (%) YMR (%) | References |
|---|---|---|---|---|---|---|---|---|
| Dehydrogenation of ethylbenzene to styrene | CMR | water, ethyl-benzene | 600 °C sweep gas: nitrogen | Fe-MFI/α-Al2O3 tube | Xethylbenzene = 45.1 Sstyrene = 92.8 Ystyrene = 41.9 | Xethylbenzene = 60.1 Sstyrene = 96.9 Ystyrene = 58.6 | [144] | |
| Dehydrogenation of i-butane | PBMR | i-butane, hydrogen, balance nitrogen | 730 K p = 100–170 kPa sweep gas: nitrogen | PtIn/MFI 0.8 wt. % In 0.5 wt. % Pt | MFI/α-Al2O3 tube | n.r. | n.r. | [145] |
| Dehydrogenation of i-butane | PBMR | pure i-butane | 510 °C WHSV = 0.5–1.6 h−1 sweep gas: nitrogen | Cr2O3/Al2O3 | MFI/α-Al2O3 tube | Xi-butane = 29.1 Si-butene = ~90 | Xi-butane = 41.7–48.6 Si-butene = 96 | [146] |
| Dehydrogenation of i-butane | PBMR | pure i-butane | 712–762 K pfeed = 101 kPa sweep gas: nitrogen | Cr2O3/Al2O3 | DD3R/α-Al2O3 tube | Yi-butene = 0.28 at 762 °C | Yi-butene = 0.41 at 762 °C | [147] |
| Dehydrogenation of cyclohexane | PBMR | cyclo-hexane diluted in argon | 423–523 K p = 101.3 kPa Sweep gas: argon | Pt/Al2O3 1 wt. % Pt | FAU/α-Al2O3 tube | Xcyclohexane = 32.2 | Xcyclohexane = 72.1 | [148,149] |
| Dehydrogenation of ethylbenzene to styrene | PBMR | water and ethyl-benzene | 580–640 °C ∆p = 0.8 atm sweep gas: nitrogen | Fe2O3 | silicalite-1/stainless steel tube | Xethylbenzene = 67.5 at 610 °C | Xethylbenzene = 74.8 at 610 °C | [150] |
| Dehydrogenation of ethane | PBMR | pure ethane | 500–550 °C pfeed = 104 kPa pperm = 101.3 kPa sweep gas: argon | Pt-Sn/Al2O3 1 wt. % Pt, 0.3 wt. % Sn | natural mordenite disk | Xethane = 9.7 Sethylene = 92.2 Yethylene = 9 at 550 °C | Xethane = 10.5 Sethylene = 93.7 Yethylene = 9.8 at 550 °C | [151] |
| High-temperature water gas shift reaction | PBMR | carbon monoxide, water steam | 400–550 °C H2O/CO = 1.0–3.5 sweep gas: nitrogen | Fe/Ce | MFI/α-Al2O3 tube | XCO = 62.5 | XCO = 81.7 | [152] |
| Low-temperature water gas shift reaction | PBMR | carbon monoxide and water steam diluted in nitrogen | 220–290 °C p = 6 bar GHSV = 1000–7500 LN/kgcat sweep gas: nitrogen | CuO-ZnO/Al2O3 | MFI/α-Al2O3 disc | XCO = 89.1 | XCO = 95.4 | [153] |
| High-temperature water gas shift reaction | PBMR | carbon monoxide, water steam and nitrogen | 400–550 °C H2O/CO = 1.0–3.5 WHSV = 7500–60,000 h−1 p = 2–6 atm sweep gas: nitrogen | Fe/Ce | MFI/α-Al2O3 disc | XCO = ~90 | XCO > 95 | [154,155] |
| Water gas shift reaction | PBMR | carbon monoxide, water steam | 500 °C p = 5 atm H2O/CO = 3.0 GHSV = 72,000 h−1 sweep gas: argon | Fe-Cr-Cu | ZSM-5/silicalite bilayer/α-Al2O3 | n.r. | XCO = 89.8 | [156] |
| High-temperature water gas shift reaction | PBMR | carbon monoxide, hydrogen, preheated steam | 300–450 °C pfeed = 0.1–0.15 MPa pperm = 0.1 MPa sweep gas: steam | Fe2O3/Cr2O3/Al2O3 | MFI/α-Al2O3 hollow fibre | XCO = 63.4 | XCO = 73.6 | [157] |
| Xylene isomerization | PBMR | m-xylene diluted in nitrogen | 577 K sweep gas: nitrogen in counter-current mode | Pt on zeolite | MFI/α-Al2O3 tube | Sp-xylene = 58 Yp-xylene = 21 | Sperm. only = 100 Sperm.+Ret. = 65 Yp-xylene = 23 | [158] |
| Xylene isomerization | CMR | pure m-xylene; carrier gas: nitrogen | 300–400 °C sweep gas: nitrogen | H-ZSM-5/316L stainless steel disc | Xm-xylene = 5.87 Sp-xylene = 55.6 So-xylene = 44.4 | Xm-xylene = 6.9 Sp-xylene = 66.7 So-xylene = 33.3 | [159] | |
| Xylene isomerization | CMR | m-xylene diluted in helium | 370 °C sweep gas: nitrogen | Pt/H-ZSM-5/stainless steel tube | n.r. | Sp-xylene = 67 | [160] | |
| Xylene isomerization | PBMR | mixture of m-, p- and o-xylene carrier gas: hydrogen | 340–390 °C WHSV = 550 h−1 | Pt/H-ZSM-5 | Ba-ZSM-5/ Stainless steel | Sp-xylene = 52 | Sp-xylene = 69 | [160] |
| m-xylene isomerization | PBMR | m-xylene diluted in helium | 270–390 °C sweep gas: helium diverse packing configurations | HZSM-5 | silicalite-1/ α-Al2O3 disc | GHSV = 1574 h−1 | - | [161] |
| Xm-xylene = 51.9 | Xm-xylene = 47.8 | |||||||
| Sp-xylene = 35.7 | Sp-xylene = 44.6 | |||||||
| GHSV = 4722 h−1 | - | |||||||
| Xm-xylene = 36.5 | Xm-xylene = 36.1 | |||||||
| Sp-xylene = 47.3 | Sp-xylene = 49.6 | |||||||
| m-xylene isomerization | PBMR | m-xylene, carrier gas: nitrogen | 473–573 K sweep gas: nitrogen | Pt-HZSM-5 | MFI/α-Al2O3 tube | Sp-xylene = 42 Yp-xylene = 27 | Sp-xylene = 49 Yp-xylene = 23 | [162,163] |
| xylene isomerization | CMR | m-xylene diluted in hydrogen | 355–450 °C p = 101 kPa sweep gas: nitrogen | acid-functionalized silicalite-1/ α-Al2O3 disc propylsulfonic and arenesulfonic acid sites | n.r. | Xm-xylene = 52 Yp-xylene = 32 at 450 °C | [164] | |
| m-xylene isomerization | CMR | m-xylene diluted in helium | 270 °C weep gas: helium | H-MFI/α-Al2O3 disc | n.r. | Xm-xylene = 6.5 Sp-xylene = 92.1 | [165] | |
| Double-bond isomerization of 1-butene | CMR | 1-butene diluted in nitrogen | 120–250 °C p = 1 bar sweep gas: nitrogen | [B]MFI/α-Al2O3 tube | n.r. | X = 44.5 ratio trans/cis = 2.2 at 250 °C | [166] | |
| Esterification of ethanol with acetic acid | CMR | ethanol, acetic acid | 333–363 K ∆p = 0–1 bar sweep gas: He | H-ZSM-5/α-Al2O3 or stainless steel tubes | X = 49.4 | X = 63.1 | [167] | |
| Esterification of acetic acid with ethanol | PBMR | ethanol, acetic acid | 358 K pret = 1.3 bar pperm = 2 mbar | Amberlyst 15 | modernite/α-Al2O3 zeolite A/α-Al2O3 | X = 66.9 | X = ~90 | [168] |
| Catalytic dehydration of methanol | PBMR | methanol | 150–250 °C WHSV= 0.5–2.6 h−1 pfeed = 1–1.7 bar pperm= 1 mbar | γ-alumina | NaA/stainless steel wire mesh | XCH3OH = 61 at 230 °C | XCH3OH = 85 at 230 °C | [169] |
| CO2 hydrogenation into methanol | PBMR | carbon dioxide, hydrogen | 200–263 °C p = 20–24 bar H2/CO2 = 3–7 | Cu/ZnO/Al2O3 | NaA/α-Al2O3 tube | XCO2 = 5 SCH3OH = 48 YCH3OH = 2.4 | XCO2 = 11.6 SCH3OH = 75 YCH3OH = 8.7 | [170] |
| Metathesis of propene and geometrical isomerization of cis-2-butene | PBMR | pure propene | 296 K sweep gas: helium | Re2O7/γ-Al2O3 | silicalite-1/stainless steel disc | Xpropene = 33.4 Xcis-2-butene = 76.1 | Xpropene = 38.4 Xcis-2-butene = 79.4 Ytrans-2-butene = 79 | [93,94] |
| Hydro-isomerization of C6 | PBMR | n-hexane, 2-methyl-pentane (MP); carrier gas: helium | 393 K WHSV = 0.21 gHC/(gcat h) sweep gas: hydrogen | Pt-chlorinated alumina (AT-2G) | silicalite-1/TiO2/stainless steel tube | n.r. | Xn-hexane = 71.8 at 393 K | [171] |
3.2. Distributor Type Zeolite Membrane Reactors
3.3. Contactor Type Membrane Reactors
4. Applications in Packed Bed Membrane Reactors (PBMR)

4.1. Product Removal: Enhanced Conversions by Shifting the Chemical Equilibrium
4.1.1. Equilibrium Shift by Water Removal
4.1.2. Hydrogen Permeation in Dehydrogenation Reactions
4.1.3. Hydrogen Permeation in Water Gas Shift Reaction


4.1.4. Hydrogen Permeation in Syngas Production
4.1.5. Hydrogenation
4.1.6. Metathesis of Propene
4.2. Product Removal: Enhanced Selectivity by Displacing the Chemical Equilibrium

4.3. Selectivity Enhancement through Selective Distribution of Reactants or Removal of Intermediate Products
5. Reaction Processing Using Permselective Catalytic Membrane Reactors (CMR)

6. Zeolite Membrane Coatings on Catalyst Particles
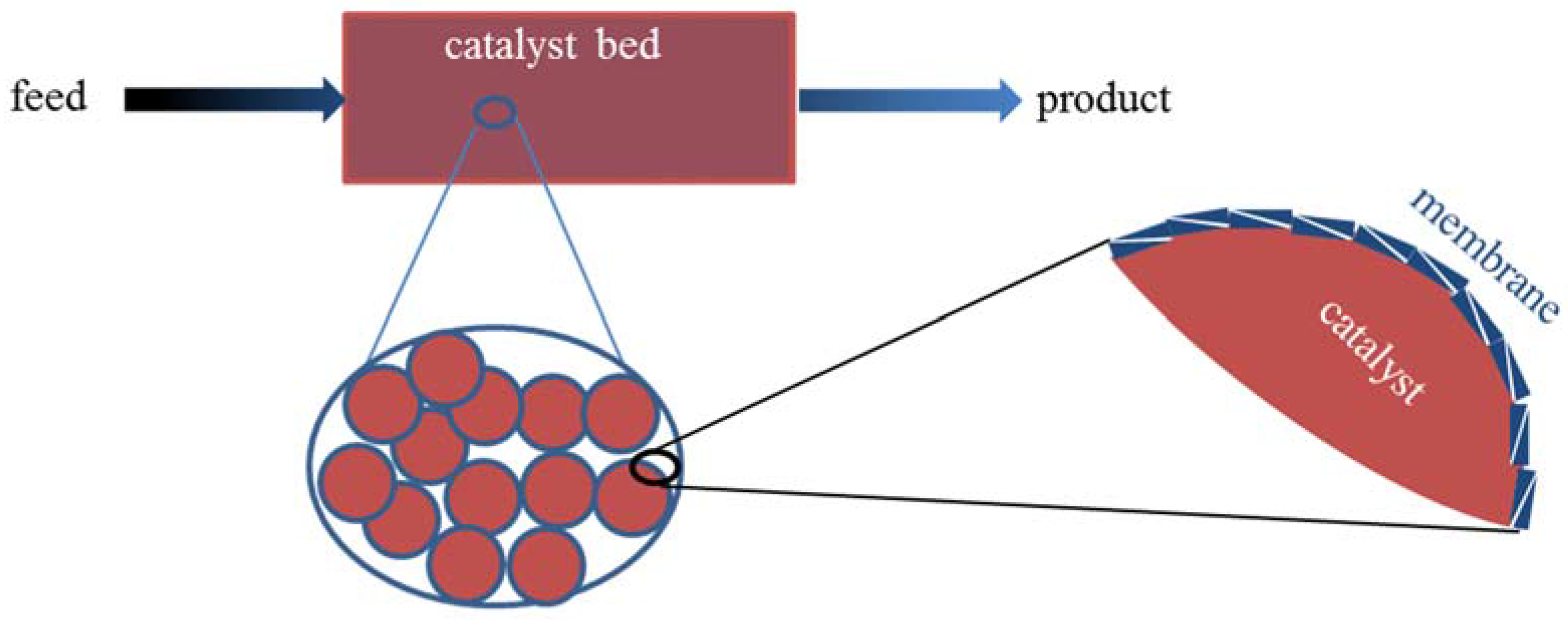
| Reaction | Reactor Type | Feed | Operating Conditions | Core-Shell Catalyst | Xmixed (%) Smixed (%) Ymixed (%) | XMR. (%) SMR. (%) YMR. (%) | References |
|---|---|---|---|---|---|---|---|
| Disproportiona-tion of toluene | PLMR product selective | toluene | 723–823 K p = 101.3 kPa WHSV = 0.1 h−1 | silicalite coated on silica-alumina catalyst | Sp-xylene = 22 | Sp-xylene > 91 | [245] |
| Alkylation of toluene | PLMR product selective | toluene, methanol | 673 K | silicalite coated on H-ZSM-5 crystals with different Si/Al ratios | Xtoluene = 63 Sp-xylene = 40 | Xtoluene = 42 Sp-xylene > 99.9 | [246,247] |
| Hydro-formylation of 1-hexene | batch type reactor product selective | 1-hexene, carbon monoxide, hydrogen | 130 °C H2/CO = 1 | silicalite-1 coated on Pd–Co/activated carbon | X = 75.7 | X = 54 | [248] |
| Shexan = 13.3 | Shexan = 28.3 | ||||||
| Sisomer = 15.4 | Sisomer = 21.9 | ||||||
| Si-hept. = 37.1 | Si-hept. = 13.9 | ||||||
| Sn-nept. = 33.1 | Sn-nept. = 35.9 | ||||||
| Hydrogenation of linear and branched alkenes | PLMR reactant selective | 1-hexene, 3,3-dimethyl-but-1-ene | 323–373 K p = 101.3 kPa | silicalite-1 coated on Pt/TiO2 particles | X1-hex > 90 X3,3-DMB > 90 S = 1–1.2 | X1-hex > 90 X3,3-DMB < 10 S = 12–20 | [249,250] |
| Oxidation of CO and n-butane | PLMR reactant selective | air, carbon monoxide and n-butane | 483 K p = 101.3 kPa | zeolite-4A coated on spherical Pt/γ-Al2O3 particles (two-steps hydrothermal synthesis) | Xn-butane = 95 XCO = 93 | Xn-butane = 0 XCO > 90 | [251] |
| Shape-selective hydrogenation of xylene isomers | PLMR reactant selective | p-/o-xylene or p-/m- xylene | 473 K p = 1.0 MPa WHSV = 1.0 h−1 | silicalite-1 coated on Pt/Al2O3 pellets | - | Sp/o = 17 Sp/m = 13.6 | [252] |
