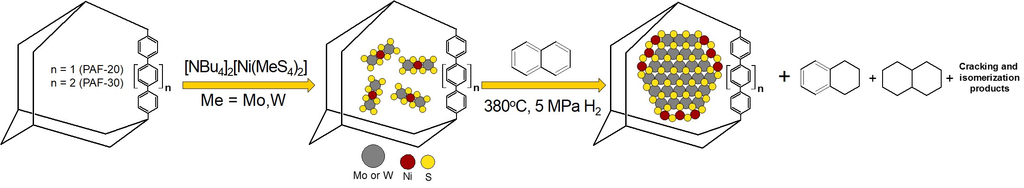
Sulfide Catalysts Supported on Porous Aromatic Frameworks for Naphthalene Hydroprocessing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Precursors and Characterization of Catalysts
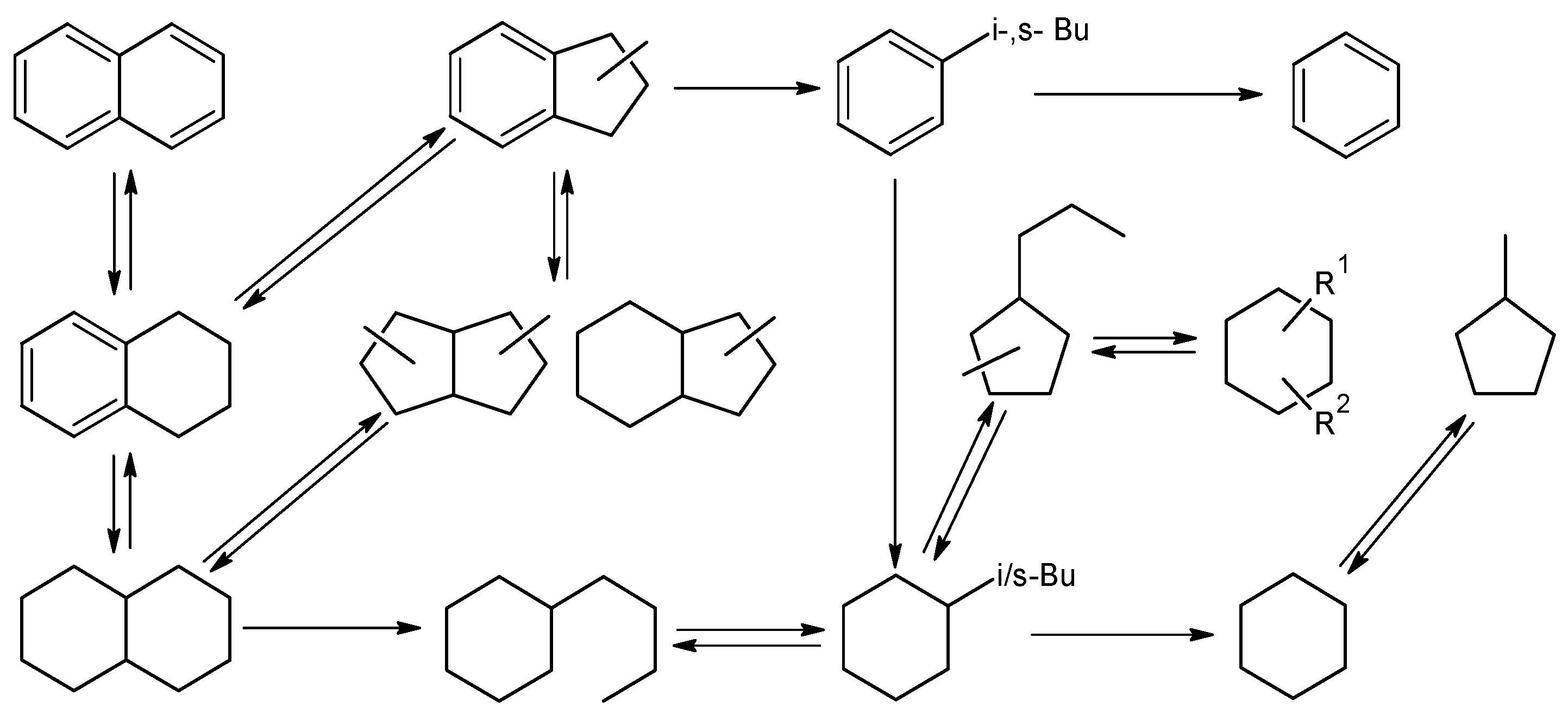
2.2. Catalytic Experiments
3. Experimental
3.1. Materials
3.2. Synthesis of Precursors
3.3. Characterization of Catalysts
3.4. Catalytic Testing Procedure
4. Conclusions
- 1)
- The porous properties of the support have an impact on the performance of the catalysts. It was revealed that catalysts supported by the framework with a larger pore size were more active than those based on the framework with a smaller pore size;
- 2)
- Typically, in the reactions without the additive of sulfur, the predominant phases on a particle surface are Mo6+ and W6+; the addition of sulfur enhanced the catalytic performance of the systems by conversion of the Me6+ form to the more active MeS2 form;
- 3)
- Molybdenum catalysts were found to be more active in the presence of sulfur than tungsten catalysts due to the more facile transition from the oxide form to the sulfide form.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farag, H.; Whitehurst, D.D.; Mochida, I. Synthesis of active hydrodesulfurization carbon-supported Co-Mo catalysts. Relationships between preparation methods and activity/selectivity. Ind. Eng. Chem. Res. 1998, 37, 3533–3539. [Google Scholar] [CrossRef]
- Álvarez, L.; Berhault, G.; Alonso-Nuñez, G. Unsupported NiMo sulfide catalysts obtained from nickel/ammonium and nickel/tetraalkylammonium thiomolybdates: Synthesis and application in the hydrodesulfurization of dibenzothiophene. Catal. Lett. 2008, 125, 35–45. [Google Scholar] [CrossRef]
