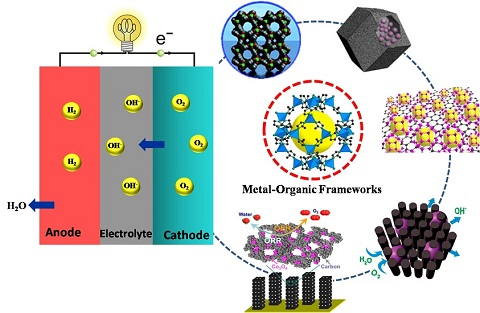
Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells
Abstract
:1. Introduction
1.1. Introduction and Challenges Facing Fuel Cell Devices
1.2. A Possible Solution with the Use of MOF-Derived Nanomaterials
2. MOF-Derived Heteroatom-Doped Nanocarbon Electrocatalysts
2.1. N-Doped Nanocarbon Electrocatalysts
2.2. Multi-Doped Nanocarbon Electrocatalysts
2.3. MOF-Derived Nanocarbon Composite Electrocatalysts
3. MOF-Derived Transition Metal/Metal Oxide-Nanocarbon Electrocatalysts
3.1. MOF-Derived Cobalt/Cobalt Oxide-Nanocarbon Electrocatalysts
3.2. MOF-Derived Iron/Iron Oxide-Nanocarbon Electrocatalysts
3.3. Other MOF-Derived Metal-Nanocarbon Electrocatalysts
4. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-H.; Yu, S.-H. Engineering interface and surface of noble metal nanoparticle nanotubes toward enhanced catalytic activity for fuel cell applications. Acc. Chem. Res. 2013, 46, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Zhong, C.-J.; Luo, J.; Njoki, P.N.; Mott, D.; Wanjala, B.; Loukrakpam, R.; Lim, S.; Wang, L.; Fang, B.; Xu, Z. Fuel cell technology: Nano-engineered multimetallic catalysts. Energy Environ. Sci. 2008, 1, 454–466. [Google Scholar] [CrossRef]
- Cheng, N.; Banis, M.N.; Liu, J.; Riese, A.; Li, X.; Li, R.; Ye, S.; Knights, S.; Sun, X. Extremely stable platinum nanoparticles encapsulated in a zirconia nanocage by area-selective atomic layer deposition for the oxygen reduction reaction. Adv. Mater. 2015, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Banis, M.N.; Liu, J.; Riese, A.; Mu, S.; Li, R.; Sham, T.-K.; Sun, X. Atomic scale enhancement of metal–support interactions between Pt and ZrC for highly stable electrocatalysts. Energy Environ. Sci. 2015, 8, 1450–1455. [Google Scholar] [CrossRef]
- Rabis, A.; Rodriguez, P.; Schmidt, T.J. Electrocatalysis for polymer electrolyte fuel cells: Recent achievements and future challenges. Acs Catal. 2012, 2, 864–890. [Google Scholar] [CrossRef]
- Cheng, N.; Mu, S.; Pan, M.; Edwards, P.P. Improved lifetime of PEM fuel cell catalysts through polymer stabilization. Electrochem. Commun. 2009, 11, 1610–1614. [Google Scholar] [CrossRef]
- Jiang, Z.-Z.; Wang, Z.-B.; Chu, Y.-Y.; Gu, D.-M.; Yin, G.-P. Carbon riveted microcapsule Pt/MWCNTs-TiO2 catalyst prepared by in situ carbonized glucose with ultrahigh stability for proton exchange membrane fuel cell. Energy Environ. Sci. 2011, 4, 2558–2566. [Google Scholar] [CrossRef]
- Jung, N.; Chung, D.Y.; Ryu, J.; Yoo, S.J.; Sung, Y.-E. Pt-based nanoarchitecture and catalyst design for fuel cell applications. Nano Today 2014, 9, 433–456. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [PubMed]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Li, C.; Ji, Q.; Jiang, B.; Tominaka, S.; Ide, Y.; Hill, J.P.; Ariga, K.; Yamauchi, Y. Self-construction from 2D to 3D: One-pot layer-by-layer assembly of graphene oxide sheets held together by coordination polymers. Angew. Chem. 2016, 55, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.-T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal-nitrogen coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yin, H.; Du, L.; He, L.; Zhao, K.; Chang, L.; Yin, G.; Zhao, H.; Liu, S.; Tang, Z. Carbonized nanoscale metal–organic frameworks as high performance electrocatalyst for oxygen reduction reaction. ACS Nano 2014, 8, 12660–12668. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.D.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Li, C.; Ji, W.; Yang, M.; Huang, H.; Liu, Y.; Kang, Z. Tunable ternary (N, P, B)-doped porous nanocarbons and their catalytic properties for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2014, 6, 22297–22304. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, R.; Hong, M. Structural evolution from metal–organic framework to hybrids of nitrogen-doped porous carbon and carbon nanotubes for enhanced oxygen reduction activity. Chem. Mater. 2015, 27, 7610–7618. [Google Scholar] [CrossRef]
