Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene
Abstract
:1. Introduction
2. Results and Discussion
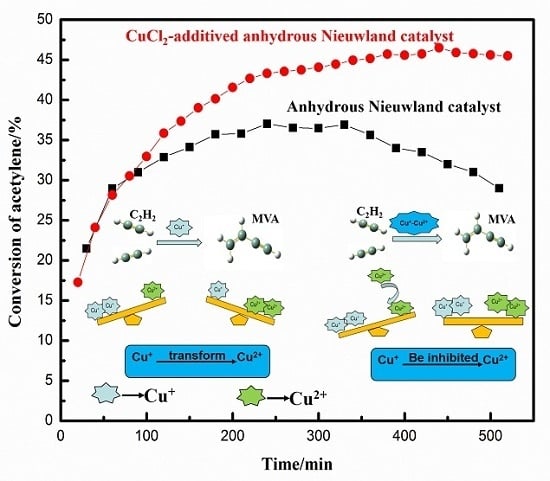
2.1. Effect of Cu+/Cu2+ Ratio on the Dimerization Reaction of C2H2 by an Anhydrous Catalyst
2.2. Relationship between the Deactivation Mechanism and Concentration of Cu2+ during Acetylene Dimerization
3. Materials and Methods
3.1. Reaction Equipment
3.2. Materials, Catalyst Preparation, and Acetylene Dimerization
3.3. Dark Red Precipitate Separation and cat-a, cat-b, cat-c, and cat-d Preparation
3.4. Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef] [PubMed]
- Rattanasom, N.; Kueseng, P.; Deeprasertkul, C. Improvement of the mechanical and thermal properties of silica-filled polychloroprene vulcanizates prepared from latex system. Appl. Polym. Sci. 2012, 124, 2657–2668. [Google Scholar] [CrossRef]
- Ismail, H.; Leong, H.C. Curing characteristics and mechanical properties of natural rubber/chloroprene rubber and epoxidized natural rubber/chloroprene rubber blends. Polym. Test. 2001, 20, 509–516. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Sun, L. Status and development proposal on synthetic rubber industry in China I. Rubber Ind. (Hechen Xiangjiao Gongye) 2006, 29, 81–85. (In Chinese) [Google Scholar]
- Ramesan, M.T.; Mathew, G.; Kuriakose, B.; Alex, R. Role of dichlorocarbene modified styrene butadiene rubber in compatibilisation of styrene butadiene rubber and chloroprene rubber blends. Eur. Polym. J. 2001, 37, 719–728. [Google Scholar] [CrossRef]
- Arutyunyan, R.M.; Pogosyan, V.S.; Simonyan, E.H.; Atoyants, A.L.; Djigardjian, E.M. In situ monitoring of the ambient air around the chloroprene rubber industrial plant using the Tradescantia-stamen-hair mutation assay. Mutat. Res. 1999, 426, 117–120. [Google Scholar] [CrossRef]
- Liu, J.G.; Han, M.H.; Zuo, Y.Z.; Wang, Z.W. Research progress of dimerization of acetylene to monovinylacetylene. Chem. Ind. Eng. Prog. (Huagong Jinzhan) 2011, 30, 942–947. (In Chinese) [Google Scholar]
- Tokita, Y.; Okamoto, A.; Nishiwaki, K.; Kobayashi, M.; Nakamura, E. Kinetics of copper(I)-catalyzed dimerization and hydration of acetylene in water. Bull. Chem. Soc. Jpn. 2004, 77, 1395–1399. [Google Scholar] [CrossRef]
- Trotus, I.-T.; Zimmermann, T.; Schüth, F. Catalytic reactions of acetylene: a feedstock for the chemical industry revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, K.; Kobayashi, M.; Takeuchi, T.; Matuoto, K.; Osakada, K. Nieuwland catalysts: Investigation of structure in the solid state and in solution and performance in the dimerization of acetylene. J. Mol. Catal. A Chem. 2001, 175, 73–81. [Google Scholar] [CrossRef]
- Tachiyama, T.; Yoshida, M.; Aoyagi, T.; Fukuzumi, S. Deuterium kinetic isotope effects and H/D exchange in dimerization of acetylene with a Nieuwland catalyst in aqueous media. J. Phys. Org. Chem. 2008, 21, 510–515. [Google Scholar] [CrossRef]
- Tachiyamaa, T.; Yoshidaa, M.; Aoyagia, T.; Fukuzumi, S. Mechanistic study on dimerization of acetylene with a Nieuwland catalyst. Appl. Organomet. Chem. 2008, 22, 205–210. [Google Scholar] [CrossRef]
- Liu, J.G.; Zuo, Y.Z.; Han, M.H.; Wang, Z.W.; Wang, D.Z. Stability improvement of the Nieuwland catalyst in the dimerization of acetylene to monovinylacetylene. J. Nat. Gas. Chem. 2012, 21, 495–500. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yu, Y.L.; Tao, C.Y.; Du, J.; Liu, R.L.; Fan, X.; Peng, M.; Sun, D.G.; Zuo, Z.H.; Ma, J.X.; et al. A Method of Dimerization of Acetylene to Monovinylacetylene. CN Patent 102775266 A, 14 November 2012. [Google Scholar]
- Liu, Z.H.; Yu, Y.L.; Tao, C.Y.; Du, J.; Liu, R.L.; Fan, X.; Peng, M.; Sun, D.G.; Zuo, Z.H.; Ma, J.X.; et al. A Method of Synthesizing Monovinylacetylene in Aqueous Solution. CN Patent 102731241 A, 17 October 2012. [Google Scholar]
- Du, J.; Liu, Z.H.; Tao, C.Y. A Method of Synthesizing Monovinylacetylene. CN Patent 102964198 A, 13 March 2013. [Google Scholar]
