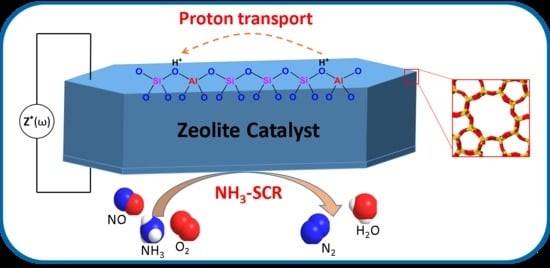
In Situ Spectroscopic Studies of Proton Transport in Zeolite Catalysts for NH3-SCR
Abstract
:1. Introduction
2. Theory and Instruments
2.1. Theory of Impedance Spectroscopy
2.2. Instruments for In Situ IS and In Situ IS-DRIFTS
3. Proton Transport in Zeolite Catalysts for NH3-SCR
3.1. NH3-Supported Proton Transport
3.2. Factors Influencing the Proton Transport in Zeolite Catalysts
3.2.1. Metal Cation Type
3.2.2. Metal Exchange Level
3.2.3. Zeolite Framework Type
3.2.4. Formation of NH4+ Intermediates
3.2.5. H2O Vapor
3.2.6. Zeolite Crystallite Size
4. Summary and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Granger, P.; Parvulescu, V.I. Catalytic NOx Abatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent Advances in Automotive Catalysis for NOx Emission Control by Small-Pore Microporous Materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A.M. Local Environment and Nature of Cu Active Sites in Zeolite-Based Catalysts for the Selective Catalytic Reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Yu, T.; Hao, T.; Fan, D.; Wang, J.; Shen, M.; Li, W. Recent NH3-SCR Mechanism Research over Cu/SAPO-34 Catalyst. J. Phys. Chem. C 2014, 118, 6565–6575. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.; Song, W.; Liu, J.; Zhao, Z.; Gao, M.; Wei, Y.; Zhao, L. Nature of Cu Species in Cu-SAPO-18 Catalyst for NH3–SCR: Combination of Experiments and DFT Calculations. J. Phys. Chem. C 2016, 120, 14669–14680. [Google Scholar] [CrossRef]
- Lezcano-Gonzalez, I.; Deka, U.; Arstad, B.; Van Yperen-De Deyne, A.; Hemelsoet, K.; Waroquier, M.; Van Speybroeck, V.; Weckhuysen, B.M.; Beale, A.M. Determining the Storage, Availability and Reactivity of NH3 within Cu-Chabazite-based Ammonia Selective Catalytic Reduction Systems. Phys. Chem. Chem. Phys. 2014, 16, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, S.; Kröcher, O.; Wokaun, A.; Tissler, A.; Althoff, R. The Role of Brønsted Acidity in the Selective Catalytic Reduction of NO with Ammonia over Fe-ZSM-5. J. Catal. 2009, 268, 297–306. [Google Scholar] [CrossRef]
- Maier, S.M.; Jentys, A.; Janousch, M.; van Bokhoven, J.A.; Lercher, J.A. Unique Dynamic Changes of Fe Cationic Species under NH3-SCR Conditions. J. Phys. Chem. C 2012, 116, 5846–5856. [Google Scholar] [CrossRef]
- Boubnov, A.; Carvalho, H.W.; Doronkin, D.E.; Gunter, T.; Gallo, E.; Atkins, A.J.; Jacob, C.R.; Grunwaldt, J.D. Selective Catalytic Reduction of NO over Fe-ZSM-5: Mechanistic Insights by Operando HERFD-XANES and Valence-to-core X-ray Emission Spectroscopy. J. Am. Chem. Soc. 2014, 136, 13006–13015. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin Caballero, J.D.; Shih, A.J.; Anggara, T.; Delgass, W.N.; Miller, J.T.; et al. Catalysis in a Cage: Condition-Dependent Speciation and Dynamics of Exchanged Cu Cations in SSZ-13 Zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, M.; Hueso, B.; Boronat, M.; Blasco, T.; Corma, A. Ammonia-Containing Species Formed in Cu-Chabazite As Per In Situ EPR, Solid-State NMR, and DFT Calculations. J. Phys. Chem. Lett. 2015, 6, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Borfecchia, E.; Lomachenko, K.A.; Giordanino, F.; Falsig, H.; Beato, P.; Soldatov, A.V.; Bordiga, S.; Lamberti, C. Revisiting the Nature of Cu Sites in the Activated Cu-SSZ-13 Catalyst for SCR Reaction. Chem. Sci. 2015, 6, 548–563. [Google Scholar] [CrossRef]
- Svelle, S.; Tuma, C.; Rozanska, X.; Kerber, T.; Sauer, J. Quantum Chemical Modeling of Zeolite-catalyzed Methylation Reactions: Toward Chemical Accuracy for Barriers. J. Am. Chem. Soc. 2009, 131, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Vogt, E.T.; Weckhuysen, B.M. Fluid Catalytic Cracking: Recent Developments on the Grand Old Lady of Zeolite Catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J. Proton Transfer in Zeolites. In Hydrogen-Transfer Reactions; Hynes, J.T., Klinman, J.P., Limbach, H.-H., Schowen, R.L., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 685–707. [Google Scholar]