- Yang, W.; Yue, X.; Liu, X.; Zhai, J.; Jia, J. IL-derived N, S co-doped ordered mesoporous carbon for high-performance oxygen reduction. Nanoscale 2015, 7, 11956–11961. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liu, S.; Ouyang, C.; Strasser, P.; Yang, R. Nitrogen-and phosphorus-doped biocarbon with enhanced electrocatalytic activity for oxygen reduction. ACS Catal. 2015, 5, 920–927. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, Y.; Xiang, Z.; Liu, G.; Cao, D. Phosphorous–nitrogen-codoped carbon materials derived from metal–organic frameworks as efficient electrocatalysts for oxygen reduction reactions. Eur. J. Inorg. Chem. 2016, 2016, 2100–2105. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal organic frameworks for energy storage and conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Fujita, M.; Kwon, Y.J.; Washizu, S.; Ogura, K. Preparation, clathration ability, and catalysis of a two-dimensional square network material composed of cadmium (II) and 4, 4’-bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Metal–organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Y.; Liang, Y.; Kong, B.; Zhang, J.; Song, J.; Bao, Q.; Simon, G.P.; Jiang, S.P.; Wang, H. Nitrogen-Doped Nanoporous Carbon/Graphene Nano-Sandwiches: Synthesis and Application for Efficient Oxygen Reduction. Adv. Funct. Mater. 2015, 25, 5768–5777. [Google Scholar] [CrossRef]
- Ge, L.; Yang, Y.; Wang, L.; Zhou, W.; De Marco, R.; Chen, Z.; Zou, J.; Zhu, Z. High activity electrocatalysts from metal–organic framework-carbon nanotube templates for the oxygen reduction reaction. Carbon 2015, 82, 417–424. [Google Scholar] [CrossRef]
- Zhong, H.-X.; Wang, J.; Zhang, Y.-W.; Xu, W.-L.; Xing, W.; Xu, D.; Zhang, Y.-F.; Zhang, X.-B. ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 14235–14239. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.-U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.B.; Madhavi, S.; Hng, H.H.; Lou, X.W. Formation of Fe2O3 microboxes with hierarchical shell structures from metal–organic frameworks and their lithium storage properties. J. Am. Chem. Soc. 2012, 134, 17388–17391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, F.; Su, P.; Li, M.; Chen, J.; Yang, Q.; Li, C. Spinel ZnMn2O4 nanoplate assemblies fabricated via “escape-by-crafty-scheme” strategy. J. Mater. Chem. 2012, 22, 13328–13333. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Zhang, F.; Chen, Q. CuO/Cu2O composite hollow polyhedrons fabricated from metal–organic framework templates for lithium-ion battery anodes with a long cycling life. Nanoscale 2013, 5, 4186–4190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, Z.; Jiang, F.; Yang, L.; Qian, J.; Zhou, Y.; Li, W.; Hong, M. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions. Nanoscale 2014, 6, 6590–6602. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-W.; Peng, F.; Wang, H.-J.; Yu, H.; Zheng, W.-X.; Yang, J. Phosphorus-doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium. Angew. Chem. Int. Ed. 2011, 50, 3257–3261. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Jong, Z.; Qu, W.; Geng, D.; Sun, X.; Wang, H. Nitrogen-doped carbon nanotubes with high activity for oxygen reduction in alkaline media. Int. J. Hydrog. Energy 2011, 36, 2258–2265. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Waje, M.M.; Chen, Z.; Yan, Y.; Bozhilov, K.N.; Feng, P. Sulfonated ordered mesoporous carbon as a stable and highly active protonic acid catalyst. Chem. Mater. 2007, 19, 2395–2397. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Tang, Y.; Li, S.; Dong, H.; Li, K.; Han, M.; Lan, Y.-Q.; Bao, J.; Dai, Z. Metal-organic framework templated nitrogen and sulfur co-doped porous carbons as highly efficient metal-free electrocatalysts for oxygen reduction reactions. J. Mater. Chem. A 2014, 2, 6316–6319. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. 2010, 122, 2619–2623. [Google Scholar] [CrossRef]
