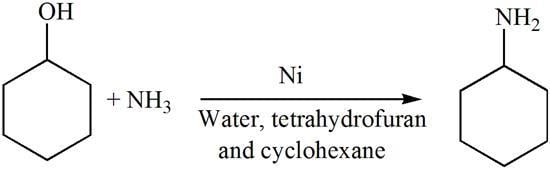
Nickel Catalyzed Conversion of Cyclohexanol into Cyclohexylamine in Water and Low Boiling Point Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Raney Ni in Aqueous Ammonia
2.2. Ni/Al2O3 and Ni/C Systems
2.3. Recycling of the Catalysts
2.4. Conversion in Low Boiling Point Solvents
3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Typical Procedures for the Reactions
3.5. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Imm, S.; Bähn, S.; Neubert, L.; Neumann, H.; Beller, M. An efficient and general synthesis of primary amines by ruthenium catalyzed amination of secondary alcohols with ammonia. Angew. Chem. Int. Ed. 2010, 49, 8126–8129. [Google Scholar] [CrossRef] [PubMed]
- Pingen, D.; Müller, C.; Vogt, D. Direct amination of secondary alcohols using ammonia. Angew. Chem. Int. Ed. 2010, 49, 8130–8133. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Selective synthesis of primary amines directly from alcohols and ammonia. Angew. Chem. Int. Ed. 2008, 120, 8789–8792. [Google Scholar] [CrossRef]
- Pingen, D.; Diebolt, O.; Vogt, D. Direct amination of bio-alcohols using ammonia. ChemCatChem 2013, 5, 2905–2912. [Google Scholar] [CrossRef]
- Walther, G.; Deutsch, J.; Martin, A.; Baumann, F.-E.; Fridag, D.; Franke, R.; Kçckritz, A. α, ω-functionalized C19 Monomers. ChemSusChem 2011, 4, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Kirumakki, S.R.; Papadaki, M.; Chary, K.V.R.; Nagaraju, N. Reductive amination of cyclohexanone in the presence of cyclohexanol over zeolites Hβ and HY. J. Mol. Catal. A Chem. 2010, 321, 15–21. [Google Scholar] [CrossRef]
- Becker, J.; Niederer, J.P.M.; Keller, M.; Hölderich, W.F. Amination of cyclohexanone and cyclohexanol/cyclohexanone in the presence of ammonia and hydrogen using copper or a group VIII metal supported on a carrier as the catalyst. Appl. Catal. A Gen. 2000, 197, 229–238. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Seela, K.K.; Naresh, D.; Ramakanth, P. Characterization and reductive amination of cyclohexanol and cyclohexanone over Cu/ZrO2 catalysts. Catal. Commun. 2008, 9, 75–81. [Google Scholar] [CrossRef]
- Baumann, W.; Spannenberg, A.; Pfeffer, J.; Haas, T.; Kçckritz, A.; Martin, A.; Deutsch, J. Utilization of common ligands for the ruthenium-catalyzed amination of alcohols. Chem. Eur. J. 2013, 19, 17702–17706. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Q.; Yu, Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dai, X.; Deng, Y.; Shi, F. Development of a general non-noble metal catalyst for the benign amination of alcohols with amines and ammonia. Chem. Eur. J. 2013, 19, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.S. Industrial processes for manufacturing amines. Appl. Catal. A Gen. 2001, 221, 187–195. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Kon, K.; Onodera, W.; Yamazaki, H.; Kondo, J.N. Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia. ACS Catal. 2013, 3, 112–117. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Enoki, Y.; Yamaguchi, R. Cp*Ir-catalyzed N-alkylation of amines with alcohols. A versatile and atom economical method for the synthesis of amines. Tetrahedron 2008, 64, 1943–1954. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Fujii, T.; Yamaguchi, R. Cp*Ir complex-catalyzed N-heterocyclization of primary amines with diols: A new catalytic system for environmentally benign synthesis of cyclic amines. Org. Lett. 2004, 6, 3525–3528. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-L.; He, L.; Zhao, S.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Hydrogen-independent reductive transformation of carbohydrate biomass into γ-valerolactone and pyrrolidone derivatives with supported gold catalysts. Angew. Chem. Int. Ed. 2011, 50, 7815–7819. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Bell, A.T. A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids. Green Chem. 2010, 12, 1253–1262. [Google Scholar] [CrossRef]