| Steam reforming of methane and toluene | PLMR reactant selective | methane or toluene, steam, helium | 780–840 °C p = 1 bar CH4/H2O = 1 H2O/C7H8 = 7 | Hβ zeolite coated on Ni/Mg/Ce0.6Zr0.4O2 pellets | XCH4 increases with temperature up to ~20XC7H8 ~58 | XCH4 increases with temperature up to ~30 XC7H8 ~ 22 | [242] |
| Direct synthesis of middle i-paraffins | PLMR catalytic | hydrogen and carbon monoxide | 533 K p = 1.0 MPa H2/CO = 2 | H-ZSM-5 coated on Co/SiO2 pellets with different size | XCO = 93.6 | XCO = 89.1 | [234,253,254] |
| SCH4 = 16.9 | SCH4 = 22.4 | ||||||
| SCO2 = 8 | SCO2 = 6.9 | ||||||
| Ci/Cn = 0.49 | Ci/Cn = 0.74 | ||||||
| Direct synthesis of i-paraffins | PLMR catalytic | hydrogen and carbon monoxide | 533 K p = 1 MPa H2/CO = 2 | H-β zeolite coated on Co/Al2O3 catalyst pellets with different size | XCO = 80.8 | XCO = 74.3 | [239] |
| SCH4 = 16.6 | SCH4 = 13.6 | ||||||
| SCO2 = 3.9 | SCO2 = 2.7 | ||||||
| Ci/Cn = 1.4 | Ci/Cn = 2.3 | ||||||
| Direct synthesis of i-paraffins | PLMR catalytic | hydrogen and carbon monoxide | 533 K p = 1 MPa H2/CO = 2 | H-ZSM-5 coated on Ru/SiO2 catalyst pellets with different size | XCO = 82.1 | XCO = 81.7 | [255] |
| SCH4 = 17.1 | SCH4 = 20.5 | ||||||
| SCO2 = 5 | SCO2 = 6.1 | ||||||
| Ci/Cn = 0.42 | Ci/Cn = 1.5 | ||||||
| Direct synthesis of i-paraffins | PLMR catalytic | hydrogen and carbon monoxide | 533 K p = 1 MPa H2/CO = 2 | H-ZSM-5 coated on Pd/SiO2 | - | XCO = 86.1 | [256] |
| SCH4 = 37.4 | |||||||
| SCO2 = 7.0 | |||||||
| Ci/Cn = 1.88 | |||||||
| Direct synthesis of middle i-paraffins | PLMR catalytic | hydrogen and carbon monoxide | 573 K p = 1.0 MPa H2/CO = 1 | H-ZSM-5 crystalized on fused-iron catalyst pellet | XCO = 96.7 | XCO = 96.9 | [257] |
| SCH4 = 12.8 | SCH4 = 8.7 | ||||||
| SCO2 = 44.7 | SCO2 = 33.9 | ||||||
| Ci/Cn = 2.31 | Ci/Cn = 4.17 | ||||||
| Synthesis of gasoline-range i-paraffins | PLMR catalytic | hydrogen and carbon monixide | 483–533 K p = 2.0 MPa H2/CO = 2 GHSV = 1000 h−1 | H-ZSM-5 coated on CoZr catalyst particles | XCO = 97.4 | XCO = 82.3 | [258] |
| SCH4 = 16 | SCH4 = 14.8 | ||||||
| S18+ = 5.6 | S18+ = 0.3 | ||||||
| Si-C5-11 = 16.7 | Si-C5-11 = 24.7 | ||||||
| Direct synthesis of light i-paraffins | PLMR catalytic | hydrogen and carbon monoxide | 553 K p = 1 Mpa H2/CO = 2 | H-ZSM-5 zeolite coated on Co/SiO2 | XCO = 98.5 | XCO = 99.1 | [259] |
| SCH4 = 23.7 | SCH4 = 20.1 | ||||||
| SCO2 = 16 | SCO2 = 18.2 | ||||||
| Sn = 53.4 | Sn = 47.6 | ||||||
| Si = 36.2 | Si = 43.8 | ||||||
| Direct synthesis of middle i-paraffins | PLMR catalytic | hydrogen and carbon monoxide | 300 °C p = 1 MPa H2/CO = 1 | H-ZSM-5 coated on Fe/SBA-15 | XCO = 63.9 | XCO = 57.6 | [244] |
| SCO2 = 43.8 | SCO2 = 37.3 | ||||||
| SCH4 = 19.2 | SCH4 = 15.3 | ||||||
| Sn = 56 | Sn = 36.7 | ||||||
| Si = 33.9 | Si = 46.5 | ||||||
| Direct synthesis of i-paraffins | PMLR catalytic | carbon monoxide, hydrogen | 280 °C p = 1 MPa H2/CO = 1 | Silicalite-1 and H-ZSM-5 coated on Fe/SiO2 dual-membrane coated catalyst | XCO = 60 | XCO = 54.8 | [260] |
| SCO2 = 29.9 | SCO2 = 33.8 | ||||||
| SCH4 = 7 | SCH4 = 14.9 | ||||||
| Si = 12.9 | Si = 29.8 | ||||||
| Dimethyl ether direct synthesis | PLMR catalytic | hydrogen, carbon monoxide, carbon dioxide and argon | 523 K p = 5.0 MPa | H-ZSM-5 coated on Cu/ZnO/Al2O3 | XCO = 58.07 | XCO = 30.4 | [261] |
| SMeOH = 57.29 | SMeOH = 21.43 | ||||||
| SDME = 40.51 | SDME = 78.57 | ||||||
| Dimethyl ether direct synthesis | PLMR catalytic | hydrogen, carbon monoxide, carbon dioxide and argon | 573-623 K p = 5.0 MPa | Double layer H-ZSM-5/Silicalite-1 membrane coated on Cr/ZnO core catalyst | XCO = 45.16 | XCO = 9.53 | [262] |
| SMeOH = 12.12 | SMeOH = 21.23 | ||||||
| SDME = 0.47 | SDME = 50.84 | ||||||
| Dimethyl ether direct synthesis | PLMR catalytic | hydrogen, carbon monoxide, carbon dioxide, argon | 523 K p = 5.0 MPa | Double layer H-ZSM-5/Silicalite-1 membrane coated on Pd/SiO2 core catalyst | XCO = 12.84 | XCO = 9.48 | [263] |
| SCH4 = 1.47 | SCH4 = 16.8 | ||||||
| SMeOH = 16.51 | SMeOH = 4.76 | ||||||
| SDME = 48.40 | SDME = 68.70 | ||||||
| Dimethyl ether direct synthesis | PLMR catalytic | hydrogen, carbon monoxide, carbon dioxide, argon | 350 °C p = 5 MPa | SAPO-46 zeolite shell encapsulated Cr/ZnO catalyst | XCO = 4.7 | XCO = 6.9 | [243] |
| SCH4 = 3.7 | SCH4 = 4.7 | ||||||
| SMeOH = 71.7 | SMeOH = 52.2 | ||||||
| SDME = 16.5 | SDME = 37.0 | ||||||
| Dimethyl ether direct synthesis | PLMR catalytic | hydrogen, carbon monoxide, carbon dioxide, argon | 250 °C p = 5 MPa | SAPO11 coated on Cu/ZnO/Al2O3 | XCO = 64.9 | XCO = 92 | [241] |
| SMeOH = 51.4 | SMeOH = 9.2 | ||||||
| SDME = 46.6 | SDME = 90.3 | ||||||
| YDME = 30.2 | YDME = 83.1 | ||||||
| Carbon dioxide hydrogenation to dimethyl ether | PLMR | carbon dioxide and hydrogen | 270 °C p = 3.0 MPa SV = 1800 mL·g·cat−1·h−1 H2/CO = 3 | H-ZSM-5 coated on CuO-ZnO-Al2O3 nanoparticles | XCO2 = ~24 | XCO2 = 48.3 | [264] |
| SDME = ~26 | SDME = 48.5 | ||||||
| YDME = ~ 6 | YDME = 23.4 |
6.1. Application in Reactant-Selective or Product-Selective Reactions (Non-Catalytic Membranes)


6.2. Application as Catalytic Membranes


7. Conclusions
Author Contributions
Conflicts of Interest
References
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Kulprathipanja, S. Zeolites in Industrial Separation and Catalysis; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Jasra, R.V.; Choudary, N.V.; Bhat, S.G.T. Separation of Gases by Pressure Swin. Sep. Sci. Technol. 1991, 26, 885–930. [Google Scholar] [CrossRef]
- Reiß, G. Status and development of oxygen generation processes on molecular sieve zeolites. Gas Sep. Purif. 1994, 8, 95–99. [Google Scholar] [CrossRef]
- Tagliabue, M.; Farrusseng, D.; Valencia, S.; Aguado, S.; Ravon, U.; Rizzo, C.; Corma, A.; Mirodatos, C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Hedström, A. Ion Exchange of Ammonium in Zeolites: A Literature Review. J. Environ. Eng. 2001, 127, 673–681. [Google Scholar] [CrossRef]
- Bein, T. Synthesis and Applications of Molecular Sieve Layers and Membranes. Chem. Mater. 1996, 8, 1636–1653. [Google Scholar] [CrossRef]
- Tavolaro, A.; Drioli, E. Zeolite membranes. Adv. Mater. 1999, 11, 975–996. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P.; Schäfer, R. Zeolite membranes—State of their development and perspective. Microporous Mesoporous Mater. 2000, 38, 3–24. [Google Scholar] [CrossRef]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P. Zeolite Membranes: From the Laboratory Scale to Technical Applications. Adsorption 2005, 11, 215–227. [Google Scholar] [CrossRef]
- Snyder, M.A.; Tsapatsis, M. Hierarchical Nanomanufacturing: From Shaped Zeolite Nanoparticles to High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2007, 46, 7560–7573. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.; Noack, M. Zeolite membranes—Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Yu, M.; Noble, R.D.; Falconer, J.L. Zeolite Membranes: Microstructure Characterization and Permeation Mechanisms. Acc. Chem. Res. 2011, 44, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.; Kapteijn, F.; Zornoza, B.; Sebastian, V.; Casado, C.; Coronas, J. Practical Approach to Zeolitic Membranes and Coatings: State of the Art, Opportunities, Barriers, and Future Perspectives. Chem. Mater. 2012, 24, 2829–2844. [Google Scholar] [CrossRef]
- Nasir, R.; Mukhtar, H.; Man, Z.; Mohshim, D.F. Material Advancements in Fabrication of Mixed-Matrix Membranes. Chem. Eng. Technol. 2013, 36, 717–727. [Google Scholar] [CrossRef]
- Miachon, S.; Dalmon, J.-A. Catalysis in Membrane Reactors: What About the Catalyst? Top. Catal. 2004, 29, 59–65. [Google Scholar] [CrossRef]
- Caro, J. 3.01—Basic Aspects of Membrane Reactors. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 1–24. [Google Scholar]
- McLeary, E.E.; Jansen, J.C.; Kapteijn, F. Zeolite based films, membranes and membrane reactors: Progress and prospects. Microporous Mesoporous Mater. 2006, 90, 198–220. [Google Scholar] [CrossRef]
- Gascon, J.; van Ommen, J.R.; Moulijn, J.A.; Kapteijn, F. Structuring catalyst and reactor—An inviting avenue to process intensification. Catal. Sci. Technol. 2015, 5, 807–817. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and New Perspectives on Integrated Membrane Operations for Sustainable Industrial Growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Coronas, J.; Santamarı́a, J. Catalytic reactors based on porous ceramic membranes. Catal. Today 1999, 51, 377–389. [Google Scholar] [CrossRef]
- Armor, J.N. Membrane catalysis: Where is it now, what needs to be done? Catal. Today 1995, 25, 199–207. [Google Scholar] [CrossRef]
- Daramola, M.O.; Aransiola, E.F.; Ojumu, T.V. Potential applications of zeolite membranes in reaction coupling separation processes. Materials 2012, 5, 2101–2136. [Google Scholar] [CrossRef]
