Experimental and Modeling Studies on the Conversion of Inulin to 5-Hydroxymethylfurfural Using Metal Salts in Water
Abstract
:1. Introduction
| Substrate | Csugar, (M) | Catalysts | Conditions | XC6-sugar d | YHMFmax (mol %) | Other Products | Ref. |
|---|---|---|---|---|---|---|---|
| Monophasic (water) | |||||||
| Fructose | 0.3 | ZnCl2/HCl = 1/1 mol/mol | 120 °C, 0–500 min | 97.3 | 53.3 | humins | [22] |
| Fructose | 0.5 | AlCl3 (0.17 M)/HCl (1 M) | 88 °C, 0–500 min | 60 | 20 | LA (45%), humins | [23] |
| Fructose | 0.3 | DyCl3 2 mM | 140 °C, 120 min | n/a | <1 | humins, LA | [24] |
| Fructose | 5 a | AlCl3 50 mol % | 120 °C, 20 min | n/a | 55.7 | LA, FA, humins | [25] |
| Fructose | 30 a | AlCl3 0.87 M/Boric acid 100 g·L−1 | 150 °C, 45 min | 100 | 21 a | LA, FA, humins | [26] |
| Fructose | 30 a | FeCl3 0.87 M/Boric acid 100 g·L−1 | 150 °C, 45 min | 99 | 36 a | LA, FA, humins | [26] |
| Fructose | 30 a | MgCl2 0.87 M/Boric acid 100 g·L−1 | 150 °C, 45 min | 81 | 52 a | LA, FA, humins | [26] |
| Fructose | 10 a | CaP2O6 10 wt. % | 200 °C, 5 min | 82 | 34 a | n/a | [27] |
| Fructose | 10 a | α-Sr(PO3)2 10 wt. % | 200 °C, 5 min | 88 | 39 a | n/a | [27] |
| Fructose | 10 a | CrCl3 | 140 °C, 60 min | 87 | 20 | LA (17% at 180 min) | [28] |
| Fructose | 10 a | CrCl3-HCl | 140 °C, 25 min | 82 | 30 | LA (45% at 6 h) | [28] |
| Fructose | 5 a | InCl3 3 wt. % | 180 °C, 15 min | 100 | 79.5 | LA (45% at 1 h) | [29] |
| Monophasic (water) | |||||||
| Glucose | 0.3 | ZnCl2/HCl = 1/1 mol/mol | 120 °C, 0–500 min | 80.5 | 32.3 | humins | [22] |
| Glucose | 0.3 | DyCl3 2 mM | 140 °C, 120 min | n/a | <1 | char, LA | [24] |
| Glucose | 5 a | AlCl3 50 mol % | 120 °C, 20 min | n/a | 40.3 | LA, FA, humins | [25] |
| Glucose | 10 a | CaP2O6 10 wt. % | 220 °C, 5 min | 70 | 20 a | n/a | [27] |
| Glucose | 10 a | α-Sr(PO3)2 10 wt. % | 220 °C, 5 min | 60 | 21 a | n/a | [27] |
| Glucose | 10 a | CrCl3 | 140 °C, 90 min | 78 | 18 | LA (17% at 180 min) | [28] |
| Glucose | 10 a | CrCl3-HCl | 140 °C, 50 min | 55 | 16 | LA (42% at 6 h) | [28] |
| Glucose | 10 a | InCl3 3 wt. % | 180 °C, 10 min | 91.4 | 59.8 | LA (27% at 10 min) | [29] |
| Glucose | 0.1 | CrSO4, 5 mM | 140 °C, 6 h | 13 | 13 | lactic acid (<3%), LA (13%), fructose (<3%), humins c | [30] |
| Glucose | 0.25 | AlCl3·6H2O | 160 °C, 10 min | 98 | 22 | LA (10%), lactic acid (17%) | [31] |
| Glucose | 0.25 | AlCl3·6H2O | 160 °C, 10 min, H2O-NaCl | 98 | 17 | LA (29%) | [31] |
| Glucose | 5 a | MgCl2 0.8 M | 160 °C, 70 min | 15.6 | 3.9 | n/a | [32] |
| Glucose | 5a | MgSO4 0.8 M | 160 °C, 70 min | 45.1 | 7.7 | n/a | [32] |
| Glucose | 5 a | NaCl(0.8M)-HCl | 160 °C, 70 min | 13.4 | 3.3 | n/a | [32] |
| Monophasic/water | |||||||