- Lu, J.L.; Xie, J.W.; Liu, H.Y.; Liu, P.; Liu, Z.Y.; Dai, B. Strontium chloride modified Nieuwland catalyst in the dimerization of acetylene to monovinylacetylene. Asian J. Chem. 2014, 26, 8211–8214. [Google Scholar]
- Lu, J.L.; Liu, H.Y.; Xie, J.W.; Liu, P.; Liu, Z.Y.; Dai, B. Study on catalytic activity of zinc(II)-copper(I) collaborative bimetallic catalysis in acetylene dimerization reaction. J. Shihezi Univ. (Nat. Sci. Ed.) 2014, 32, 213–217. [Google Scholar]
- Lu, J.L.; Liu, H.Y.; Xie, J.W.; Liu, P.; Dai, B.; Liu, Z.Y. Effect of polyethylene glycol/Nieuwland catalyst on acetylene dimerization reaction. Chem. Eng. 2015, 43, 60–64. (In Chinese) [Google Scholar]
- Deng, G.C.; Wang, Z.P.; Liu, G.D.; Mu, R.C.; Li, B.C.; Chen, R.T. Non-aqueous catalyst for dimerization of acetylene. Chin. Synth. Rubber Ind. (Hecheng Xiangjiao Gongye) 1991, 14, 106–108. (In Chinese) [Google Scholar]
- Liu, J.G.; Han, M.H.; Wang, Z.W. Effect of solvent on catalytic performance of anhydrous catalyst in acetylene dimerization to monovinylacetylene. J. Energy Chem. 2013, 22, 599–604. [Google Scholar] [CrossRef]
- Tao, C.Y.; Du, J.; Fan, X.; Liu, Z.H.; Sun, D.G.; Shen, H.N.; Liu, R.L.; Zuo, Z.H.; Xiao, C.C.; Zhen, X.X.; et al. A Method of Preparing Catalyst of Synthesizing Monovinylacetylene and Application. CN Patent 101786022 A, 28 July 2010. [Google Scholar]
- Tao, C.Y.; Du, J.; Fan, X.; Liu, Z.H.; Xiao, C.C.; Ma, J.X.; Xue, H.T.; Shen, H.N.; Sun, D.G.; Liu, R.L.; et al. A Method of Preparing Hydrous Catalyst of Synthesizing Monovinylacetylene. CN Patent 101940953 A, 12 January 2011. [Google Scholar]
- Han, M.H.; Liu, J.G.; Zuo, Y.Z.; Wang, Z.W. The Method and Application of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. CN Patent 103285925 A, 11 September 2013. [Google Scholar]
- Han, M.H.; Liu, J.G.; Zuo, Y.Z.; Wang, Z.W. Monovinylacetylene product and preparation method of monovinylacetylene. CN Patent 103288572 A, 11 September 2013. [Google Scholar]
- Liu, H.Y.; Xie, J.W.; Liu, P.; Liu, Z.Y.; Dai, B. Study on anhydrous catalyst in acetylene dimerization to monovinylacetylene. J. Shihezi Univ. (Nat. Sci. Ed.) 2015, 33, 622–627. [Google Scholar]
- Liu, J.G.; Han, M.H.; Wang, Z.W. Studies on the catalytic performance of the Nieuwland catalyst and anhydrous catalyst in the dimerization of acetylene to monovinylacetylene. Adv. Mater. Res. 2012, 550–553, 312–316. [Google Scholar] [CrossRef]
- Liu, J.G.; Zuo, Y.Z.; Han, M.H.; Wang, Z.W. Improvement of anhydrous catalyst stability in acetylene dimerization by regulating acidity. J. Chem. Technol. Biotechnol. 2013, 88, 408–414. [Google Scholar] [CrossRef]
- Nieuwland, J.A.; Calott, W.S.; Downing, F.B.; Carter, A.S. Acetylene polymers and their derivatives. I. The controlled polymerization of acetylene. J. Am. Chem. Soc. 1931, 53, 4197–4202. [Google Scholar] [CrossRef]









| Mole Ratio of Cu+/Cu2+ | Composition of Outlet Gas (%) | C2H2 Conversion (%) | MVA Selectivity (%) | |||
|---|---|---|---|---|---|---|
| MVA | DVA | Acetaldehyde | C2H2 (Recovered) | |||
| 5.6:1 | 12.72 | 2.88 | 0.12 | 84.28 | 28.9 | 74.4 |
| 4:1 | 15.15 | 3.58 | 0.12 | 81.15 | 33.7 | 73.6 |
| 3:1 | 16.95 | 4.33 | 0.16 | 78.56 | 37.5 | 72.1 |
| 2:1 | 17.94 | 4.42 | 0.18 | 77.46 | 38.9 | 72.7 |
| 1:1 | 16.27 | 3.51 | 0.30 | 79.92 | 35.2 | 75.0 |
| 1:2 | 11.07 | 2.43 | 0.10 | 86.40 | 25.5 | 75.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xie, J.; Liu, P.; Dai, B. Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. Catalysts 2016, 6, 120. https://doi.org/10.3390/catal6080120
Liu H, Xie J, Liu P, Dai B. Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. Catalysts. 2016; 6(8):120. https://doi.org/10.3390/catal6080120
Chicago/Turabian StyleLiu, Haiyue, Jianwei Xie, Ping Liu, and Bin Dai. 2016. "Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene" Catalysts 6, no. 8: 120. https://doi.org/10.3390/catal6080120
APA StyleLiu, H., Xie, J., Liu, P., & Dai, B. (2016). Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. Catalysts, 6(8), 120. https://doi.org/10.3390/catal6080120