- Yang, W.; Fellinger, T.; Antonietti, M. Efficient metal-free oxygen reduction in alkaline medium on high-surface-area mesoporous nitrogen-doped carbons made from ionic liquids and nucleobases. J. Am. Chem. Soc. 2010, 133, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Xiang, Z.; Shen, Z.; Yun, J.; Cao, D. ZIF-derived in situ nitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 442–450. [Google Scholar] [CrossRef]
- Pandiaraj, S.; Aiyappa, H.B.; Banerjee, R.; Kurungot, S. Post modification of MOF derived carbon via g-C3N4 entrapment for an efficient metal-free oxygen reduction reaction. Chem. Commun. 2014, 50, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Park, S.H.; Woo, S.I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 2012, 6, 7084–7091. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar Sahraie, N.; Paraknowitsch, J.P.; Gobel, C.; Thomas, A.; Strasser, P. Noble-metal-free electrocatalysts with enhanced ORR performance by task-specific functionalization of carbon using ionic liquid precursor systems. J. Am. Chem. Soc. 2014, 136, 14486–14497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dai, L. Carbon nanomaterials as metal-free catalysts in next generation fuel cells. Nano Energy 2012, 1, 514–517. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Fu, H.; Li, W.; Fan, X.; Xin, G.; Zheng, J.; Li, X. MOF derived catalysts for electrochemical oxygen reduction. J. Mater. Chem. A 2014, 2, 14064–14070. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, C.; Wu, Z.Y.; Xiong, Y.; Xu, Q.; Yu, S.H.; Jiang, H.L. From Bimetallic Metal-Organic Framework to Porous Carbon: High Surface Area and Multicomponent Active Dopants for Excellent Electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Xia, Y.; Xu, Y.; Xiao, J.; Wang, S. (Fe, Co)@ nitrogen-doped graphitic carbon nanocubes derived from polydopamine-encapsulated metal–organic frameworks as a highly stable and selective non-precious oxygen reduction electrocatalyst. Chem. Commun. 2015, 51, 10479–10482. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zou, R.; An, L.; Xia, D.; Guo, S. A metal-organic framework route to in situ encapsulation of Co@Co3O4@C core@bishell nanoparticles into a highly ordered porous carbon matrix for oxygen reduction. Energy Environ. Sci. 2015, 8, 568–576. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Han, M.; Dai, Z.-H.; Bao, J.-C.; Lan, Y.-Q. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: Efficient bifunctional electrocatalysts for ORR and OER. Chem. Commun. 2015, 51, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Huang, T.; Wen, Z.; Mao, S.; Cui, S.; Chen, J. Metal-Organic Framework-Derived Nitrogen-Doped Core-Shell-Structured Porous Fe/Fe3C@ C Nanoboxes Supported on Graphene Sheets for Efficient Oxygen Reduction Reactions. Adv. Energy Mater. 2014, 4, 1400337. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Lin, X.; Tian, M.; An, X.; Fu, Y.; Li, R.; Jin, J.; Ma, J. Metal–organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar]
- Xia, W.; Zou, R.; An, L.; Xia, D.; Guo, S. MOF derived Co3O4 nanoparticles embedded in N-doped mesoporous carbon layer/MWCNT hybrids: Extraordinary bi-functional electrocatalysts for OER and ORR. J. Mater. Chem. A 2015, 3, 17392–17402. [Google Scholar]