| Glucose | 5 a | KCl (0.8M)-HCl | 160 °C, 70 min | 14.4 | 3.4 | n/a | [32] |
| Glucose | 5 a | MgCl2(0.8M)-HCl | 160 °C, 70 min | 11.1 | 2.7 | n/a | [32] |
| Glucose | 5 a | CaCl2(0.8M)-HCl | 160 °C, 70 min | 18 | 3.8 | n/a | [32] |
| Glucose | 10 a | CrCl2, 12 mol % | 120 °C, 3 h | 65 | <3% | humins | [33] |
| Glucose | 10 a | AlCl3, 12 mol % | 120 °C, 3 h | 74 | <3% | humins | [33] |
| Glucose | 10 a | FeCl3, 12 mol % | 120 °C, 3 h | 7 | 0 | humins | [33] |
| Glucose | 10 a | CuCl2, 12 mol % | 120 °C, 3 h | 4 | 0 | humins | [33] |
| Glucose | 0.2 | CrCl3·6H2O, 12 mol % | 140 °C, 1 h, 80 bar | 99 | 13% | LA (13%) | [34] |
| Glucose | 0.2 | AlCl3·6H2O, 12 mol % | 140 °C, 1 h, 80 bar | 88 | 19% | LA (6%) | [34] |
| Glucose | 0.2 | ZrCl4, 12 mol % | 140 °C, 1 h, 80 bar | 69 | 5% | LA (13%) | [34] |
| Glucose | 0.2 | CuCl2·2H2O, 12 mol % | 140 °C, 1 h, 80 bar | 23 | 6% | LA (2%) | [34] |
| Glucose | 0.2 | BiCl3, 12 mol % | 140 °C, 1 h, 80 bar | 17 | 5% | LA (3%) | [34] |
| Glucose | 0.2 | FeCl3·6H2O, 12 mol % | 140 °C, 1 h, 80 bar | 12 | 3% | LA (0%) | [34] |
| Glucose | 0.2 | MgCl2, 12 mol % | 140 °C, 1 h, 80 bar | 22 | 0.8% | LA (0%) | [34] |
| Biphasic | |||||||
| Glucose | 10 a | CrCl3-HCl | 140 °C, 180 min, THF | 97 | 59 | LA (5% at 180 min) | [28] |
| Glucose | 0.25 | AlCl3.6H2O | 160 °C, 10 min, H2O/THF | 99 | 52 | LA (trace), Lactic acid (13%) | [31] |
| Glucose | 0.25 | AlCl3.6H2O | 160 °C, 10 min, H2O-NaCl/THF | 99 | 61 | LA (1%) | [31] |
| Glucose | 5 a | AlCl3-HCl | 170 °C, 40 min, SBP b | 91 | 62 | LA | [35] |
| Glucose | 5 a | VCl3-HCl | 170 °C, 90 min, SBP b | 92 | 49 | LA | [35] |
| Glucose | 5 a | GaCl3-HCl | 170 °C, 2 h, SBP b | 90 | 45 | LA | [35] |
| Glucose | 5 a | InCl3-HCl | 170 °C, 150 min, SBP b | 86 | 45 | LA | [35] |
| Glucose | 5 a | YbCl3-HCl | 170 °C, 2 h, SBP b | 93 | 43 | LA | [35] |
| Glucose | 5 a | DyCl3-HCl | 170 °C, 160 min, SBP b | 93 | 38 | LA | [35] |
| Glucose | 5 a | SnCl4-HCl | 170 °C, 45 min, SBP b | 90 | 52 | LA | [35] |

2. Results and Discussion
2.1. Inulin Characterisation
2.2. Catalyst Screening Experiments

| Salt | pH a | YHMF (wt. %) | Salt | pH a | YHMF (wt. %) |
|---|---|---|---|---|---|
| Al2(SO4)3 | 4.04 | 18 ± 1.5 | FeSO4 | 2.90 | 25 ± 1.5 |
| AlCl3 | 4.34 | 19 ± 1.5 | FeCl3 | 2.96 | 27 ± 1.5 |
| SnSO4 | 2.97 | 21 ± 1.5 | CuSO4 | 5.54 | 28 ± 1.5 |
| SnCl2 | 3.11 | 22 ± 1.5 | CuCl2 | 5.71 | 30 ± 1.5 |

2.3. Exploratory Experiments on the Conversion of Inulin to HMF Using CuCl2
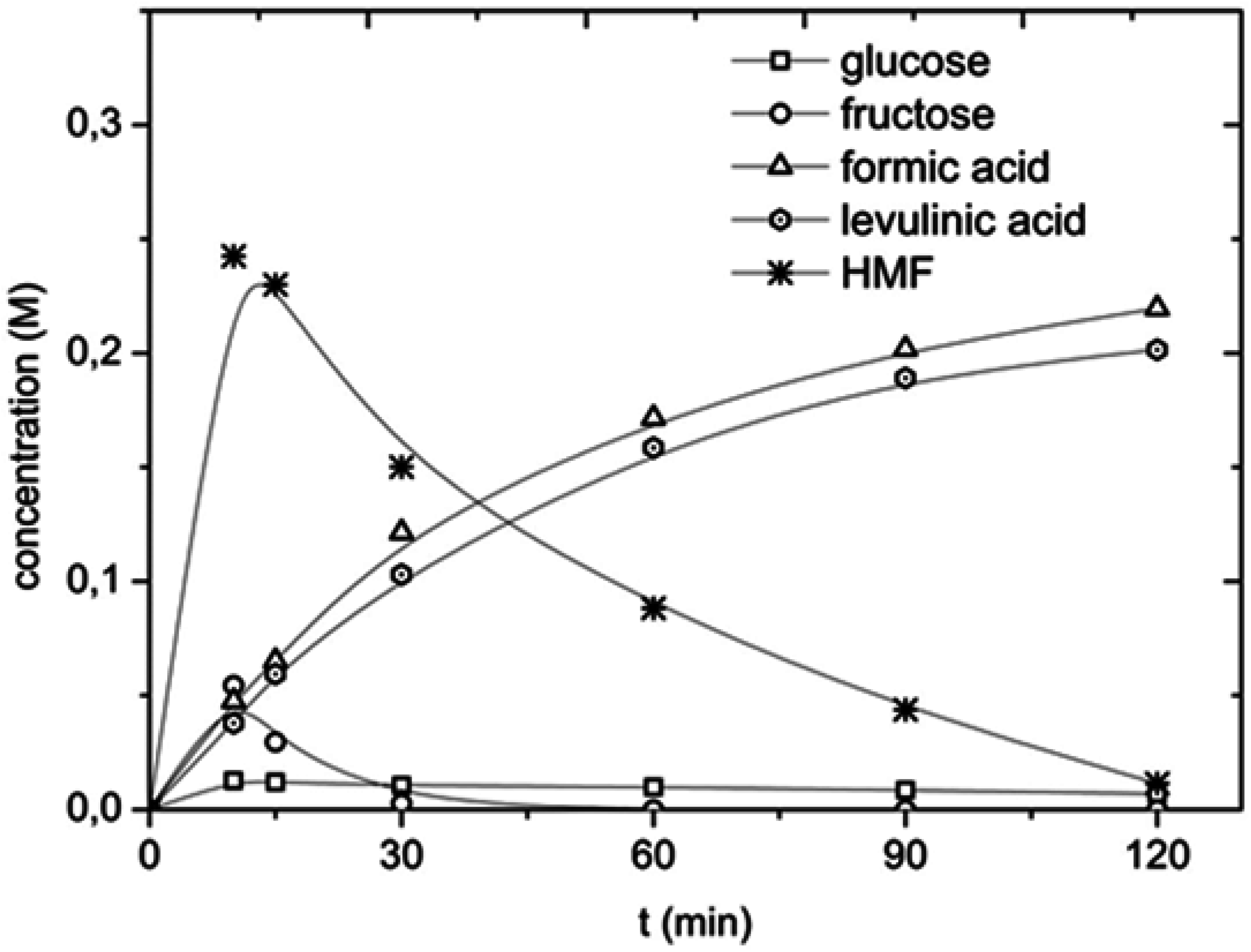


2.4. Effect of Process Conditions on HMF and LA Yields for Inulin Using CuCl2 Solutions



2.5. Quantification of Process Conditions on HMF and LA Yield Using Experimental Design and Statistical Modelling