- Chen, P.; Jabłońska, M.; Weide, P.; Caumanns, T.; Weirich, T.; Muhler, M.; Moos, R.; Palkovits, R.; Simon, U. Formation and Effect of NH4+ Intermediates in NH3–SCR over Fe-ZSM-5 Zeolite Catalysts. ACS Catal. 2016, 6, 7696–7700. [Google Scholar] [CrossRef]
- Sierka, M.; Sauer, J. Proton Mobility in Chabazite, Faujasite, and ZSM-5 Zeolite Catalysts, Comparison Based on Ab Initio Calculations. J. Phys. Chem. B 2001, 105, 1603–1613. [Google Scholar] [CrossRef]
- Kanellopoulos, J.; Gottert, C.; Schneider, D.; Knorr, B.; Prager, D.; Ernst, H.; Freude, D. NMR Investigation of Proton Mobility in Zeolites. J. Catal. 2008, 255, 68–78. [Google Scholar] [CrossRef]
- Liu, N.; Chen, B.; Li, Y.; Zhang, R.; Liang, X.; Li, Y.; Lei, Z. Charge Transfer Analysis on the Direct Decomposition of Nitrous Oxide over Fe-BEA Zeolite: An Experimental and Density Functional Study. J. Phys. Chem. C 2011, 115, 12883–12890. [Google Scholar] [CrossRef]
- Huo, H.; Peng, L.; Grey, C.P. Low Temperature 1H MAS NMR Spectroscopy Studies of Proton Motion in Zeolite HZSM-5. J. Phys. Chem. C 2009, 113, 8211–8219. [Google Scholar] [CrossRef]
- Derouane, E.G.; Védrine, J.C.; Pinto, R.R.; Borges, P.M.; Costa, L.; Lemos, M.; Lemos, F.; Ribeiro, F.R. The Acidity of Zeolites: Concepts, Measurements and Relation to Catalysis: A Review on Experimental and Theoretical Methods for the Study of Zeolite Acidity. Catal. Rev. 2013, 55, 454–515. [Google Scholar] [CrossRef]
- Brüggemann, T.C.; Keil, F.J. Theoretical Investigation of the Mechanism of the Oxidation of Nitrogen Oxide on Iron-Form Zeolites in the Presence of Water. J. Phys. Chem. C 2011, 115, 2114–2133. [Google Scholar] [CrossRef]
- Sajith, P.K.; Shiota, Y.; Yoshizawa, K. Role of Acidic Proton in the Decomposition of NO over Dimeric Cu(I) Active Sites in Cu-ZSM-5 Catalyst: A QM/MM Study. ACS Catal. 2014, 4, 2075–2085. [Google Scholar] [CrossRef]
- Chen, P.; Schönebaum, S.; Simons, T.; Rauch, D.; Moos, R.; Simon, U. In Situ Monitoring of DeNOx-SCR on Zeolite Catalysts by Means of Simultaneous Impedance and DRIFT Spectroscopy. Procedia Eng. 2015, 120, 257–260. [Google Scholar] [CrossRef]
- Chen, P.; Schönebaum, S.; Simons, T.; Rauch, D.; Dietrich, M.; Moos, R.; Simon, U. Correlating the Integral Sensing Properties of Zeolites with Molecular Processes by Combining Broadband Impedance and DRIFT Spectroscopy-A New Approach for Bridging the Scales. Sensors 2015, 15, 28915–28941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Simböck, J.; Schönebaum, S.; Rauch, D.; Simons, T.; Palkovits, R.; Moos, R.; Simon, U. Monitoring NH3 Storage and Conversion in Cu-ZSM-5 and Cu-SAPO-34 Catalysts for NH3-SCR by Simultaneous Impedance and DRIFT Spectroscopy. Sens. Actuators B 2016, 236, 1075–1082. [Google Scholar] [CrossRef]
- Simons, T.; Chen, P.; Rauch, D.; Moos, R.; Simon, U. Sensing Catalytic Conversion: Simultaneous DRIFT and Impedance Spectroscopy for in Situ Monitoring of NH3–SCR on Zeolites. Sens. Actuators B 2016, 224, 492–499. [Google Scholar] [CrossRef]
- Chen, P.; Rauch, D.; Weide, P.; Schönebaum, S.; Simons, T.; Muhler, M.; Moos, R.; Simon, U. The Effect of Cu and Fe Cations on NH3-supported Proton Transport in DeNOx-SCR Zeolite Catalysts. Catal. Sci. Technol. 2016, 6, 3362–3366. [Google Scholar] [CrossRef]
- Simons, T.; Simon, U. Zeolites as Nanoporous, Gas-sensitive Materials for in Situ Monitoring of DeNOx-SCR. Beilstein J. Nanotechnol. 2012, 3, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Moos, R.; Simon, U. Metal Loading Affects the Proton Transport Properties and the Reaction Monitoring Performance of Fe-ZSM-5 and Cu-ZSM-5 in NH3-SCR. J. Phys. Chem. C 2016, 120, 25361–25370. [Google Scholar] [CrossRef]
