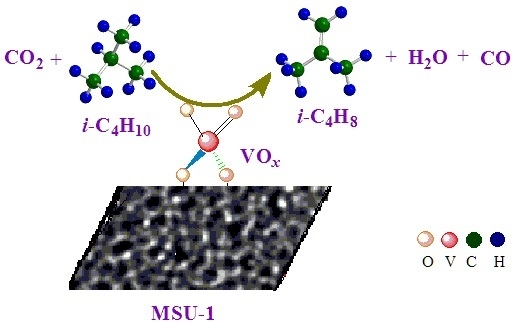
Vanadium Oxide Supported on MSU-1 as a Highly Active Catalyst for Dehydrogenation of Isobutane with CO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Performance
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.B.; Murata, K.; Hayakawa, T.; Hamakawa, S.; Suzuki, K. Dehydrogenation of ethane with carbon dioxide over supported chromium oxide catalysts. Appl. Catal. A 2000, 196, 1–8. [Google Scholar] [CrossRef]
- Tasbihi, M.; Feyzi, F.; Amlashi, M.A.; Abdullah, A.Z.; Mohamed, A.R. Effect of the addition of potassium and lithium in Pt-Sn/Al2O3 catalysts for the dehydrogenation of isobutane. Fuel Process. Technol. 2007, 88, 883–889. [Google Scholar] [CrossRef]
- Wang, M.G.; Zhong, S.H. Pd/V2O5-SiO2 catalyst for oxidative dehydrogenation of isobutane with CO2 to isobutene. Chin. J. Catal. 2007, 28, 124–130. [Google Scholar]
- Rombi, E.; Gazzoli, D.; Cutrufello, M.G.; De Rossi, S.; Ferino, I. Modifications induced by potassium addition on chromia/alumina catalysts and their influence on the catalytic activity for the oxidative dehydrogenation of propane. Appl. Surf. Sci. 2010, 256, 5576–5580. [Google Scholar] [CrossRef]
- Seddon, D. Reformulated gasoline, opportunities for new catalyst technology. Catal. Today 1992, 15, 1–21. [Google Scholar] [CrossRef]
- Iannazzo, V.; Neri, G.; Galvagno, S.; Di Serio, M.; Tesser, R.; Santacesaria, E. Oxidative dehydrogenation of isobutane over V2O5-based catalysts prepared by grafting vanadyl alkoxides on TiO2-SiO2 supports. Appl. Catal. A 2003, 246, 49–68. [Google Scholar] [CrossRef]
- Resasco, D.E.; Marcus, B.K.; Huang, C.S.; Durante, V.A. Isobutane dehydrogenation over sulfided nickel catalysts. J. Catal. 1994, 146, 40–55. [Google Scholar] [CrossRef]
- De Rossi, S.; Ferraris, G.; Fremiotti, S.; Indovina, V.; Cimino, A. Isobutane dehydrogenation on chromia/zirconia catalysts. Appl. Catal. A 1993, 106, 125–141. [Google Scholar] [CrossRef]
- Gniot, I.; Kirszensztejn, P.; Kozlowski, M. Oxidative dehydrogenation of isobutane using modified activated carbons as catalysts. Appl. Catal. A 2009, 362, 67–74. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, J.; Dai, H.; Au, C.T. Binary Cr-Mo oxide catalysts supported on MgO-coated polyhedral three-dimensional mesoporous SBA-16 for the oxidative dehydrogenation of iso-butane. Appl. Catal. A 2009, 354, 72–81. [Google Scholar] [CrossRef]
- Neri, G.; Pistone, A.; De Rossi, S.; Rombi, E.; Milone, C.; Galvagno, S. Ca-doped chromium oxide catalysts supported on alumina for the oxidative dehydrogenation of isobutane. Appl. Catal. A 2004, 260, 75–86. [Google Scholar] [CrossRef]
- Castro, A.J.R.; Soares, J.M.; Filho, J.M.; Oliveira, A.C.; Campos, A.; Milet, É.R.C. Oxidative dehydrogenation of ethylbenzene with CO2 for styrene production over porous iron-based catalysts. Fuel 2013, 108, 740–748. [Google Scholar] [CrossRef]
- Chen, S.W.; Qin, Z.F.; Wang, G.F.; Dong, M.; Wang, J.G. Promoting effect of carbon dioxide on the dehydrogenation of ethylbenzene over silica-supported vanadium catalysts. Fuel 2013, 109, 43–48. [Google Scholar] [CrossRef]
- Wu, R.; Xie, P.; Cheng, Y.; Yue, Y.; Gu, S.; Yang, W.; Miao, C.; Hua, W.; Gao, Z. Hydrothermally prepared Cr2O3–ZrO2 as a novel efficient catalyst for dehydrogenation of propane with CO2. Catal. Commun. 2013, 39, 20–23. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenation-a review. Energy Fuels 2004, 18, 1126–1139. [Google Scholar] [CrossRef]