- Coronas, J.; Santamaria, J. State-of-the-art in zeolite membrane reactors. Top. Catal. 2004, 29, 29–44. [Google Scholar] [CrossRef]
- Saracco, G.; Specchia, V. Catalytic Inorganic-Membrane Reactors: Present Experience and Future Opportunities. Catal. Rev. 1994, 36, 305–384. [Google Scholar] [CrossRef]
- Michalkiewicz, B.; Koren, Z. Zeolite membranes for hydrogen production from natural gas: State of the art. J. Porous Mater. 2015, 22, 635–646. [Google Scholar] [CrossRef]
- Van den Bergh, J.; Nishiyama, N.; Kapteijn, F. Zeolite Membranes in Catalysis: What Is New and How Bright Is the Future? In Novel Concepts in Catalysis and Chemical Reactors; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 211–237. [Google Scholar]
- Tsapatsis, M. Toward High-Throughput Zeolite Membranes. Science 2011, 334, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.V.; Topuz, B.; Pham, T.C.T.; Nguyen, T.H.; Sauer, N.; Rangnekar, N.; Zhang, H.; Narasimharao, K.; Basahel, S.N.; Francis, L.F.; et al. Oriented MFI Membranes by Gel-Less Secondary Growth of Sub-100 nm MFI-Nanosheet Seed Layers. Adv. Mater. 2015, 27, 3243–3249. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.T.; Nguyen, T.H.; Yoon, K.B. Gel-Free Secondary Growth of Uniformly Oriented Silica MFI Zeolite Films and Application for Xylene Separation. Angew. Chem. Int. Ed. 2013, 52, 8693–8698. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, T.; Nian, P.; Zhang, Q.; Yao, J.; Li, S.; Gao, Z.; Yue, X. Fabrication of a Highly b-Oriented MFI-Type Zeolite Film by the Langmuir-Blodgett Method. Langmuir 2014, 30, 4531–4534. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, E.; Sandström, L.; Öhrman, O.G.W.; Hedlund, J. Separation of CO2 from black liquor derived syngas using an MFI membrane. J. Membr. Sci. 2013, 443, 131–137. [Google Scholar] [CrossRef]
- Zhou, M.; Korelskiy, D.; Ye, P.; Grahn, M.; Hedlund, J. A Uniformly Oriented MFI Membrane for Improved CO2 Separation. Angew. Chem. Int. Ed. 2014, 53, 3492–3495. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Zeolites from a Materials Chemistry Perspective. Chem. Mater. 2014, 26, 239–245. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, H.; Wang, Z.; Yan, Y. Microstructural optimization of MFI-type zeolite membranes for ethanol-water separation. J. Mater. Chem. A 2014, 2, 16093–16100. [Google Scholar] [CrossRef]
- Iyoki, K.; Itabashi, K.; Okubo, T. Progress in seed-assisted synthesis of zeolites without using organic structure-directing agents. Microporous Mesoporous Mater. 2014, 189, 22–30. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Morigami, Y.; Kondo, M.; Abe, J.; Kita, H.; Okamoto, K. The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane. Sep. Purif. Technol. 2001, 25, 251–260. [Google Scholar] [CrossRef]
- Gallego-Lizon, T.; Edwards, E.; Lobiundo, G.; Freitas dos Santos, L. Dehydration of water/t-butanol mixtures by pervaporation: Comparative study of commercially available polymeric, microporous silica and zeolite membranes. J. Membr. Sci. 2002, 197, 309–319. [Google Scholar] [CrossRef]
- Urtiaga, A.; Gorri, E.D.; Casado, C.; Ortiz, I. Pervaporative dehydration of industrial solvents using a zeolite NaA commercial membrane. Sep. Purif. Technol. 2003, 32, 207–213. [Google Scholar] [CrossRef]
- Richter, H.; Voigt, I.; Kühnert, J.-T. Dewatering of ethanol by pervaporation and vapour permeation with industrial scale NaA-membranes. Desalination 2006, 199, 92–93. [Google Scholar] [CrossRef]
- Sato, K.; Aoki, K.; Sugimoto, K.; Izumi, K.; Inoue, S.; Saito, J.; Ikeda, S.; Nakane, T. Dehydrating performance of commercial LTA zeolite membranes and application to fuel grade bio-ethanol production by hybrid distillation/vapor permeation process. Microporous Mesoporous Mater. 2008, 115, 184–188. [Google Scholar] [CrossRef]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Jia, M.-D.; Falconer, J.L.; Noble, R.D. Preparation and separation properties of silicalite composite membranes. J. Membr. Sci. 1995, 105, 79–87. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y.S. In Situ Synthesis of P-Type Zeolite Membranes on Porous α-Alumina Supports. Ind. Eng. Chem. Res. 1998, 37, 2404–2409. [Google Scholar] [CrossRef]
- Yan, Y.; Davis, M.E.; Gavalas, G.R. Preparation of Zeolite ZSM-5 Membranes by In-situ Crystallization on Porous α-Al2O3. Ind. Eng. Chem. Res. 1995, 34, 1652–1661. [Google Scholar] [CrossRef]
- Tuan, V.A.; Li, S.; Falconer, J.L.; Noble, R.D. In situ Crystallization of Beta Zeolite Membranes and Their Permeation and Separation Properties. Chem. Mater. 2002, 14, 489–492. [Google Scholar] [CrossRef]
- Lin, X.; Chen, X.; Kita, H.; Okamoto, K. Synthesis of silicalite tubular membranes by in situ crystallization. AIChE J. 2003, 49, 237–247. [Google Scholar] [CrossRef]
- Vilaseca, M.; Mateo, E.; Palacio, L.; Prádanos, P.; Hernández, A.; Paniagua, A.; Coronas, J.N.; Santamarı́a, J. AFM characterization of the growth of MFI-type zeolite films on alumina substrates. Microporous Mesoporous Mater. 2004, 71, 33–37. [Google Scholar] [CrossRef]
- Guillou, F.; Rouleau, L.; Pirngruber, G.; Valtchev, V. Synthesis of FAU-type zeolite membrane: An original in situ process focusing on the rheological control of gel-like precursor species. Microporous Mesoporous Mater. 2009, 119, 1–8. [Google Scholar] [CrossRef]
- Huang, A.; Liang, F.; Steinbach, F.; Caro, J. Preparation and separation properties of LTA membranes by using 3-aminopropyltriethoxysilane as covalent linker. J. Membr. Sci. 2010, 350, 5–9. [Google Scholar] [CrossRef]
- Huang, A.; Wang, N.; Caro, J. Seeding-free synthesis of dense zeolite FAU membranes on 3-aminopropyltriethoxysilane-functionalized alumina supports. J. Membr. Sci. 2012, 389, 272–279. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y. Controlling crystal orientation in zeolite MFI thin films by direct in situ crystallization. Chem. Mater. 2001, 13, 1101–1107. [Google Scholar] [CrossRef]
- Zhang, F.-Z.; Fuji, M.; Takahashi, M. In situ growth of continuous b-oriented MFI zeolite membranes on porous α-alumina substrates precoated with a mesoporous silica sublayer. Chem. Mater. 2005, 17, 1167–1173. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y. Oriented zeolite MFI monolayer films on metal substrates by in situ crystallization. Microporous Mesoporous Mater. 2001, 48, 229–238. [Google Scholar] [CrossRef]
- Li, S.; Demmelmaier, C.; Itkis, M.; Liu, Z.; Haddon, R.C.; Yan, Y. Micropatterned oriented zeolite monolayer films by direct in situ crystallization. Chem. Mater. 2003, 15, 2687–2689. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Gouzinis, A.; Nair, S.; Okubo, T.; He, M.; Overney, R.M.; Tsapatsis, M. Growth, microstructure, and permeation properties of supported zeolite (MFI) films and membranes prepared by secondary growth. Chem. Eng. Sci. 1999, 54, 3521–3531. [Google Scholar] [CrossRef]
- Bernal, M.A.P.; Xomeritakis, G.; Tsapatsis, M. Tubular MFI zeolite membranes made by secondary (seeded) growth. Catal. Today 2001, 67, 101–107. [Google Scholar] [CrossRef]
- Nair, S.; Lai, Z.; Nikolakis, V.; Xomeritakis, G.; Bonilla, G.; Tsapatsis, M. Separation of close-boiling hydrocarbon mixtures by MFI and FAU membranes made by secondary growth. Microporous Mesoporous Mater. 2001, 48, 219–228. [Google Scholar] [CrossRef]
- Tomita, T.; Nakayama, K.; Sakai, H. Gas separation characteristics of DDR type zeolite membrane. Microporous Mesoporous Mater. 2004, 68, 71–75. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Yu, C.; Gu, X.; Xu, N. Effect of seeding methods on growth of NaA zeolite membranes. Microporous Mesoporous Mater. 2011, 143, 348–356. [Google Scholar] [CrossRef]
- Wohlrab, S.; Meyer, T.; Stöhr, M.; Hecker, C.; Lubenau, U.; Oßmann, A. On the performance of customized MFI membranes for the separation of n-butane from methane. J. Membr. Sci. 2011, 369, 96–104. [Google Scholar] [CrossRef]
- Fan, W.; Snyder, M.A.; Kumar, S.; Lee, P.-S.; Yoo, W.C.; McCormick, A.V.; Lee Penn, R.; Stein, A.; Tsapatsis, M. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity. Nat. Mater. 2008, 7, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.-S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal Synthesis of Zeolites with Three-Dimensionally Ordered Mesoporous-Imprinted Structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef] [PubMed]
- Diaz, U.; Corma, A. Layered zeolitic materials: An approach to designing versatile functional solids. Dalton Trans. 2014, 43, 10292–10316. [Google Scholar] [CrossRef] [PubMed]
- Morawetz, K.; Reiche, J.; Kamusewitz, H.; Kosmella, H.; Ries, R.; Noack, M.; Brehmer, L. Zeolite films prepared via the Langmuir-Blodgett technique. Colloids Surf. A 2002, 198–200, 409–414. [Google Scholar] [CrossRef]
- Pan, M.; Lin, Y.S. Template-free secondary growth synthesis of MFI type zeolite membranes. Microporous Mesoporous Mater. 2001, 43, 319–327. [Google Scholar] [CrossRef]