- Franke, M.E.; Simon, U.; Moos, R.; Knezevic, A.; Muller, R.; Plog, C. Development and Working Principle of an Ammonia Gas Sensor Based on a Refined Model for Solvate Supported Proton Transport in Zeolites. Phys. Chem. Chem. Phys. 2003, 5, 5195–5198. [Google Scholar] [CrossRef]
- Franke, M.E.; Simon, U. Solvate-supported Proton Transport in Zeolites. ChemPhysChem 2004, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, L.; Rodriguez-Castellon, E.; Jimenez-Lopez, A.; Simon, U. Correlation of TPD and Impedance Measurements on the Desorption of NH3 from Zeolite H-ZSM-5. Solid State Ion. 2008, 179, 1968–1973. [Google Scholar] [CrossRef]
- Rauch, D.; Dietrich, M.; Simons, T.; Simon, U.; Porch, A.; Moos, R. Microwave Cavity Perturbation Studies on H-form and Cu Ion-Exchanged SCR Catalyst Materials: Correlation of Ammonia Storage and Dielectric Properties. Top. Catal. 2016. [Google Scholar] [CrossRef]
- Rauch, D.; Kubinski, D.; Simon, U.; Moos, R. Detection of the Ammonia Loading of a Cu Chabazite SCR Catalyst by a Radio Frequency-based Method. Sens. Actuators B 2014, 205, 88–93. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Simon, U.; Franke, M.E. Electrical Properties of Nanoscaled Host-guest Compounds. Microporous Mesoporous Mater. 2000, 41, 1–36. [Google Scholar] [CrossRef]
- Simon, U.; Flesch, U. Cation-cation Interaction in Dehydrated Zeolites X and Y Monitored by Modulus Spectroscopy. J. Porous Mater. 1999, 6, 33–40. [Google Scholar] [CrossRef]
- Dragomirova, R.; Wohlrab, S. Zeolite Membranes in Catalysis—From Separate Units to Particle Coatings. Catalysts 2015, 5, 2161–2222. [Google Scholar] [CrossRef]
- Tolle, P.; Kohler, C.; Marschall, R.; Sharifi, M.; Wark, M.; Frauenheim, T. Proton Transport in Functionalised Additives for PEM Fuel Cells: Contributions from Atomistic Simulations. Chem. Soc. Rev. 2012, 41, 5143–5159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, X.; Dutta, P.K. Exploitation of Unique Properties of Zeolites in the Development of Gas Sensors. Sensors 2012, 12, 5170–5194. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, L.; Simon, U. NH3-TPD Measurements Using a Zeolite-based Sensor. Meas. Sci. Technol. 2010, 21, 027003. [Google Scholar] [CrossRef]
- Paolucci, C.; Verma, A.A.; Bates, S.A.; Kispersky, V.F.; Miller, J.T.; Gounder, R.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Isolation of the Copper Redox Steps in the Standard Selective Catalytic Reduction on Cu-SSZ-13. Angew. Chem. Int. Ed. 2014, 53, 11828–11833. [Google Scholar] [CrossRef] [PubMed]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrøm, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Zheng, Y.; Kukkadapu, R.K.; Wang, Y.; Walter, E.D.; Schwenzer, B.; Szanyi, J.; Peden, C.H.F. Iron Loading Effects in Fe/SSZ-13 NH3-SCR Catalysts: Nature of the Fe Ions and Structure–Function Relationships. ACS Catal. 2016, 6, 2939–2954. [Google Scholar] [CrossRef]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The Effect of Iron Loading and Hydrothermal Aging on One-pot Synthesized Fe/SAPO-34 for Ammonia SCR. Appl. Catal. B 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The Determination of the Activities of Different Iron Species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure–Activity Relationships in NH3-SCR over Cu-SSZ-13 as Probed by Reaction Kinetics and EPR Studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al Ratio on Cu/SSZ-13 NH3-SCR Catalysts: Implications for the Active Cu Species and the Roles of Brønsted Acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, J.H.; Burton, S.D.; Lipton, A.S.; Peden, C.H.; Szanyi, J. A Common Intermediate for N2 Formation in Enzymes and Zeolites: Side-on Cu-nitrosyl Complexes. Angew. Chem. Int. Ed. 2013, 52, 9985–9989. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.P.; Selleri, T.; Colombo, M.; Nova, I.; Tronconi, E. Identification of Nitrites/HONO as Primary Products of NO Oxidation over Fe-ZSM-5 and Their Role in the Standard SCR Mechanism: A Chemical Trapping Study. J. Catal. 2014, 311, 266–270. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Kollár, M.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. A Comparative Kinetics Study between Cu/SSZ-13 and Fe/SSZ-13 SCR Catalysts. Catal. Today 2015, 258, 347–358. [Google Scholar] [CrossRef]
- Gunter, T.; Carvalho, H.W.; Doronkin, D.E.; Sheppard, T.; Glatzel, P.; Atkins, A.J.; Rudolph, J.; Jacob, C.R.; Casapu, M.; Grunwaldt, J.D. Structural Snapshots of the SCR Reaction mechanism on Cu-SSZ-13. Chem. Commun. 2015, 51, 9227–9230. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.; Müller, R.; Plog, C.; Knezevic, A.; Leye, H.; Irion, E.; Braun, T.; Marquardt, K.-J.; Binder, K. Selective Ammonia Exhaust Gas Sensor for Automotive Applications. Sens. Actuators B 2002, 83, 181–189. [Google Scholar] [CrossRef]
- Takata, T.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Nanosized CHA Zeolites with High Thermal and Hydrothermal Stability Derived from the Hydrothermal Conversion of FAU Zeolite. Microporous Mesoporous Mater. 2016, 225, 524–533. [Google Scholar] [CrossRef]
- Hu, X.; Yang, M.; Fan, D.; Qi, G.; Wang, J.; Wang, J.; Yu, T.; Li, W.; Shen, M. The Role of Pore Diffusion in Determining NH3 SCR Active Sites over Cu/SAPO-34 Catalysts. J. Catal. 2016, 341, 55–66. [Google Scholar] [CrossRef]
- Feng, B.; Wang, Z.; Sun, Y.; Zhang, C.; Tang, S.; Li, X.; Huang, X. Size Controlled ZSM-5 on the Structure and Performance of Fe Catalyst in the Selective Catalytic Reduction of NOx with NH3. Catal. Commun. 2016, 80, 20–23. [Google Scholar] [CrossRef]
- Severance, M.; Zheng, Y.; Heck, E.; Dutta, P.K. Influence of Crystallite Size on Cation Conductivity in Faujasitic Zeolites. J. Phys. Chem. A 2013, 117, 13704–13711. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S.; Jaber, M.; Valtchev, V. Nanosized Microporous Crystals: Emerging Applications. Chem. Soc. Rev. 2015, 44, 7207–7233. [Google Scholar] [CrossRef] [PubMed]
- Dapsens, P.Y.; Mondelli, C.; Perez-Ramirez, J. Design of Lewis-acid Centres in Zeolitic Matrices for the Conversion of Renewables. Chem. Soc. Rev. 2015, 44, 7025–7043. [Google Scholar] [CrossRef] [PubMed]
- Buurmans, I.L.; Weckhuysen, B.M. Heterogeneities of Individual Catalyst Particles in Space and Time as Monitored by Spectroscopy. Nat. Chem. 2012, 4, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Saltzmann, T.; Bornhofft, M.; Mayer, J.; Simon, U. Shape without Structure: An Intriguing Formation Mechanism in the Solvothermal Synthesis of the Phase-Change Material Sb2Te3. Angew. Chem. Int. Ed. 2015, 54, 6632–6636. [Google Scholar] [CrossRef] [PubMed]











© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Simon, U. In Situ Spectroscopic Studies of Proton Transport in Zeolite Catalysts for NH3-SCR. Catalysts 2016, 6, 204. https://doi.org/10.3390/catal6120204
Chen P, Simon U. In Situ Spectroscopic Studies of Proton Transport in Zeolite Catalysts for NH3-SCR. Catalysts. 2016; 6(12):204. https://doi.org/10.3390/catal6120204
Chicago/Turabian StyleChen, Peirong, and Ulrich Simon. 2016. "In Situ Spectroscopic Studies of Proton Transport in Zeolite Catalysts for NH3-SCR" Catalysts 6, no. 12: 204. https://doi.org/10.3390/catal6120204
APA StyleChen, P., & Simon, U. (2016). In Situ Spectroscopic Studies of Proton Transport in Zeolite Catalysts for NH3-SCR. Catalysts, 6(12), 204. https://doi.org/10.3390/catal6120204