| Entry | NaOH/g | Conversion/% | Yield/% |
|---|---|---|---|
| 1 | 0 | 44 | 41 |
| 2 | 0.3 | 93 | 79 |
| 3 | 0.4 | 95 | 78 |
| 4 | 0.5 | 95 | 84 |
| 5 | 0.6 | 96 | 90 |
| 6 | 0.7 | 96 | 89 |
| 7 b | 0.7 | 57 | 52 |
| Entry | Time/h | Conversion/% | Yield/% | Selectivity/% |
|---|---|---|---|---|
| 1 | 4 | 71 | 68 | 95 |
| 2 | 6 | 83 | 76 | 91 |
| 3 | 8 | 94 | 81 | 86 |
| 4 | 12 | 95 | 82 | 86 |
| 5 | 17 | 96 | 82 | 85 |
| 6 | 24 | 98 | 83 | 84 |
| 7 | 30 | 98 | 83 | 84 |
| Entry | Alcohol/g | Conversion/% | Yield/% | Selectivity/% |
|---|---|---|---|---|
| 1 | 1 | 96 | 88 | 93 |
| 2 | 1.5 | 93 | 86 | 92 |
| 3 | 2 | 89 | 76 | 85 |
| 4 | 2.5 | 93 | 72 | 77 |
| Entry | Catalyst | T/°C | t/h | H2/MPa | NaOH/g | Conversion/% | Yield/% |
|---|---|---|---|---|---|---|---|
| 1 | Ni/Al2O3 | 160 | 17 | 0 | 0.3 | 51 | 42 |
| 2 | Ni/Al2O3 | 180 | 17 | 0 | 0.3 | 54 | 49 |
| 3 | Ni/Al2O3 | 200 | 17 | 0 | 0.3 | 71 | 65 |
| 4 | Ni/Al2O3 | 200 | 8 | 1 | 0.3 | 63 | 54 |
| 5 | Ni/Al2O3 | 200 | 14 | 1 | 0.3 | 82 | 72 |
| 6 | Ni/Al2O3 | 200 | 17 | 1 | 0.3 | 87 | 79 |
| 7 | Ni/Al2O3 | 200 | 17 | 0 | 0 | 48 | 41 |
| 8 | Ni/C | 160 | 17 | 0 | 0 | 37 | 34 |
| 9 | Ni/C | 160 | 17 | 0 | 0.3 | 87 | 81 |
| 10 | Ni/C | 160 | 17 | 1 | 0.3 | 92 | 85 |
| 11 | Ni/C | 160 | 6 | 1 | 0.3 | 76 | 69 |
| 12 | Ni/C | 160 | 24 | 1 | 0.3 | 91 | 81 |
| 13 | Ni/C | 180 | 17 | 1 | 0.3 | 91 | 86 |
| Entry | Solvents | T/°C | H2/MPa | NaOH/g | Conversion/% | Yield/% |
|---|---|---|---|---|---|---|
| 1 | THF | 160 | 0 | 0 | 13 | 11 |
| 2 | THF | 160 | 1 | 0 | 11 | 9 |
| 3 | THF | 160 | 0 | 0.3 | 50 | 46 |
| 4 | THF | 160 | 1 | 0.3 | 50 | 41 |
| 5 | THF | 180 | 1 | 0.3 | 85 | 76 |
| 6 | cyclohexane | 180 | 0 | 0 | 31 | 28 |
| 7 | cyclohexane | 180 | 1 | 0 | 96 | 87 |
| Entry | Solvent | T/°C | H2/MPa | NaOH/g | Conversion/% | Yield/% |
|---|---|---|---|---|---|---|
| 1 | THF | 160 | 0 | 0 | 11 | 9 |
| 2 | THF | 160 | 0 | 0.3 | 22 | 20 |
| 3 | THF | 160 | 1 | 0 | 12 | 10 |
| 4 | THF | 180 | 1 | 0.3 | 32 | 28 |
| 5 | cyclohexane | 180 | 1 | 0.3 | 42 | 35 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Yu, H.; Cao, Q.; Dong, B.; Mu, X.; Mao, A. Nickel Catalyzed Conversion of Cyclohexanol into Cyclohexylamine in Water and Low Boiling Point Solvents. Catalysts 2016, 6, 63. https://doi.org/10.3390/catal6050063
Qi Y, Yu H, Cao Q, Dong B, Mu X, Mao A. Nickel Catalyzed Conversion of Cyclohexanol into Cyclohexylamine in Water and Low Boiling Point Solvents. Catalysts. 2016; 6(5):63. https://doi.org/10.3390/catal6050063
Chicago/Turabian StyleQi, Yunfei, Haiyun Yu, Quan Cao, Bo Dong, Xindong Mu, and Aiqing Mao. 2016. "Nickel Catalyzed Conversion of Cyclohexanol into Cyclohexylamine in Water and Low Boiling Point Solvents" Catalysts 6, no. 5: 63. https://doi.org/10.3390/catal6050063
APA StyleQi, Y., Yu, H., Cao, Q., Dong, B., Mu, X., & Mao, A. (2016). Nickel Catalyzed Conversion of Cyclohexanol into Cyclohexylamine in Water and Low Boiling Point Solvents. Catalysts, 6(5), 63. https://doi.org/10.3390/catal6050063