- Huang, A.; Lin, Y.S.; Yang, W. Synthesis and properties of A-type zeolite membranes by secondary growth method with vacuum seeding. J. Membr. Sci. 2004, 245, 41–51. [Google Scholar] [CrossRef]
- Li, G.; Kikuchi, E.; Matsukata, M. The control of phase and orientation in zeolite membranes by the secondary growth method. Microporous Mesoporous Mater. 2003, 62, 211–220. [Google Scholar] [CrossRef]
- Lovallo, M.C.; Gouzinis, A.; Tsapatsis, M. Synthesis and characterization of oriented MFI membranes prepared by secondary growth. AIChE J. 1998, 44, 1903–1913. [Google Scholar] [CrossRef]
- Choi, J.; Ghosh, S.; Lai, Z.; Tsapatsis, M. Uniformly a-oriented MFI zeolite films by secondary growth. Angew. Chem. 2006, 118, 1172–1176. [Google Scholar] [CrossRef]
- Gouzinis, A.; Tsapatsis, M. On the preferred orientation and microstructural manipulation of molecular sieve films prepared by secondary growth. Chem. Mater. 1998, 10, 2497–2504. [Google Scholar] [CrossRef]
- Lai, Z.; Tsapatsis, M.; Nicolich, J.P. Siliceous ZSM-5 Membranes by Secondary Growth of b-Oriented Seed Layers. Adv. Funct. Mater. 2004, 14, 716–729. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yang, W. Fabrication of Highly b-Oriented MFI Film with Molecular Sieving Properties by Controlled in-Plane Secondary Growth. J. Am. Chem. Soc. 2010, 132, 1768–1769. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, J.; Schoeman, B.; Sterte, J. Ultrathin oriented zeolite LTA films. Chem. Commun. 1997. [Google Scholar] [CrossRef]
- Pham, T.C.T.; Kim, H.S.; Yoon, K.B. Growth of Uniformly Oriented Silica MFI and BEA Zeolite Films on Substrates. Science 2011, 334, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dong, J.; Li, J.; Li, J.; Wu, F. A novel method for the preparation of zeolite ZSM-5. Chem. Commun. 1990, 755–756. [Google Scholar] [CrossRef]
- Nishiyama, N.; Ueyama, K.; Matsukata, M. Synthesis of defect-free zeolite-alumina composite membranes by a vapor-phase transport method. Microporous Mater. 1996, 7, 299–308. [Google Scholar] [CrossRef]
- Matsufuji, T.; Nishiyama, N.; Matsukata, M.; Ueyama, K. Separation of butane and xylene isomers with MFI-type zeolitic membrane synthesized by a vapor-phase transport method. J. Membr. Sci. 2000, 178, 25–34. [Google Scholar] [CrossRef]
- Kikuchi, E.; Yamashita, K.; Hiromoto, S.; Ueyama, K.; Matsukata, M. Synthesis of a zeolitic thin layer by a vapor-phase transport method: Appearance of a preferential orientation of MFI zeolite. Microporous Mater. 1997, 11, 107–116. [Google Scholar] [CrossRef]
- Matsufuji, T.; Nishiyama, N.; Ueyama, K.; Matsukata, M. Crystallization of ferrierite (FER) on a porous alumina support by a vapor-phase transport method. Microporous Mesoporous Mater. 1999, 32, 159–168. [Google Scholar] [CrossRef]
- Nishiyama, N.; Matsufuji, T.; Ueyama, K.; Matsukata, M. FER membrane synthesized by a vapor-phase transport method: Its structure and separation characteristics. Microporous Mater. 1997, 12, 293–303. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W. Microwave synthesis of zeolite membranes: A review. J. Membr. Sci. 2008, 316, 3–17. [Google Scholar] [CrossRef]
- Weisz, P.B. The Science of the Possible. Chemtech 1982, 12, 689–690. [Google Scholar]
- Boudart, M. Surface Time Yields of Membrane Reactors. In CaTTech; Springer Science & Business: Berlin, Germany, 1997. [Google Scholar]
- Yan, S.; Maeda, H.; Kusakabe, K.; Morooka, S. Thin Palladium Membrane Formed in Support Pores by Metal-Organic Chemical Vapor Deposition Method and Application to Hydrogen Separation. Ind. Eng. Chem. Res. 1994, 33, 616–622. [Google Scholar] [CrossRef]
- Graaf, J.M.V.; Zwiep, M.; Kapteijn, F.; Moulijn, J.A. Application of a silicalite-1 membrane reactor in metathesis reactions. Appl. Catal. A 1999, 178, 225–241. [Google Scholar] [CrossRef]
- Graaf, J.M.V.D.; Zwiep, M.; Kapteijn, F.; Moulijn, J.A. Application of a zeolite membrane reactor in the metathesis of propene. Chem. Eng. Sci. 1999, 54, 1441–1445. [Google Scholar] [CrossRef]
- Moon, W.S.; Park, S.B. Design guide of a membrane for a membrane reactor in terms of permeability and selectivity. J. Membr. Sci. 2000, 170, 43–51. [Google Scholar] [CrossRef]
- Battersby, S.; Teixeira, P.W.; Beltramini, J.; Duke, M.C.; Rudolph, V.; Diniz da Costa, J.C. An analysis of the Peclet and Damkohler numbers for dehydrogenation reactions using molecular sieve silica (MSS) membrane reactors. Catal. Today 2006, 116, 12–17. [Google Scholar] [CrossRef]
- Choi, S.-W.; Jones, C.W.; Nair, S.; Sholl, D.S.; Moore, J.S.; Liu, Y.; Dixit, R.S.; Pendergast, J.G. Material properties and operating configurations of membrane reactors for propane dehydrogenation. AIChE J. 2015, 61, 922–935. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technologies and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2004. [Google Scholar]
- Krishna, R.; van den Broeke, L.J.P. The Maxwell-Stefan description of mass transport across zeolite membranes. Chem. Eng. J. Biochem. Eng. J. 1995, 57, 155–162. [Google Scholar] [CrossRef]
- Krishna, R.; Wesselingh, J.A. The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A.; Krishna, R. The generalized Maxwell-Stefan model for diffusion in zeolites: Sorbate molecules with different saturation loadings. Chem. Eng. Sci. 2000, 55, 2923–2930. [Google Scholar] [CrossRef]
- Krishna, R.; Baur, R. Modelling issues in zeolite based separation processes. Sep. Purif. Technol. 2003, 33, 213–254. [Google Scholar] [CrossRef]
- Krishna, R. The Maxwell-Stefan description of mixture diffusion in nanoporous crystalline materials. Microporous Mesoporous Mater. 2014, 185, 30–50. [Google Scholar] [CrossRef]
- Yuan, W.; Lin, Y.S.; Yang, W. Molecular Sieving MFI-Type Zeolite Membranes for Pervaporation Separation of Xylene Isomers. J. Am. Chem. Soc. 2004, 126, 4776–4777. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Kusakabe, K.; Morooka, S. Gas permeation properties of A-type zeolite membrane formed on porous substrate by hydrothermal synthesis. J. Membr. Sci. 1998, 141, 197–205. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Song, C.; Yang, W.; Liu, J.; Lin, L. Synthesis, characterization and single gas permeation properties of NaA zeolite membrane. J. Membr. Sci. 2005, 249, 51–64. [Google Scholar] [CrossRef]
- Van de Graaf, J.M.; van der Bijl, E.; Stol, A.; Kapteijn, F.; Moulijn, J.A. Effect of Operating Conditions and Membrane Quality on the Separation Performance of Composite Silicalite-1 Membranes. Ind. Eng. Chem. Res. 1998, 37, 4071–4083. [Google Scholar] [CrossRef]
- Vroon, Z.A.E.P.; Keizer, K.; Burggraaf, A.J.; Verweij, H. Preparation and characterization of thin zeolite MFI membranes on porous supports. J. Membr. Sci. 1998, 144, 65–76. [Google Scholar] [CrossRef]
- Keizer, K.; Burggraaf, A.J.; Vroon, Z.A.E.P.; Verweij, H. Two component permeation through thin zeolite MFI membranes. J. Membr. Sci. 1998, 147, 159–172. [Google Scholar] [CrossRef]
- Coronas, J.; Falconer, J.L.; Noble, R.D. Characterization and permeation properties of ZSM-5 tubular membranes. AIChE J. 1997, 43, 1797–1812. [Google Scholar] [CrossRef]
- Jiang, M.; Eic, M.; Miachon, S.; Dalmon, J.-A.; Kocirik, M. Diffusion of n-butane, isobutane and ethane in a MFI-zeolite membrane investigated by gas permeation and ZLC measurements. Sep. Purif. Technol. 2001, 25, 287–295. [Google Scholar] [CrossRef]
- Barrer, R.M. Porous crystal membranes. J. Chem. Soc. Faraday Trans. 1990, 86, 1123–1130. [Google Scholar] [CrossRef]
- Bakker, W.J.W.; Kapteijn, F.; Poppe, J.; Moulijn, J.A. Permeation characteristics of a metal-supported silicalite-1 zeolite membrane. J. Membr. Sci. 1996, 117, 57–78. [Google Scholar] [CrossRef]
- Neubauer, K.; Lubenau, U.; Hecker, C.; Lücke, B.; Paschek, D.; Wohlrab, S. Abreicherung von Flüssiggas aus Erdgas mittels Zeolithmembranen; Depletion of Liquefied Petroleum Gas from Natural Gas by Zeolite Membranes. Chem. Ing. Tech. 2013, 85, 713–722. [Google Scholar] [CrossRef]
- Dragomirova, R.; Stohr, M.; Hecker, C.; Lubenau, U.; Paschek, D.; Wohlrab, S. Desorption-controlled separation of natural gas alkanes by zeolite membranes. RSC Adv. 2014, 4, 59831–59834. [Google Scholar] [CrossRef]
- Neubauer, K.; Dragomirova, R.; Stöhr, M.; Mothes, R.; Lubenau, U.; Paschek, D.; Wohlrab, S. Combination of membrane separation and gas condensation for advanced natural gas conditioning. J. Membr. Sci. 2014, 453, 100–107. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Arruebo, M.; Coronas, J.; Menéndez, M.; Santamarı́a, J. Separation of hydrocarbons from natural gas using silicalite membranes. Sep. Purif. Technol. 2001, 25, 275–286. [Google Scholar] [CrossRef]
- Dragomirova, R.; Kreft, S.; Georgi, G.; Seeburg, D.; Wohlrab, S. Liquefied Petroleum Gas Enrichment by Zeolite Membranes for Low-Temperature Steam Reforming of Natural Gas. In Proceedings of Synthesis Gas Chemistry, 2015-2, DGMK International Conference, Dresden, Germany, 7–9 October 2015; pp. 273–280.