- Chaikittisilp, W.; Torad, N.L.; Li, C.; Imura, M.; Suzuki, N.; Ishihara, S.; Ariga, K.; Yamauchi, Y. Synthesis of Nanoporous Carbon–Cobalt-Oxide Hybrid Electrocatalysts by Thermal Conversion of Metal–Organic Frameworks. Chem. A Eur. J. 2014, 20, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.-i.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene Oxide and Copper-Centered Metal Organic Framework Composite as a Tri-Functional Catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Lin, Q.; Bu, X.; Kong, A.; Mao, C.; Zhao, X.; Bu, F.; Feng, P. New Heterometallic Zirconium Metalloporphyrin Frameworks and Their Heteroatom-Activated High-Surface-Area Carbon Derivatives. J. Am. Chem. Soc. 2015, 137, 2235–2238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-W.; Li, Y.-A.; Wang, X.-R.; Chen, G.-J.; Liu, Q.-K.; Ma, J.-P.; Dong, Y.-B. Fabrication of Cd (ii)-MOF-based ternary photocatalytic composite materials for H 2 production via a gel-to-crystal approach. Chem. Commun. 2015, 51, 15906–15909. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, R.; Huang, J.; Shi, Y.; Zhang, D.; Zhong, X.; Wang, D.; Wu, Y.; Li, Y. Platinum-nickel frame within metal-organic framework fabricated in situ for hydrogen enrichment and molecular sieving. Nat. Commun. 2015, 6, 8248. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yang, L.; Yu, P.; Wei, X.; Mao, L. Electrocatalytic four-electron reduction of oxygen with Copper (II)-based metal-organic frameworks. Electrochem. Commun. 2012, 19, 29–31. [Google Scholar] [CrossRef]
- Li, J.-S.; Tang, Y.-J.; Liu, C.-H.; Li, S.-L.; Li, R.-H.; Dong, L.-Z.; Dai, Z.-H.; Bao, J.-C.; Lan, Y.-Q. Polyoxometalate-based metal-organic framework-derived hybrid electrocatalysts for highly efficient hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 1202–1207. [Google Scholar] [CrossRef]
- Tang, Y.J.; Gao, M.R.; Liu, C.H.; Li, S.L.; Jiang, H.L.; Lan, Y.Q.; Han, M.; Yu, S.H. Porous Molybdenum-Based Hybrid Catalysts for Highly Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 12928–12932. [Google Scholar] [CrossRef] [PubMed]
- Katrib, A.; Leflaive, P.; Hilaire, L.; Maire, G. Molybdenum based catalysts. I. MoO2 as the active species in the reforming of hydrocarbons. Catal. Lett. 1996, 38, 95–99. [Google Scholar] [CrossRef]











| MOF-Derived Nanocarbon Catalysts | Onset Potential | Limiting Current Density (mA·cm−2) | Electron Transfer Numbers | Ref. |
|---|---|---|---|---|
| ZIF-67 derived NCNTFs-700 | 0.97 V (vs. RHE) | - | 3.97 | [18] |
| NGPC/NCNT-900 | −0.051 V (vs. Ag/AgCl) | 5.06 | 4.0 | [21] |
| P-N-Carbon-950 | - | 4.86 | 3.82 | [24] |
| Zn-ZIF/GO-800 | 0.92 V (vs. RHE) | 5.2 | 4.0 | [29] |
| ZIF-8 derived NGPC | −0.02 V (vs. Ag/AgCl) | 4.3 | 3.84 | [37] |
| N, S-MOF-5-C | −0.005 V (vs. Ag/AgCl) | - | 3.4–3.8 | [46] |
| ZIF-derived carbon-L | 0.86 V (vs. RHE) | 4.6 | 3.68 | [50] |
| MOF-5-CN900 | 0.035 V (vs. Hg/HgO) | 4.2 | 3.12 | [51] |
| MOF-Derived Catalysts | Onset Potential | Limiting Current Density (mA·cm−2) | Electron Transfer Numbers | Ref. |
|---|---|---|---|---|
| MIL-88B-Fe CNPs | 1.03 V (vs. RHE) | 8.31 | 3.97 | [17] |
| ZIF-67-900 | 0.91 V (vs. RHE) | ~5.0 | - | [55] |
| P-CNCo-20 | −0.04 V (vs. Ag/AgCl) | 6.0 | 3.9 | [56] |
| (Fe,Co)@GNC | 0.91 V (vs. RHE) | - | 3.7 | [57] |
| Co@Co3O4@C–CM | 0.93 V (vs. RHE) | - | 3.8–3.9 | [58] |
| (r-GO-50 wt %-FeP)n-MOFs | −0.23 V (vs. Ag/AgCl) | 6.2 | 4.0 | [59] |
| (Fe/Fe3C@NGL-NCNT) | 0.04 V (vs. Ag/AgCl) | - | 3.6 | [60] |
| N-doped Fe/Fe3C@C/RGO | 1.0 V (vs. RHE) | 10.12 | 3.08–3.52 | [61] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Cheng, N.; Lushington, A.; Sun, X. Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells. Catalysts 2016, 6, 116. https://doi.org/10.3390/catal6080116
Song Z, Cheng N, Lushington A, Sun X. Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells. Catalysts. 2016; 6(8):116. https://doi.org/10.3390/catal6080116
Chicago/Turabian StyleSong, Zhongxin, Niancai Cheng, Andrew Lushington, and Xueliang Sun. 2016. "Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells" Catalysts 6, no. 8: 116. https://doi.org/10.3390/catal6080116
APA StyleSong, Z., Cheng, N., Lushington, A., & Sun, X. (2016). Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells. Catalysts, 6(8), 116. https://doi.org/10.3390/catal6080116





_Sun.png)