- Ajayi, B.P.; Jermy, B.R.; Ogunronbi, K.E.; Abussaud, B.A.; Al-Khattaf, S. n-Butane dehydrogenation over mono and bimetallic MCM-41 catalysts under oxygen free atmosphere. Catal. Today 2013, 204, 189–196. [Google Scholar] [CrossRef]
- Ascoop, I.; Galvita, V.V.; Alexopoulos, K.; Reyniers, M.; Van Der Voort, P.; Bliznuk c, V.; Marin, G.B. The role of CO2 in the dehydrogenation of propane over WOx-VOx/SiO2. J. Catal. 2016, 335, 1–10. [Google Scholar]
- Ding, J.F.; Qin, Z.F.; Li, X.K.; Wang, G.F.; Wang, J.G. Catalytic dehydrogenation of isobutane in the presence of carbon dioxide over nickel supported on active carbon. J. Mol. Catal. A 2010, 315, 221–225. [Google Scholar] [CrossRef]
- Ogonowski, J.; Skrzyńska, K. Carbon dioxide in the dehydrogenation of isobutane over VMgOx. Catal. Commun. 2009, 11, 132–136. [Google Scholar] [CrossRef]
- Carrazán, S.R.G.; Peres, C.; Bernard, J.P.; Ruwet, M.; Ruiz, P.; Delmon, B. Catalytic synergy in the oxidative dehydrogenation of propane over MgVO catalysts. J. Catal. 1996, 158, 452–476. [Google Scholar] [CrossRef]
- Takita, Y.; Xia, Q.; Kikutani, K.; Soda, K.; Takami, H.; Nishigushi, H.; Nagaoka, K. Anaerobic oxidation of isobutane: II. Catalysis by Mg-V complex oxides. J. Mol. Catal. A 2006, 248, 61–69. [Google Scholar] [CrossRef]
- Fu, Y.; Ma, H.; Wang, Z.; Zhu, W.; Wu, T.; Wang, G. Characterization and reactivity of SnO2-doped V2O5/γ-Al2O3 catalysts in dehydrogenation of isobutane to isobutene. J. Mol. Catal. A 2004, 221, 163–168. [Google Scholar] [CrossRef]
- Ogonowski, J.; Skrzyńska, E. Deactivation of VMgOx catalysts by coke in the process of isobutane dehydrogenation with carbon dioxide. Catal. Lett. 2008, 121, 234–240. [Google Scholar] [CrossRef]
- Ogonowski, J.; Skrzyńska, E. Activity of vanadium magnesium oxide supported catalysts in the dehydrogenation of isobutane. Catal. Lett. 2006, 111, 79–85. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Wang, Z.; Zhu, W.; Wang, G. Study of V2O5/γ-Al2O3 catalyst for dehydrogenation of isobutane. Chin. J. Appl. Chem. 2002, 19, 290–294. [Google Scholar]
- Prouzet, É.; Boissière, C. A review on the synthesis, structure and applications in separation processes of mesoporous MSU-x silica obtained with the two-step process. Comptes Rendus Chim. 2005, 8, 579–596. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhang, Y. A comparative study on catalytic performances of chromium incorporated and supported mesoporous MSU-x catalysts for theoxidehydrogenation of ethane to ethylene with carbon dioxide. Catal. Today 2006, 115, 235–241. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhang, Y. Mesoporous silica-supported chromium catalyst: Characterization and excellent performance in dehydrogenation of propaneto propylene with carbon dioxide. Catal. Comm. 2007, 8, 565–570. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Yang, W.; Wang, X.; Zhang, Y. Effect of Cr content on catalytic performance of Cr/MSU-1 catalysts in oxidative dehydrogenation of propane to propylene with CO2. Acta Chim. Sin. 2009, 15, 1749–1753. [Google Scholar]
- Liu, L.; Li, H.; Zhang, Y. Effect of synthesis parameters on the chromium content and catalytic activities of mesoporous Cr-MSU-x prepared under acidic conditions. J. Phys. Chem. B 2006, 110, 15478–15485. [Google Scholar] [CrossRef] [PubMed]
- Sierra, L.; Guth, J.L. Synthesis of mesoporous silica with tunable pore size from sodium silicate solutions and a polyethylene oxide surfactant. Micropor. Mesopor. Mater. 1999, 27, 243–253. [Google Scholar] [CrossRef]