| Run | Temperature (T, °C) | Inulin Intake (Cinulin, g/mL) | CuCl2 Concentration, (Ccat, M) | Reaction Time (t, min) | yHMF (wt. %) a | yLA (wt. %) b |
|---|---|---|---|---|---|---|
| 1 | 170 | 0.15 | 0.010 | 65 | 18.2 | 8.3 |
| 2 | 160 | 0.15 | 0.005 | 10 | 9.8 | 1.4 |
| 3 | 170 | 0.10 | 0.010 | 65 | 20.6 | 9.7 |
| 4 | 170 | 0.10 | 0.010 | 10 | 21.6 | 3.0 |
| 5 | 180 | 0.15 | 0.005 | 10 | 25.8 | 2.6 |
| 6 | 160 | 0.05 | 0.015 | 10 | 23.5 | 5.2 |
| 7 | 160 | 0.05 | 0.015 | 120 | 25 | 10.0 |
| 8 | 160 | 0.05 | 0.005 | 10 | 19.7 | 6.3 |
| 9 | 170 | 0.10 | 0.005 | 65 | 18.7 | 7.8 |
| 10 | 170 | 0.10 | 0.010 | 65 | 23.0 | 13.1 |
| 11 | 160 | 0.15 | 0.005 | 120 | 3.3 | 7.2 |
| 12 | 180 | 0.10 | 0.010 | 65 | 18.4 | 19.0 |
| 13 | 160 | 0.15 | 0.015 | 120 | 10 | 8.3 |
| 14 | 170 | 0.10 | 0.010 | 120 | 7.9 | 16.1 |
| 15 | 160 | 0.15 | 0.015 | 10 | 9.7 | 4.0 |
| 16 | 170 | 0.10 | 0.010 | 65 | 19.0 | 9.7 |
| 17 | 180 | 0.15 | 0.015 | 10 | 17.0 | 5.3 |
| 18 | 180 | 0.05 | 0.015 | 10 | 21.4 | 9.8 |
| 19 | 170 | 0.10 | 0.010 | 65 | 22.0 | 11.0 |
| 20 | 160 | 0.10 | 0.010 | 65 | 23.5 | 5.2 |
| 21 | 160 | 0.05 | 0.005 | 120 | 13.6 | 4.7 |
| 22 | 170 | 0.10 | 0.015 | 65 | 18.4 | 14.8 |
| 23 | 180 | 0.15 | 0.005 | 120 | 1.0 | 21.6 |
| 24 | 170 | 0.05 | 0.010 | 65 | 25.4 | 13.3 |
| 25 | 170 | 0.10 | 0.010 | 65 | 20.7 | 10.7 |
| 26 | 180 | 0.05 | 0.005 | 10 | 30.3 | 6.5 |
| 27 | 180 | 0.05 | 0.015 | 120 | 2.0 | 28.9 |
| 28 | 180 | 0.15 | 0.015 | 120 | 3.4 | 27.1 |
| 29 | 170 | 0.10 | 0.010 | 65 | 19.9 | 10.6 |
| 30 | 180 | 0.05 | 0.005 | 120 | 4.4 | 27.8 |
| Source | Sum of Squares | df | Mean Square | F-value | p-Value |
|---|---|---|---|---|---|
| Prob > F | |||||
| Model | 1831.98 | 10 | 183.20 | 78.50 | <0.0001 (significant) |
| A-T | 11.81 | 1 | 11.81 | 5.06 | 0.0365 |
| B-Cload | 247.48 | 1 | 247.48 | 106.04 | <0.0001 |
| C-Ccat. | 0.92 | 1 | 0.92 | 0.39 | 0.5373 |
| D-t | 647.07 | 1 | 647.07 | 277.25 | <0.0001 |
| AB | 91.95 | 1 | 91.95 | 39.40 | <0.0001 |
| AC | 95.09 | 1 | 95.09 | 40.74 | <0.0001 |
| AD | 330.29 | 1 | 330.29 | 141.52 | <0.0001 |
| CD | 63.23 | 1 | 63.23 | 27.09 | <0.0001 |
| C2 | 10.46 | 1 | 10.46 | 4.48 | 0.0477 |
| D2 | 106.06 | 1 | 106.06 | 45.45 | <0.0001 |
| Residual | 44.34 | 19 | - | - | - |



| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Prob > F | |||||
| Model | 1538.79 | 6 | 256.47 | 106.20 | <0.0001 (significant) |
| A-T | 515.98 | 1 | 515.98 | 213.29 | <0.0001 |
| B-Cload | 39.77 | 1 | 39.77 | 16.44 | 0.0005 |
| C-Ccat. | 41.54 | 1 | 41.54 | 17.17 | 0.0004 |
| D-t | 644.84 | 1 | 644.84 | 266.56 | <0.0001 |
| AB | 7.60 | 1 | 7.60 | 3.14 | 0.0895 |
| AD | 289.06 | 1 | 289.06 | 119.49 | <0.0001 |