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Varela-Gandía, F.J.; Berenguer-Murcia, Á.; Lozano-Castelló, D.; Cazorla-Amorós, D. Zeolite A/carbon membranes for H2 purification from a simulated gas reformer mixture. J. Membr. Sci. 2011, 378, 407–414. [Google Scholar] [CrossRef]
- Gallo, M.; Nenoff, T.M.; Mitchell, M.C. Selectivities for binary mixtures of hydrogen/methane and hydrogen/carbon dioxide in silicalite and ETS-10 by Grand Canonical Monte Carlo techniques. Fluid Phase Equilibria 2006, 247, 135–142. [Google Scholar] [CrossRef]
- Mitchell, M.C.; Autry, J.D.; Nenoff, T.M. Molecular dynamics simulations of binary mixtures of methane and hydrogen in zeolite A and a novel zinc phosphate. Mol. Phys. 2001, 99, 1831–1837. [Google Scholar] [CrossRef]
- Tang, Z.; Dong, J.; Nenoff, T.M. Internal Surface Modification of MFI-Type Zeolite Membranes for High Selectivity and High Flux for Hydrogen. Langmuir 2009, 25, 4848–4852. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.-L.; Tye, C.-T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Lima, S.Y.; Parkb, B.; Hunga, F.; Sahimia, M.; Tsotsisa, T.T. Design issues of pervaporation membrane reactors for esterification. Chem. Eng. Sci. 2002, 57, 4933–4946. [Google Scholar] [CrossRef]
- Jafar, J.J.; Budd, P.M.; Hughes, R. Enhancement of esterification reaction yield using zeolite—A vapour permeation membrane. J. Membr. Sci. 2002, 199, 117–123. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of a sodalite membrane reactor in esterification—Coupling reaction and separation. Catal. Today 2010, 156, 132–139. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Mizukami, F.; Kowata, Y.; Hanaoka, T. Application of a CHA-type zeolite membrane to the esterification of adipic acid with isopropyl alcohol using sulfuric acid catalyst. J. Membr. Sci. 2012, 415, 368–374. [Google Scholar] [CrossRef]
- Okamoto, K.-I.; Kita, H.; Horii, K. Zeolite NaA Membrane: Preparation, Single-Gas Permeation, and Pervaporation and Vapor Permeation of Water/Organic Liquid Mixtures. Ind. Eng. Chem. Res. 2001, 40, 163–175. [Google Scholar] [CrossRef]
- Kita, H.; Horii, K.; Ohtoshi, Y.; Tanaka, K.; Okamoto, K.-I. Synthesis of a zeolite NaA membrane for pervaporation of water/organic liquid mixtures. J. Mater. Sci. Lett. 1995, 14, 206–208. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nagase, T.; Kiyozumi, Y.; Hanaoka, T.; Mizukami, F. Influence of acid on the permeation properties of NaA-type zeolite membranes. J. Membr. Sci. 2010, 349, 189–194. [Google Scholar] [CrossRef]
- Inoue, T.; Nagase, T.; Hasegawa, Y.; Kiyozumi, Y.; Sato, K.; Nishioka, M.; Hamakawa, S.; Mizukami, F. Stoichiometric ester condensation reaction processes by pervaporative water removal via acid-tolerant zeolite membranes. Ind. Eng. Chem. Res. 2007, 46, 3743–3750. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of hydroxy sodalite films as novel water selective membranes. J. Membr. Sci. 2009, 326, 153–160. [Google Scholar] [CrossRef]
- Zhang, W.; Na, S.; Li, W.; Xing, W. Kinetic Modeling of Pervaporation Aided Esterification of Propionic Acid and Ethanol Using T-Type Zeolite Membrane. Ind. Eng. Chem. Res. 2015, 54, 4940–4946. [Google Scholar] [CrossRef]
- Aiouache, F.; Goto, S. Reactive distillation-pervaporation hybrid column for tert-amyl alcohol etherification with ethanol. Chem. Eng. Sci. 2003, 58, 2465–2477. [Google Scholar] [CrossRef]
- Thomas, S.; Hamel, C.; Seidel-Morgenstern, A. Basic Problems of Chemical Reaction Engineering and Potential of Membrane Reactors. In Membrane Reactors; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 1–27. [Google Scholar]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A.; Hai, F.I. Introduction—A Review of Membrane Reactors. In Membranes for Membrane Reactors; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 1–61. [Google Scholar]
- Téllez, C.; Menéndez, M. Zeolite Membrane Reactors. In Membranes for Membrane Reactors; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 243–273. [Google Scholar]
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for membranes and membrane processes. Pure Appl. Chem. 1999, 68, 1479–1489. [Google Scholar]
- Dittmeyer, R.; Hollein, V.; Daub, K. Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium. J. Mol. Catal. A 2001, 173, 135–184. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Caro, J. Catalytic Membrane Reactors. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinhein, Germany, 2008. [Google Scholar]
- Xiongfu, Z.; Yongsheng, L.; Jinqu, W.; Huairong, T.; Changhou, L. Synthesis and characterization of Fe-MFI zeolite membrane on a porous g-Al2O3 tube. Sep. Purif. Technol. 2001, 25, 269–274. [Google Scholar] [CrossRef]
- Ciavarella, P.; Casanave, D.; Moueddeb, H.; Miachon, S.; Fiaty, K.; Dalmon, J.A. Isobutane dehydrogenation in a membrane reactor—Influence of the operating conditions on the performance. Catal. Today 2001, 67, 177–184. [Google Scholar] [CrossRef]
- Illgen, U.; Schäfer, R.; Noack, M.; Kölsch, P.; Kühnle, A.; Caro, J. Membrane supported catalytic dehydrogenation of iso-butane using an MFI zeolite membrane reactor. Catal. Commun. 2001, 2, 339–345. [Google Scholar] [CrossRef]
- Bergh, J.V.D.; Gücüyener, C.; Gascon, J.; Kapteijn, F. Isobutane dehydrogenation in a DD3R zeolite membrane reactor. Chem. Eng. J. 2011, 166, 368–377. [Google Scholar] [CrossRef]
- Jeong, B.H.; Sotowa, K.I.; Kusakabe, K. Catalytic dehydrogenation of cyclohexane in an FAU-type zeolite membrane reactor. J. Membr. Sci. 2003, 224, 151–158. [Google Scholar] [CrossRef]
- Jeong, B.H.; Sotowa, K.I.; Kusakabe, K. Modeling of an FAU-type zeolite membrane reactor for the catalytic dehydrogenation of cyclohexane. Chem. Eng. J. 2004, 103, 69–75. [Google Scholar] [CrossRef]
- Kong, C.L.; Lu, J.M.; Yang, H.H.; Wang, J.Q. Catalytic dehydrogenation of ethylbenzene to styrene in a zeolite silicalite-1 membrane reactor. J. Membr. Sci. 2007, 306, 29–35. [Google Scholar] [CrossRef]
- Avila, A.M.; Yu, Z.; Fazli, S.; Sawada, J.A.; Kuznicki, S.M. Hydrogen-selective natural mordenite in a membrane reactor for ethane dehydrogenation. Microporous Mesoporous Mater. 2014, 190, 301–308. [Google Scholar] [CrossRef]
- Tang, Z.; Kim, S.J.; Reddy, G.K.; Dong, J.H.; Smirniotis, P. Modified zeolite membrane reactor for high temperature water gas shift reaction. J. Membr. Sci. 2010, 354, 114–122. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wu, Z.J.; Hong, Z.; Gu, X.H.; Xu, N.P. Hydrogen-selective zeolite membrane reactor for low temperature water gas shift reaction. Chem. Eng. J. 2012, 197, 314–321. [Google Scholar] [CrossRef]
- Kim, S.J.; Xu, Z.; Reddy, G.K.; Smirniotis, P.; Dong, J.H. Effect of Pressure on High-Temperature Water Gas Shift Reaction in Microporous Zeolite Membrane Reactor. Ind. Eng. Chem. Res. 2012, 51, 1364–1375. [Google Scholar] [CrossRef]
- Kim, S.J.; Yang, S.W.; Reddy, G.K.; Smirniotis, P.; Dong, J.H. Zeolite Membrane Reactor for High-Temperature Water-Gas Shift Reaction: Effects of Membrane Properties and Operating Conditions. Energy Fuels 2013, 27, 4471–4480. [Google Scholar] [CrossRef]
- Dong, X.L.; Wang, H.B.; Rui, Z.B.; Lin, Y.S. Tubular dual-layer MFI zeolite membrane reactor for hydrogen production via the WGS reaction: Experimental and modeling studies. Chem. Eng. J. 2015, 268, 219–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Gu, X. Pure H2 production through hollow fiber hydrogen-selective MFI zeolite membranes using steam as sweep gas. AIChE J. 2015. [Google Scholar] [CrossRef]
- Dyk, L.V.; Lorenzen, L.; Miachon, S.; Dalmon, J.-A. Xylene isomerization in an extractor type catalytic membrane reactor. Catal. Today 2005, 104, 274–280. [Google Scholar] [CrossRef]
- Haag, S.; Hanebuth, M.; Mabande, G.T.P.; Avhale, A.; Schwieger, W.; Dittmeyer, R. On the use of a catalytic H-ZSM-5 membrane for xylene isomerization. Microporous Mesoporous Mater. 2006, 96, 168–176. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Horowitz, G.I.; Lombardo, E.A. Xylene isomerization in a ZSM-5/SS membrane reactor. Catal. Lett. 2008, 123, 7–15. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, Z.; Gu, X.H.; Zhong, Z.X.; Jin, W.Q.; Xu, N.P. Silicalite-1 Zeolite Membrane Reactor Packed with HZSM-5 Catalyst for meta-Xylene Isomerization. Ind. Eng. Chem. Res. 2009, 48, 4293–4299. [Google Scholar] [CrossRef]
- Daramola, M.O.; Deng, Z.; Pera-Titus, M.; Giroir-Fendler, A.; Miachon, S.; Burger, A.J.; Lorenzen, L.; Guo, Y. Nanocomposite MFI-alumina membranes prepared via pore-pugging synthesis: Application as packed-bed membrane reactors for m-xylene isomerization over a Pt-HZSM-5 catalyst. Catal. Today 2010, 261–267. [Google Scholar] [CrossRef]
- Daramola, M.O.; Burger, A.J.; Giroir-Fendler, A.; Miachon, S.; Lorenzen, L. Extractor-type catalytic membrane reactor with nanocomposite MFI-alumina membrane tube as separation unit: Prospect for ultra-pure para-Xylene production from m-Xylene isomerization over Pt-HZSM-5 catalyst. Appl. Catal. A 2010, 386, 109–115. [Google Scholar] [CrossRef]
- Yeong, Y.F.; Abdullah, A.Z.; Ahmad, A.L.; Bhatia, S. Xylene isomerization kinetic over acid-functionalized silicalite-1 catalytic membranes: Experimental and modeling studies. Chem. Eng. J. 2010, 157, 579–589. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, Z.; Chen, J.; Gu, X.H.; Jin, W.; Xu, N. Catalytic MFI zeolite membranes supported on Al2O3 substrates for m-xylene isomerization. J. Membr. Sci. 2012, 389, 451–458. [Google Scholar] [CrossRef]
- Mihályi, R.M.; Patis, A.; Nikolakis, V.; Kollár, M.; Valyon, J. [B]MFI membrane: Synthesis, physico-chemical properties and catalytic behavior in the double-bond isomerization of 1-butene. Sep. Purif. Technol. 2013, 118, 135–143. [Google Scholar] [CrossRef]