- Rossetti, I.; Fabbrini, L.; Ballarini, N.; Oliva, C.; Cavani, F.; Cericola, A.; Bonelli, B.; Piumetti, M.; Garrone, E.; Dyrbeck, H.; et al. V2O5-SiO2 systems prepared by flame pyrolysis as catalysts for the oxidative dehydrogenation of propane. J. Catal. 2008, 256, 45–61. [Google Scholar] [CrossRef]
- Koranne, M.M.; Goodwin, J.G.; Marcelin, G. Characterization of silica- and alumina-supported vanadia catalysts using temperature programmed reduction. J. Catal. 1994, 148, 369–377. [Google Scholar] [CrossRef]
- Adamski, A.; Sojka, Z.; Dyrek, K. Surface heterogeneity of zirconia-supported V2O5 catalysts. The link between structure and catalytic properties in oxidative dehydrogenation of propane. Langmuir 1999, 15, 5733–5741. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Reactivity of supported vanadium oxide catalysts: The partial oxidation of methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Ding, J.F.; Qin, Z.F.; Li, X.K.; Wang, G.F.; Wang, J.G. Coupling dehydrogenation of isobutane in the presence of carbon dioxide over chromium oxide supported on active carbon. Chin. Chem. Lett. 2008, 19, 1059–1062. [Google Scholar] [CrossRef]
- Gascón, J.; Téllez, C.; Herguido, J.; Menéndez, M. Propane dehydrogenation over a Cr2O3/Al2O3 catalyst: Transient kinetic modeling of propene and coke formation. Appl. Catal. A 2003, 248, 105–116. [Google Scholar] [CrossRef]
- Shimada, H.; Akazawa, T.; Ikenaga, N.; Suzuki, T. Dehydrogenation of isobutane to isobutene with iron-loaded activated carbon catalyst. Appl. Catal. A 1998, 168, 243–250. [Google Scholar] [CrossRef]





| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| MSU-1 | 1021.3 | 0.54 |
| 2.5 wt. % VOx/MSU-1 | 748.4 | 0.40 |
| 7.0 wt. % VOx/MSU-1 | 655.7 | 0.33 |
| 12.0 wt. % VOx/MSU-1 | 539.4 | 0.34 |
| 17.5 wt. % VOx/MSU-1 | 297.9 | 0.30 |
| Entry | VOx Loading (wt. %) | Conversion (%) | Selectivity (%) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
| i-C4H10 | CO2 | i-C4H8 | C3H6 | CH4 | i-C4H8 | ||
| 1 | 0 | 11.8 | 0.6 | 52.5 | 30.2 | 13.6 | 6.2 |
| 2 | 2.5 | 36.3 | 7.7 | 79.5 | 9.6 | 4.6 | 28.9 |
| 3 | 7.0 | 54.9 | 14.8 | 79.2 | 9.4 | 4.7 | 43.5 |
| 4 | 12.0 | 58.8 | 16.9 | 78.5 | 9.9 | 5.4 | 46.2 |
| 5 | 12.0* | 40.5 | / | 82.8 | 10.5 | 6.6 | 33.5 |
| 6 | 17.5 | 51.0 | 15.2 | 78.9 | 9.8 | 5.6 | 40.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, G.; Huang, Q.; Huang, S.; Wang, Q.; Li, H.; Liu, H.; Wan, S.; Zhang, X.; Wang, J. Vanadium Oxide Supported on MSU-1 as a Highly Active Catalyst for Dehydrogenation of Isobutane with CO2. Catalysts 2016, 6, 41. https://doi.org/10.3390/catal6030041
Sun G, Huang Q, Huang S, Wang Q, Li H, Liu H, Wan S, Zhang X, Wang J. Vanadium Oxide Supported on MSU-1 as a Highly Active Catalyst for Dehydrogenation of Isobutane with CO2. Catalysts. 2016; 6(3):41. https://doi.org/10.3390/catal6030041
Chicago/Turabian StyleSun, Guosong, Qingze Huang, Shiyong Huang, Qiuping Wang, Huiquan Li, Haitao Liu, Shijie Wan, Xuewang Zhang, and Jinshu Wang. 2016. "Vanadium Oxide Supported on MSU-1 as a Highly Active Catalyst for Dehydrogenation of Isobutane with CO2" Catalysts 6, no. 3: 41. https://doi.org/10.3390/catal6030041
APA StyleSun, G., Huang, Q., Huang, S., Wang, Q., Li, H., Liu, H., Wan, S., Zhang, X., & Wang, J. (2016). Vanadium Oxide Supported on MSU-1 as a Highly Active Catalyst for Dehydrogenation of Isobutane with CO2. Catalysts, 6(3), 41. https://doi.org/10.3390/catal6030041