| Residual | 55.64 | 23 | - | - | - |

3. Experimental Section
3.1. Chemicals
3.2. Experimental Procedures
3.2.1. Catalyst Screening Experiments
3.2.2. Experimental Design Experiments
3.3. Analyses
3.4. Definitions
3.5. Statistical Modelling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as green raw materials for the chemical. Comptes Rendus Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US department of energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, N.B. Catalytic dehydration of C6 carbohydrates for the production of Hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallet, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803–2830. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A Review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top value added chemicals from biomass. Volume I—Results of screening for potential candidates from sugars and synthesis gas. Nat. Renew. Energy Lab. 2004. [Google Scholar] [CrossRef]
- Clark, J.H.; Budarin, V.; Deswarte, F.E.; Hardy, J.J.; Kerton, F.M.; Hunt, A.J.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Rodriguez, A.; et al. Green chemistry and the biorefinery: A partnership for a sustainable future. Green Chem. 2006, 8, 853–860. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Girisuta, B.; Danon, B.; Manurung, R.; Janssen, L.P.B.M.; Heeres, H.J. Experimental and kinetic modelling studies on the acid-catalysed hydrolysis of the water hyacinth plant to levulinic acid. Bioresour. Technol. 2008, 99, 8367–8375. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.J. An examination of biorefining processes, catalysts and challenges. Catal. Today 2009, 145, 138–151. [Google Scholar] [CrossRef]
- Su, Y.; Brown, H.M.; Huang, X.; Zhou, X.; Amonette, J.E.; Zhang, Z.C. Single-step conversion of cellulose to 5-hydroxymethylfurfural (HMF), a versatile platform chemical. Appl. Catal. A 2009, 361, 117–122. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Doherty, W.O. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Bioref. 2011, 5, 198–214. [Google Scholar] [CrossRef] [Green Version]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivative. ARKIVOC 2001, 2001, 17–54. [Google Scholar]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Boisen, A.; Christensen, T.; Fu, W.; Gorban, Y.; Hansen, T.S.; Jensen, J.S.; Klitgaard, S.K.; Pedersen, S.; Riisager, A.; Ståhlberg, T.; et al. Process integration for the conversion of glucose to 2,5-furandicarboxylic acid. Chem. Eng. Res. Des. 2009, 87, 1318–1327. [Google Scholar] [CrossRef]