- Bernal, M.P.; Coronas, J.; Menendez, M.; Santamaria, J. Coupling ofreaction and separation at the microscopic level: Esterification processes in a H-ZSM-5 membrane reactor. Chem. Eng. Sci. 2002, 57, 1557–1562. [Google Scholar] [CrossRef]
- De la Iglesia, Ó.; Mallada, R.; Menéndez, M.; Coronas, J. Continuous zeolite membrane reactor for esterification of ethanol and acetic acid. Chem. Eng. J. 2007, 131, 35–39. [Google Scholar] [CrossRef]
- Fedosov, D.A.; Smirnov, A.V.; Shkirskiy, V.V.; Voskoboynikov, T.; Ivanova, I.I. Methanol dehydration in NaA zeolite membrane reactor. J. Membr. Sci. 2015, 486, 189–194. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Gora, L.; Jansen, J.C. Hydroisomerization of C6 with a zeolite membrane reactor. J. Catal. 2005, 230, 269–281. [Google Scholar] [CrossRef]
- Mota, S.; Miachon, S.; Volta, J.C.; Dalmon, J.A. Membrane reactor for selective oxidation of butane to maleic anhydride. Catal. Today 2001, 67, 169–176. [Google Scholar] [CrossRef]
- Abon, M.; Volta, J.-C. Vanadium phosphorus oxides for n-butane oxidation to maleic anhydride. Appl. Catal. A 1997, 157, 173–193. [Google Scholar] [CrossRef]
- Mallada, R.; Menéndez, M.; Santamarı́a, J. Use of membrane reactors for the oxidation of butane to maleic anhydride under high butane concentrations. Catal. Today 2000, 56, 191–197. [Google Scholar] [CrossRef]
- Xue, E.; Ross, J. The use of membrane reactors for catalytic n-butane oxidation to maleic anhydride with a butane-rich feed. Catal. Today 2000, 61, 3–8. [Google Scholar] [CrossRef]
- Cruz-López, A.M.; Guilhaume, N.; Miachon, S.; Dalmon, J.-A. Selective oxidation of butane to maleic anhydride in a catalytic membrane reactor adapted to rich butane feed. Catal. Today 2005, 2005, 949–956. [Google Scholar] [CrossRef]
- Pantazidis, A.; Dalmon, J.A.; Mirodatos, C. Oxidative Dehydrogenation of Propane on Catalytic Membrane Reactors. Catal. Today 1995, 25, 403–408. [Google Scholar] [CrossRef]
- Julbe, A.; Farrusseng, D.; Jalibert, J.C.; Mirodatos, C.; Guizard, C. Characteristics and performance in the oxidative dehydrogenation of propane of MFI and V-MFI zeolite membranes. Catal. Today 2000, 56, 199–209. [Google Scholar] [CrossRef]
- Diakov, V.; Blackwell, B.; Varma, A. Methanol oxidative dehydrogenation in a catalytic packed-bed membrane reactor: Experiments and model. Chem. Eng. Sci. 2002, 57, 1563–1569. [Google Scholar] [CrossRef]
- Diakov, V.; Varma, A. Optimal Feed Distribution in a Packed-Bed Membrane Reactor: The Case of Methanol Oxidative Dehydrogenation. Ind. Eng. Chem. Res. 2004, 43, 309–314. [Google Scholar] [CrossRef]
- Chommeloux, B.; Cimaomo, S.; Jolimaitre, E.; Uzio, D.; Magnoux, P.; Sanchez, J. New membrane for use as hydrogen distributor for hydrocarbon selective hydrogenation. Microporous Mesoporous Mater. 2008, 109, 28–37. [Google Scholar] [CrossRef]
- Torres, M.; López, L.; Domı́nguez, J.M.; Mantilla, A.; Ferrat, G.; Gutierrez, M.; Maubert, M. Olefins catalytic oligomerization on new composites of beta-zeolite films supported on α-Al2O3 membranes. Chem. Eng. J. 2003, 92, 1–6. [Google Scholar] [CrossRef]
- Torres, M.; Gutiérrez, M.; Mugica, V.; Romero, M.; López, L. Oligomerization of isobutene with a,β-zeolite membrane: Effect of the acid properties of the catalytic membrane. Catal. Today 2011, 166, 205–208. [Google Scholar] [CrossRef]
- Aguado, S.; Coronas, J.; Santamaría, J. Use of Zeolite Membrane Reactors for the Combustion of VOCs Present in Air at Low Concentrations. Chem. Eng. Res. Des. 2005, 83, 295–301. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshikawa, R.; Ying, C.; Kita, H.; Okamoto, K. Application of zeolite membranes to esterification reactions. Catal. Today 2001, 67, 121–125. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshikawa, R.; Ying, C.; Kita, H.; Okamoto, K. Application of zeolite T membrane to vapor-permeation-aided esterification of lactic acid with ethanol. Chem. Eng. Sci. 2002, 57, 1577–1584. [Google Scholar] [CrossRef]
- Salomón, M.A.; Coronas, J.; Menéndez, M.; Santamarı́a, J. Synthesis of MTBE in zeolite membrane reactors. Appl. Catal. A 2000, 200, 201–210. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Llorens, J.; Cunill, F. Technical and economical feasibility of zeolite NaA membrane-based reactors in liquid-phase etherification reactions. Chem. Eng. Process. Process Intensif. 2009, 48, 1072–1079. [Google Scholar] [CrossRef]
- Aresta, M.; Galatola, M. Life cycle analysis applied to the assessment of the environmental impact of alternative synthetic processes. The dimethylcarbonate case: Part 1. J. Clean Prod. 1999, 7, 181–193. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Tommasi, I. Developing Innovative Synthetic Technologies of Industrial Relevance Based on Carbon Dioxide as Raw Material. Energy Fuels 2001, 15, 269–273. [Google Scholar] [CrossRef]
- Diban, N.; Urtiaga, A.M.; Ortiz, I.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Influence of the membrane properties on the catalytic production of dimethyl ether with in situ water removal for the successful capture of CO2. Chem. Eng. J. 2013, 234, 140–148. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, A.T.; Ereña, J.; Sierra, I.; Olazar, M.; Bilbao, J. Deactivation and regeneration of hybrid catalysts in the single-step synthesis of dimethyl ether from syngas and CO2. Catal. Today 2005, 106, 265–270. [Google Scholar] [CrossRef]
- Diban, N.; Urtiaga, A.M.; Ortiz, I.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Improved Performance of a PBM Reactor for Simultaneous CO2 Capture and DME Synthesis. Ind. Eng. Chem. Res. 2014, 53, 19479–19487. [Google Scholar]
- Rohde, M.P.; Unruh, D.; Schaub, G. Membrane application in Fischer-Tropsch synthesis reactors—Overview of concepts. Catal. Today 2005, 106, 143–148. [Google Scholar] [CrossRef]
- Rohde, M.P.; Schaub, G.; Khajavi, S.; Jansen, J.C.; Kapteijn, F. Fischer-Tropsch synthesis with in situ H2O removal—Directions of membrane development. Microporous Mesoporous Mater. 2008, 115, 123–136. [Google Scholar] [CrossRef]
- Espinoza, R.L.; du Toit, E.; Santamaria, J.; Menendez, M.; Coronas, J.; Irusta, S. Use of membranes in Fischer-Tropsch reactors. In Studies in Surface Science and Catalysis; Avelino Corma, F.V.M.S.M., José Luis, G.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 130, pp. 389–394. [Google Scholar]
- Khajavi, S.; Kapteijn, F.; Jansen, J.C. Synthesis of thin defect-free hydroxy sodalite membranes: New candidate for activated water permeation. J. Membr. Sci. 2007, 299, 63–72. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mirvakili, A.; Paymooni, K. A novel water perm-selective membrane dual-type reactor concept for Fischer-Tropsch synthesis of GTL (gas to liquid) technology. Energy 2011, 36, 1223–1235. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mirvakili, A.; Paymooni, K.; Moghtaderi, B. A comparative study between a fluidized-bed and a fixed-bed water perm-selective membrane reactor with in situ H2O removal for Fischer-Tropsch synthesis of GTL technology. J. Nat. Gas Sci. Eng. 2011, 3, 484–495. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F. Classification of industrial catalysts and catalysis for the petrochemical industry. Catal. Today 1997, 34, 269–279. [Google Scholar] [CrossRef]
- Casanave, D.; Giroirfendler, A.; Sanchez, J.; Loutaty, R.; Dalmon, J.A. Control of Transport-Properties with a Microporous Membrane Reactor to Enhance Yields in Dehydrogenation Reactions. Catal. Today 1995, 25, 309–314. [Google Scholar] [CrossRef]
- Casanave, D.; Ciavarella, P.; Fiaty, K.; Dalmon, J.A. Zeolite membrane reactor for isobutane dehydrogenation: Experimental results and theoretical modelling. Chem. Eng. Sci. 1999, 54, 2807–2815. [Google Scholar] [CrossRef]
- Dyk, L.V.; Miachon, S.; Lorenzen, L.; Torres, M.; Fiaty, K.; Dalmon, J.-A. Comparison of microporous MFI and dense Pd membrane performances in an extractor-type CMR. Catal. Today 2003, 82, 167–177. [Google Scholar]
- Gokhale, Y.V.; Noble, R.D.; Falconer, J.L. Effects of reactant loss and membrane selectivity on a dehydrogenation reaction in a membrane-enclosed catalytic reactor. J. Membr. Sci. 1995, 103, 235–242. [Google Scholar] [CrossRef]
- Vaezi, M.J.; Babaluo, A.A.; Shafiei, S. Modeling of ethylbenzene dehydrogenation membrane reactor to investigate the potential application of a microporous dydroxy sodalite membrane. J. Chem. Pet. Eng. 2015, 49, 51–62. [Google Scholar]
- Rhodes, C.; Hutchings, G.J.; Ward, A.M. Water-gas shift reaction: Finding the mechanistic boundary. Catal. Today 1995, 23, 43–58. [Google Scholar] [CrossRef]
- Tosti, S.; Basile, A.; Chiappetta, G.; Rizzello, C.; Violante, V. Pd-Ag membrane reactors for water gas shift reaction. Chem. Eng. J. 2003, 93, 23–30. [Google Scholar] [CrossRef]
- Basile, A.; Chiappetta, G.; Tosti, S.; Violante, V. Experimental and simulation of both Pd and Pd/Ag for a water gas shift membrane reactor. Sep. Purif. Technol. 2001, 25, 549–571. [Google Scholar] [CrossRef]
- Peters, T.A.; Stange, M.; Klette, H.; Bredesen, R. High pressure performance of thin Pd-23%Ag/stainless steel composite membranes in water gas shift gas mixtures; influence of dilution, mass transfer and surface effects on the hydrogen flux. J. Membr. Sci. 2008, 316, 119–127. [Google Scholar] [CrossRef]
- Augustine, A.S.; Ma, Y.H.; Kazantzis, N.K. High pressure palladium membrane reactor for the high temperature water-gas shift reaction. Int. J. Hydrogen Energy 2011, 36, 5350–5360. [Google Scholar] [CrossRef]
- Giessler, S.; Jordan, L.; Diniz da Costa, J.C.; Lu, G.Q. Performance of hydrophobic and hydrophilic silica membrane reactors for the water gas shift reaction. Sep. Purif. Technol. 2003, 32, 255–264. [Google Scholar] [CrossRef]
- Battersby, S.; Duke, M.C.; Liu, S.; Rudolph, V.; Costa, J.C.D.D. Metal doped silica membrane reactor: Operational effects of reaction and permeation for the water gas shift reaction. J. Membr. Sci. 2008, 316, 46–52. [Google Scholar] [CrossRef]
- Gu, X.; Tang, Z.; Dong, J. On-stream modification of MFI zeolite membranes for enhancing hydrogen separation at high temperature. Microporous Mesoporous Mater. 2008, 111, 441–448. [Google Scholar] [CrossRef]
- Liu, B.S.; Au, C.T. A La2NiO4-Zeolite Membrane Reactor for the CO2 Reforming of Methane to Syngas. Catal. Lett. 2001, 77, 67–74. [Google Scholar] [CrossRef]
- Liu, B.S.; Gao, L.Z.; Au, C.T. Preparation, characterization and application of a catalytic NaA membrane for CH4/CO2 reforming to syngas. Appl. Catal. A 2002, 235, 193–206. [Google Scholar] [CrossRef]
- Barbieri, G.; Marigliano, G.; Golemme, G.; Drioli, E. Simulation of CO2 hydrogenation with CH3OH removal in a zeolite membrane reactor. Chem. Eng. J. 2002, 85, 53–59. [Google Scholar] [CrossRef]
- Lee, G.-S.; McCain, J.; Bhasin, M. Synthetic Organic Chemicals. In Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology; Kent, J., Ed.; Springer US: New York, NY, USA, 2007; pp. 345–403. [Google Scholar]
- Yan, T.Y. Separation of p-xylene and ethylbenzene from C8 aromatics using medium-pore zeolites. Ind. Eng. Chem. Res. 1989, 28, 572–576. [Google Scholar] [CrossRef]
- Daramola, M.O.; Burger, A.J.; Pera-Titus, M.; Giroir-Fendler, A.; Miachon, S.; Dalmon, J.A.; Lorenzen, L. Separation and isomerization of xylenes using zeolite membranes: A short overview. Asia-Pac. J. Chem. Eng. 2010, 5, 815–837. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Lai, Z.; Tsapatsis, M. Separation of Xylene Isomer Vapors with Oriented MFI Membranes Made by Seeded Growth. Ind. Eng. Chem. Res. 2001, 40, 544–552. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. MFI-Pnma. In Atlas of Zeolite Framework Types, 6th ed.; Baerlocher, C., Olson, L.B.M.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 212–213. [Google Scholar]
- Sakai, H.; Tomita, T.; Takahashi, T. p-Xylene separation with MFI-type zeolite membrane. Sep. Purif. Technol. 2001, 25, 297–306. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Nenoff, T.M.; Ozokwelu, D.E. Separation of p-xylene from multicomponent vapor mixtures using tubular MFI zeolite mmbranes. J. Membr. Sci. 2006, 280, 624–633. [Google Scholar] [CrossRef]
- Gump, C.J.; Tuan, V.A.; Noble, R.D.; Falconer, J.L. Aromatic Permeation through Crystalline Molecular Sieve Membranes. Ind. Eng. Chem. Res. 2001, 40, 565–577. [Google Scholar] [CrossRef]
- Lai, Z.; Bonilla, G.; Diaz, I.; Nery, J.G.; Sujaoti, K.; Amat, M.A.; Kokkoli, E.; Terasaki, O.; Thompson, R.W.; Tsapatsis, M.; et al. Microstructural optimization of a zeolite membrane for organic vapor separation. Science 2003, 300, 456–460. [Google Scholar] [PubMed]
- Daramola, M.O.; Burger, A.J.; Pera-Titus, M.; Giroir-Fendler, A.; Miachon, S.; Lorenzen, L.; Dalmon, J.A. Nanocomposite MFI-ceramic hollow fibre membranes via pore-plugging synthesis: Prospects for xylene isomer separation. J. Membr. Sci. 2009, 337, 106–112. [Google Scholar] [CrossRef]
- Maloncy, M.L.; Maschmeyer, T.; Jansen, J.C. Technical and economical evaluation of a zeolite membrane based heptane hydroisomerization process. Chem. Eng. J. 2005, 106, 187–195. [Google Scholar] [CrossRef]
- Piera, E.; Tellez, C.; Coronas, J.; Menendez, M.; SaIntamaria, J. Use of zeolite membrane reactors for selectivity enhancement: Application to the liquid-phase oligomerization of i-butene. Catal. Today 2001, 67, 127–138. [Google Scholar] [CrossRef]
- Peters, T.A.; Benes, N.E.; Keurentjes, J.T.F. Zeolite-Coated Ceramic Pervaporation Membranes; Pervaporation-Esterification Coupling and Reactor Evaluation. Ind. Eng. Chem. Res. 2005, 44, 9490–9496. [Google Scholar] [CrossRef]
- De la Iglesia, Ó.; Irusta, S.; Mallada, R.; Menéndez, M.; Coronas, J.; Santamaría, J. Preparation and characterization of two-layered mordenite-ZSM-5 bi-functional membranes. Microporous Mesoporous Mater. 2006, 93, 318–324. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Lombardo, E.A.; Avila, A.M. Xylene Permeation Transport through Composite Ba-ZSM-5/SS Tubular Membranes: Modeling the Steady-State Permeation. Ind. Eng. Chem. Res. 2008, 47, 2377–2385. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zhang, X.F.; Zhang, J.; Liu, H.O.; Zhou, L.; Yeung, K.L. Preparation of alkali-resistant, Sil-1 encapsulated nickel catalysts for direct internal reforming-molten carbonate fuel cell. Catal. Commun. 2009, 10, 1804–1807. [Google Scholar] [CrossRef]
- He, J.; Yoneyama, Y.; Xu, B.; Nishiyama, N.; Tsubaki, N. Designing a Capsule Catalyst and Its Application for Direct Synthesis of Middle Isoparaffins. Langmuir 2005, 21, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Puil, N.V.D.; Creyghton, E.J.; Rodenburg, E.C.; Sie, T.S.; Bekkum, H.v.; Jansen, J.C. Catalytic testing of TiO2/platinum/silicalite-1 composites. Faraday Trans. 1996, 96, 4609–4615. [Google Scholar] [CrossRef]
- Van der Puil, N.; Dautzenberg, F.M.; van Bekkum, H.; Jansen, J.C. Preparation and catalytic testing of zeolite coatings on preshaped alumina supports. Microporous Mesoporous Mater. 1999, 27, 95–106. [Google Scholar] [CrossRef]
- Bouizi, Y.; Rouleau, L.; Valtchev, V.P. Factors Controlling the Formation of Core-Shell Zeolite-Zeolite Composites. Chem. Mater. 2006, 18, 4959–4966. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Meng, M.; Yoneyama, Y.; Tsubaki, N. One-step synthesis of H-β zeolite-enwrapped Co/Al2O3 Fischer-Tropsch catalyst with high spatial selectivity. J. Catal. 2009, 265, 26–34. [Google Scholar] [CrossRef]
- Bao, J.; He, J.; Zhang, Y.; Yoneyama, Y.; Tsubaki, N. A Core/Shell Catalyst Produces a Spatially Confined Effect and Shape Selectivity in a Consecutive Reaction. Angew. Chem. Int. Ed. 2008, 47, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, H.; Kido, Y.; Yoneyama, Y.; Suehiro, Y.; Tsubaki, N. A capsule catalyst with a zeolite membrane prepared by direct liquid membrane crystallization. ChemSusChem 2012, 5, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Phienluphon, R.; Pinkaew, K.; Yang, G.H.; Li, J.; Wei, Q.H.; Yoneyama, Y.; Vitidsant, T.; Tsubaki, N. Designing core (Cu/ZnO/Al2O3)-shell (SAPO-11) zeolite capsule catalyst with a facile physical way for dimethyl ether direct synthesis from syngas. Chem. Eng. J. 2015, 270, 605–611. [Google Scholar] [CrossRef]
- Cimenler, U.; Joseph, B.; Kuhn, J.N. Molecular-size selective H-β zeolite-encapsulated Ce-Zr/Ni-Mg catalysts for steam reforming. Appl. Catal. A 2015, 505, 494–500. [Google Scholar] [CrossRef]
- Pinkaew, K.; Yang, G.; Vitidsant, T.; Jin, Y.; Zeng, C.; Yoneyama, Y.; Tsubaki, N. A new core-Shell-like capsule catalyst with SAPO-46 zeolite shell encapsulated Cr/ZnO for the controlled tandem synthesis of dimethyl ether from syngas. Fuel 2013, 111, 727–732. [Google Scholar] [CrossRef]
- Xing, C.; Sun, J.; Chen, Q.; Yang, G.; Muranaka, N.; Lu, P.; Shen, W.; Zhu, P.; Wei, Q.; Li, J.; et al. Tunable isoparaffin and olefin yields in Fischer-Tropsch synthesis achieved by a novel iron-based micro-capsule catalyst. Catal. Today 2015, 251, 41–46. [Google Scholar] [CrossRef]
- Nishiyama, N.; Miyamoto, M.; Egashira, Y.; Ueyama, K. Zeolite membrane on catalyst particles for selective formation of p-xylene in the disproportionation of toluene. Chem. Commun. 2001, 1746–1747. [Google Scholar] [CrossRef]
- Vu, D.V.; Miyamoto, M.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Selective formation of para-xylene over H-ZSM-5 coated with polycrystalline silicalite crystals. J. Catal. 2006, 243, 389–394. [Google Scholar]
- Vu, D.V.; Miyamoto, M.; Nishiyama, N.; Ichikawa, S.; Egashira, Y.; Ueyama, K. Catalytic activities and structures of silicalite-1/H-ZSM-5 zeolite composites. Microporous Mesoporous Mater. 2008, 115, 106–112. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Meng, F.; San, X.; Yang, G.; Meng, M.; Takahashi, M.; Tsubaki, N. Hydroformylation of 1-Hexene on Silicalite-1 Zeolite Membrane Coated Pd-Co/AC Catalyst. Top Catal. 2010, 53, 608–614. [Google Scholar] [CrossRef]
- Nishiyama, N.; Ichioka, K.; Park, D.-H.; Egashira, Y.; Ueyama, K.; Gora, L.; Zhu, W.; Kapteijn, F.; Moulijn, J.A. Reactant-Selective Hydrogenation over Composite Silicalite-1-Coated Pt/TiO2 Particles. Ind. Eng. Chem. Res. 2004, 43, 1211–1215. [Google Scholar] [CrossRef]
- Nishiyama, N.; Ichioka, K.; Miyamoto, M.; Egashira, Y.; Ueyama, K.; Gora, L.; Zhu, W.D.; Kapteijn, F.; Moulijn, J.A. Silicalite-1 coating on Pt/TiO2 particles by a two-step hydrothermal synthesis. Microporous Mesoporous Mater. 2005, 83, 244–250. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, L.; Luo, M.; Xie, Y.; Zhu, W. Fabrication of zeolite-4A membranes on a catalyst particle level. Chem. Commun. 2006, 2911–2912. [Google Scholar] [CrossRef]
- Wu, Y.; Chai, Y.; Li, J.; Guo, H.; Wena, L.; Liu, C. Preparation of silicalite-1@Pt/alumina core-shell catalyst for shape-selective hydrogenation of xylene isomers. Catal. Commun. 2015, 64, 110–113. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Yoneyama, Y.; Nishiyama, N.; Tsubaki, N. Multiple-Functional Capsule Catalysts: A Tailor-Made Confined Reaction Environment for the Direct Synthesis of Middle Isoparaffins from Syngas. Chemistry 2006, 12, 8296–8304. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; He, J.J.; Yoneyama, Y.; Tan, Y.S.; Han, Y.Z.; Tsubaki, N. Preparation, characterization and reaction performance of H-ZSM-5/cobalt/silica capsule catalysts with different sizes for direct synthesis of isoparaffins. Appl. Catal. A 2007, 329, 99–105. [Google Scholar] [CrossRef]