- Deng, T.; Cui, X.; Qi, Y.; Wang, Y.; Hou, X.; Zhu, Y. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by ZnCl2 in water. Chem. Commun. 2012, 48, 5494–5496. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, H.E.; Kieboom, A.P.G.; van Bekkum, H. The conversion of fructose and glucose in acidic media: Formation of hydroxymethylfurfural. Starch-Starke 1986, 38, 95–101. [Google Scholar] [CrossRef]
- Seri, K.; Inoue, Y.; Ishida, H. Catalytic activity of lanthanide (III) ions for the dehydration of hexose to 5-hydroxymethyl-2-furaldehyde in water. Bull. Chem. Soc. Jpn. 2001, 74, 1145–1150. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethylfurfural with aluminium chloride catalyst in water. Green Chem. 2011, 13, 2859–2868. [Google Scholar] [CrossRef]
- Hansen, T.S.; Mielby, J.; Riisager, A. Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 13, 109–114. [Google Scholar] [CrossRef]
- Daorattanachai, P.; Khemthong, P.; Viriya-empikul, N.; Laosiripojana, N.; Faungnawakij, K. Conversion of fructose, glucose, and cellulose to 5-hydroxymethylfurfural by alkaline earth phosphate catalysts in hot compressed water. Carbohydr. Res. 2012, 363, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the interplay of lewis and brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl)furfural and levulinic acid in aqueous media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, Y.; Sun, J.; Wang, B.; Xu, F.; Sun, R. Efficient conversion of monosaccharides into 5-hydroxymethylfurfural and levulinic acid in InCl3-H2O medium. Catal. Commun. 2014, 50, 17–20. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Makertihartha, I.G.; Adisasmito, S.; Heeres, H.J. Green chemicals from d-glucose: Systematic studies on catalytic effects of inorganic salts on the chemo-selectivity and yield in aqueous solutions. Top. Catal. 2010, 53, 1241–1247. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.; Abu-Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3.6H2O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Combs, E.; Cinlar, B.; Pagan-Torres, Y.; Dumesic, J.A.; Shanks, B.H. Influence of alkali and alkaline earth metal salts on glucose conversion to 5-hydroxymethylfurfural in an aqueous system. Catal. Commun. 2013, 30, 1–4. [Google Scholar] [CrossRef]
- Degirmenci, V.; Hensen, E.J.M. Development of a heterogeneous catalyst for lignocellulosic biomass conversion: Glucose dehydration by metal chlorides in a silica-supported ionic liquid layer. Environ. Prog. Sustain. Energy 2013, 33, 657–662. [Google Scholar] [CrossRef]
- Loerbroks, C.; van Rijn, J.; Ruby, M.R.; Tong, Q.; Schuth, F.; Thiel, W. Reactivity of metal catalyst in glucose-fructose conversion. Chem. Eur. J. 2014, 20, 12298–12309. [Google Scholar] [CrossRef] [PubMed]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-hydroxymethylfurfural from glucose using a combination of lewis and brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nagayama, S.; Busujima, T. Lewis acid catalyst stable in water. Correlation between catalyst activity in water and hydrolysis constants and exchange rate constants for substitution of inner-sphere water ligands. J. Am. Chem. Soc. 1998, 120, 8287–8288. [Google Scholar] [CrossRef]
- Fachri, B.A.; Abdilla, R.N.; Rasrendra, C.B.; Heeres, H.J. Experimental and modelling studies on the uncatalysed thermal conversion of inulin to 5-hydroxymethylfurfural and levulinic acid. Sustain. Chem. Process. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Fructan Storing Plants—A potential source of high fructose syrups. J. Sci. Ind. Res. 1997, 56, 447–452. [Google Scholar]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nut. 2002, 87, S287–S291. [Google Scholar] [CrossRef]
- Wu, S.; Fan, H.; Xie, Y.; Cheng, Y.; Wang, Q.; Zhang, Z.; Han, B. Effect of CO2 on conversion of inulin to 5-hydroxymethylfurfural and propylene oxide to 1,2-propanediol in water. Green Chem. 2010, 12, 1215–1219. [Google Scholar] [CrossRef]
- Benvenuti, F.; Carlini, C.; Patrono, P.; Raspolli Galletti, A.M.; Sbrana, G.; Massucci, M.A.; Galli, P. Heterogeneous zirconium and titanium catalysts for the selective synthesis of 5-hydroxymethyl-2-furaldehyde from carbohydrates. Appl. Catal. A 2000, 193, 147–153. [Google Scholar] [CrossRef]
- Carlini, C.; Giuttari, M.; Galletti, A.M.; Sbrana, G.; Armaroli, T.; Busca, G. Selective saccharides dehydration to 5-hydroxymethyl-2-furaldehyde by heterogeneous niobium catalysts. Appl. Catal. A 1999, 183, 295–302. [Google Scholar] [CrossRef]
- Carlini, C.; Patrono, P.; Gallettia, A.M.R.; Sbrana, G. Heterogeneous catalysts based on vanadyl phosphate for fructose dehydration to 5-hydroxymethyl-2-furaldehyde. Appl. Catal. A 2004, 275, 111–118. [Google Scholar] [CrossRef]
- Despax, S.; Maurer, C.; Estrini, B.; le Bras, J.; Hoffmann, N.; Marinkovic, S.; Muzart, J. Fast and efficient DMSO-mediated dehydration of carbohydrates into 5-hydroxymethylfurfural. Catal. Commun. 2014, 51, 5–9. [Google Scholar] [CrossRef]
- Harvey, D.J. Matrix-assisted laser desorption/ionisation mass spectrometry of oligosaccharides and glycoconjugates. J. Chromatogr. A 1996, 720, 429–446. [Google Scholar] [CrossRef]
- Kazmaier, T.; Roth, S.; Zapp, J.; Harding, M.; Kuhn, R. Quantitative analysis of malto-oligosaccharides by MALDI-TOF mass spectrometry, capillary electrophoresis and anion exchange chromatography. Fresenius J. Anal. Chem. 1998, 361, 473–478. [Google Scholar] [CrossRef]