- Yang, G.H.; Tan, Y.S.; Han, Y.Z.; Qiu, J.S.; Tsubaki, N. Increasing the shell thickness by controlling the core size of zeolite capsule catalyst: Application in iso-paraffin direct synthesis. Catal. Commun. 2008, 9, 2520–2524. [Google Scholar] [CrossRef]
- Yang, G.H.; He, J.J.; Zhang, Y.; Yoneyama, Y.; Tan, Y.S.; Han, Y.Z.; Vitidsant, T.; Tsubaki, N. Design and modification of zeolite capsule catalyst, a confined reaction field, and its application in one-step isoparaffin synthesis from syngas. Energy Fuels 2008, 22, 1463–1468. [Google Scholar] [CrossRef]
- Bao, J.; Yang, G.H.; Okada, C.; Yoneyama, Y.; Tsubaki, N. H-type zeolite coated iron-based multiple-functional catalyst for direct synthesis of middle isoparaffins from syngas. Appl. Catal. A 2011, 394, 195–200. [Google Scholar] [CrossRef]
- Huang, X.; Hou, B.; Wang, J.G.; Li, D.B.; Jia, L.T.; Chen, J.G.; Sun, Y.H. CoZr/H-ZSM-5 hybrid catalysts for synthesis of gasoline-range isoparaffins from syngas. Appl. Catal. A 2011, 408, 38–46. [Google Scholar] [CrossRef]
- Yang, G.; Xing, C.; Hirohama, W.; Jin, Y.; Zeng, C.; Suehiro, Y.; Wang, T.; Yoneyama, Y.; Tsubaki, N. Tandem catalytic synthesis of light isoparaffin from syngas via Fischer-Tropsch synthesis by newly developed core-shell-like zeolite capsule catalysts. Catal. Today 2013, 215, 29–35. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, G.; Chen, Q.; Niu, W.; Lu, P.; Yoneyama, Y.; Tsubaki, N. Development of dual-membrane coated Fe/SiO2 catalyst for efficient synthesis of isoparaffins directly from syngas. J. Membr. Sci. 2015, 475, 22–29. [Google Scholar] [CrossRef]
- Yang, G.; Tsubaki, N.; Shamoto, J.; Yoneyama, Y.; Zhang, Y. Confinement effect and synergistic function of H-ZSM-5/Cu-ZnO-Al2O3 capsule catalyst for one-step controlled synthesis. J. Am. Chem. Soc. 2010, 132, 8129–8136. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Thongkam, M.; Vitidsant, T.; Yoneyama, Y.; Tan, Y.; Tsubaki, N. A double-shell capsule catalyst with core-shell-like structure for one-step exactly controlled synthesis of dimethyl ether from CO2 containing syngas. Catal. Today 2011, 171, 229–235. [Google Scholar] [CrossRef]
- Yang, G.; Wang, D.; Yoneyama, Y.; Tan, Y.; Tsubaki, N. Facile synthesis of H-type zeolite shell on a silica substrate for tandem catalysis. Chem. Commun. 2012, 48, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tian, H.; Yang, A.; Zha, F.; Ding, J.; Chang, Y. Preparation of HZSM-5 membrane packed CuO-ZnO-Al2O3 nanoparticles for catalysing carbon dioxide hydrogenation to dimethyl ether. Appl. Surf. Sci. 2015, 345, 1–9. [Google Scholar] [CrossRef]
- Ren, N.; Yang, Y.H.; Shen, J.; Zhang, Y.H.; Xu, H.L.; Gao, Z.; Tang, Y. Novel, efficient hollow zeolitically microcapsulized noble metal catalysts. J. Catal. 2007, 251, 182–188. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Deactivation of metal catalysts in liquid phase organic reactions. Catal. Today 2003, 81, 547–559. [Google Scholar] [CrossRef]
- Papp, A.; Miklos, K.; Forgo, M.; Molnar, A. Heck coupling by Pd deposited onto organic-inorganic hybrid supports. J. Mol.Catal. A 2005, 229, 107–116. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Yan, X.; Lin, L.; Liu, H.; Qiu, J.; Yeung, K.L. Core-shell Pd/ZSM-5@ZIF-8 membrane micro-reactors with sizeselectivity properties for alkene hydrogenation. Catal. Today 2014, 236, 41–48. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kamei, T.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Single crystals of ZSM-5/silicalite composites. Adv. Mater. 2005, 17, 1985–1988. [Google Scholar] [CrossRef]
- Mabuchi, K.; Miyamoto, M.; Oumi, Y.; Uemiya, S. Selective formation of p-xylene in aromatization of propane over silicalite-1-coated GaAlMFI. J. Jpn. Pet. Inst. 2011, 54, 275–276. [Google Scholar] [CrossRef]
- Miyamoto, M.; Mabuchi, K.; Kamada, J.; Hirota, Y.; Oumi, Y.; Nishiyama, N.; Uemiya, S. para-Selectivity of silicalite-1 coated MFI type galloaluminosilicate in aromatization of light alkanes. J. Porous Mater. 2015, 22, 769–778. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Tong, W.Y.; Zou, W.; Qi, X.L.; Kong, D.J. Manufacture of b-Oriented ZSM-5/Silicalite-1 Core/Shell Structured Zeolite Catalyst. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45, 1356–1362. [Google Scholar] [CrossRef]
- Van der Laan, G.P.; Beenackers, A.A.C.M. Kinetics and Selectivity of the Fischer-Tropsch Synthesis: A Literature Review. Catal. Rev. 1999, 41, 255–318. [Google Scholar] [CrossRef]
- Li, X.H.; Asami, K.; Luo, M.F.; Michiki, K.; Tsubaki, N.; Fujimoto, K. Direct synthesis of middle iso-paraffins from synthesis gas. Catal. Today 2003, 84, 59–65. [Google Scholar] [CrossRef]
- Li, X.H.; Luo, M.F.; Asami, K. Direct synthesis of middle iso-paraffins from synthesis gas on hybrid catalysts. Catal. Today 2004, 89, 439–446. [Google Scholar] [CrossRef]
- Botes, F.G.; Böhringer, W. The addition of HZSM-5 to the Fischer-Tropsch process for improved gasoline production. Appl. Catal. A 2004, 267, 217–225. [Google Scholar] [CrossRef]
- Yoneyama, Y.; He, J.; Morii, Y.; Azuma, S.; Tsubaki, N. Direct synthesis of isoparaffin by modified Fischer-Tropsch synthesis using hybrid catalyst of iron catalyst and zeolite. Catal. Today 2005, 104, 37–40. [Google Scholar] [CrossRef]
- Moradi, G.R.; Basir, M.M.; Taeb, A.; Kiennemann, A. Promotion of Co/SiO2 Fischer-Tropsch catalysts with zirconium. Catal. Commun. 2003, 4, 37–32. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Y.; Liew, K.; Li, J. Catalytic performance of zirconium-modified Co/Al2O3 for Fischer-Tropsch synthesis. J. Mol. Catal. A 2005, 231, 145–151. [Google Scholar] [CrossRef]
- Koizumi, N.; Seki, H.; Hayasaka, Y.; Oda, Y.; Shindo, T.; Yamada, M. Application of liquid phase deposition method for preparation of Co/ZrOx/SiO2 catalyst with enhanced Fischer-Tropsch synthesis activity: Importance of Co-Zr interaction. Appl. Catal. A 2011, 398, 168–178. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, G.; Li, X.; Yoneyama, Y.; Wan, H.; Tsubaki, N. A Catalyst for One-step Isoparaffin Production via Fischer-Tropsch Synthesis: Growth of a H-Mordenite Shell Encapsulating a Fused Iron Core. ChemCatChem 2013, 5, 3101–3106. [Google Scholar] [CrossRef]
- Panpranot, J.; Goodwin, J.G.; Sayari, A. CO hydrogenation on Ru-promoted Co/MCM-41 catalysts. J. Catal. 2002, 211, 530–539. [Google Scholar] [CrossRef]
- Panpranot, J.; Goodwin, J.G.; Sayari, A. Effect of H2 partial pressure on surface reaction parameters during CO hydrogenation on Ru-promoted silica-supported Co catalysts. J. Catal. 2003, 213, 78–85. [Google Scholar] [CrossRef]
- Cano, L.A.; Cagnoli, M.V.; Fellenz, N.A.; Bengoa, J.F.; Gallegos, N.G.; Alvarez, A.M.; Marchetti, S.G. Fischer-Tropsch synthesis. Influence of the crystal size of iron active species on the activity and selectivity. Appl. Catal. A 2010, 379, 105–110. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer-Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Khodakov, A.Y. Fischer-Tropsch synthesis: Relations between structure of cobalt catalysts and their catalytic performance. Catal. Today 2009, 144, 251–257. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Deng, W.P.; Wang, Y. Recent advances in understanding the key catalyst factors for Fischer-Tropsch synthesis. J. Energy Chem. 2013, 22, 27–38. [Google Scholar] [CrossRef]
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Mao, D.S.; Yang, W.M.; Xia, J.C.; Zhang, B.; Song, Q.Y.; Chen, Q.L. Highly effective hybrid catalyst for the direct synthesis of dimethyl ether from syngas with magnesium oxide-modified HZSM-5 as a dehydration component. J. Catal. 2005, 230, 140–149. [Google Scholar] [CrossRef]
- Ramos, F.S.; de Farias, A.M.D.; Borges, L.E.P.; Monteiro, J.L.; Fraga, M.A.; Sousa-Aguiar, E.F.; Appel, L.G. Role of dehydration catalyst acid properties on one-step DME synthesis over physical mixtures. Catal. Today 2005, 101, 39–44. [Google Scholar] [CrossRef]
- Moradi, G.R.; Nosrati, S.; Yaripor, F. Effect of the hybrid catalysts preparation method upon direct synthesis of dimethyl ether from synthesis gas. Catal. Commun. 2007, 8, 598–606. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, B.; Hou, Z.; Fei, J.; Lou, H.; Zheng, X. Dimethyl ether synthesis via methanol and syngas over rare earth metals modified zeolite Y and dual Cu-Mn-Zn catalysts. Fuel 2007, 86, 2707–2713. [Google Scholar] [CrossRef]
- Mao, D.; Xia, J.; Zhang, B.; Lu, G. Highly efficient synthesis of dimethyl ether from syngas over the admixed catalyst of CuO-ZnO-Al2O3 and antimony oxide modified HZSM-5 zeolite. Energy Convers. Manag. 2010, 51, 1134–1139. [Google Scholar] [CrossRef]
- Pop, G.; Bozga, G.; Ganea, R.; Natu, N. Methanol Conversion to Dimethyl Ether over H-SAPO-34 Catalyst. Ind. Eng. Chem. Res. 2009, 48, 7065–7071. [Google Scholar] [CrossRef]
- Dai, W.L.; Kong, W.B.; Wu, G.J.; Li, N.; Li, L.D.; Guan, N.J. Catalytic dehydration of methanol to dimethyl ether over aluminophosphate and silico-aluminophosphate molecular sieves. Catal. Commun. 2011, 12, 535–538. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragomirova, R.; Wohlrab, S. Zeolite Membranes in Catalysis—From Separate Units to Particle Coatings. Catalysts 2015, 5, 2161-2222. https://doi.org/10.3390/catal5042161
Dragomirova R, Wohlrab S. Zeolite Membranes in Catalysis—From Separate Units to Particle Coatings. Catalysts. 2015; 5(4):2161-2222. https://doi.org/10.3390/catal5042161
Chicago/Turabian StyleDragomirova, Radostina, and Sebastian Wohlrab. 2015. "Zeolite Membranes in Catalysis—From Separate Units to Particle Coatings" Catalysts 5, no. 4: 2161-2222. https://doi.org/10.3390/catal5042161
APA StyleDragomirova, R., & Wohlrab, S. (2015). Zeolite Membranes in Catalysis—From Separate Units to Particle Coatings. Catalysts, 5(4), 2161-2222. https://doi.org/10.3390/catal5042161