- Roberfroid, M. Inulin-Type of Fructans; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Asghari, F.S.; Yoshida, H. Acid-catalyzed production of 5-hydroxymethyl furfural from d-fructose in subcritical water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Roman-Leskhov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of glucose isomerization using a solid lewis acid catalyst in water. Angew. Chem. Int. Ed. 2010, 49, 8954–8957. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Mechanism of brønsted acid-catalyzed conversion of carbohydrates. J. Catal. 2012, 295, 122–132. [Google Scholar] [CrossRef]
- Aida, T.M.; Tajima, K.; Watanabe, M.; Saito, Y.; Kuroda, K.; Nonaka, T.; Hattori, H.; Smith, R.L., Jr.; Arai, K. Reactions of d-fructose in water at temperatures up to 100 MPa. J. Supercrit. Fluids 2007, 42, 110–119. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Fachri, B.A.; Makertihartha, I.B.; Adisasmito, S.; Heeres, H.J. Catalytic conversion of dihydroxyacetone to lactic acid catalytic conversion of dihydroxyacetone to lactic acid. ChemSusChem. 2011, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, R.K.P.; van Haveren, J.; van Es, D.S.; Melián-Cabrera, I.; Meeldijk, J.D.; Heeres, H.J. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal. B 2014, 147, 92–100. [Google Scholar] [CrossRef]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions. ChemSusChem. 2013, 6, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Fachri, B.A.; Abdilla, R.M.; Bovenkamp, H.H.; Rasrendra, C.B.; Heeres, H.J. Experimental and kinetic modeling studies on the sulfuric acid-catalysed conversion of d-fructose to 5-hydroxymethylfurfural and levulinic acid in water. ACS Sustainable Chem. Eng. 2015, 3, 3024–3034. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fachri, B.A.; Rasrendra, C.B.; Heeres, H.J. Experimental and Modeling Studies on the Conversion of Inulin to 5-Hydroxymethylfurfural Using Metal Salts in Water. Catalysts 2015, 5, 2287-2308. https://doi.org/10.3390/catal5042287
Fachri BA, Rasrendra CB, Heeres HJ. Experimental and Modeling Studies on the Conversion of Inulin to 5-Hydroxymethylfurfural Using Metal Salts in Water. Catalysts. 2015; 5(4):2287-2308. https://doi.org/10.3390/catal5042287
Chicago/Turabian StyleFachri, Boy Arief, Carolus Borromeus Rasrendra, and Hero Jan Heeres. 2015. "Experimental and Modeling Studies on the Conversion of Inulin to 5-Hydroxymethylfurfural Using Metal Salts in Water" Catalysts 5, no. 4: 2287-2308. https://doi.org/10.3390/catal5042287
APA StyleFachri, B. A., Rasrendra, C. B., & Heeres, H. J. (2015). Experimental and Modeling Studies on the Conversion of Inulin to 5-Hydroxymethylfurfural Using Metal Salts in Water. Catalysts, 5(4), 2287-2308. https://doi.org/10.3390/catal5042287