- Díaz de León, J.N.; Picquart, M.; Massin, L.; Vrinat, M.; de los Reyes, J.A. Hydrodesulfurization of sulfur refractory compounds: Effect of gallium as an additive in NiWS/γ-Al2O3 catalysts. J. Mol. Catal. A Chem. 2012, 363–364, 311–321. [Google Scholar] [CrossRef]
- Ferraz, S.G.A.; Zotin, F.M.Z.; Araujo, L.R.R.; Zotin, J.L. Influence of support acidity of NiMoS catalysts in the activity for hydrogenation and hydrocracking of tetralin. Appl. Catal. A Gen. 2010, 384, 51–57. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Minaev, P.P.; Mozhaev, A.V.; Maslakov, K.I.; Kulikova, M.S.; Pimerzin, A.A. Investigation of co-effect of 12-tungstophosphoric heteropolyacid, nickel citrate and carbon-coated alumina in preparation of NiW catalysts for HDS, HYD and HDN reactions. Appl. Catal. B Environ. 2015, 176–177, 374–384. [Google Scholar] [CrossRef]
- Sizova, I.A.; Serdyukov, S.I.; Maksimov, A.L. Nickel-tungsten sulfide aromatic hydrocarbon hydrogenation catalysts synthesized in situ in a hydrocarbon medium. Pet. Chem. 2015, 55, 470–480. [Google Scholar] [CrossRef]
- Brito, J.N.L.; Severino, F.; Ninoska Delgado, N.; Laine, J. Hds activity of carbon-supported Ni-Mo catalysts derived from thiomolybdate complexes. Appl. Catal. A Gen. 1998, 173, 193–199. [Google Scholar] [CrossRef]
- Wilkinson, K.; Merchán, M.D.; Vasudevan, P.T. Characterization of supported tungsten sulfide catalysts ex ammonium tetrathiotungstate. J. Catal. 1997, 171, 325–328. [Google Scholar] [CrossRef]
- Dutta, R.P.; Schobert, H.H. Catalytic conversion of polycyclic aromatic hydrocarbons hydrogenation/dehydrogenation of polycyclic aromatic hydrocarbons using ammonium tetrathiomolybdate as catalyst precursor. Catal. Today 1996, 31, 65–77. [Google Scholar] [CrossRef]
- Brito, J.L.; Ilija, M.; Hernández, P. Thermal and reductive decomposition of ammonium thiomolybdates. Thermochim. Acta 1995, 256, 325–338. [Google Scholar] [CrossRef]
- Poisot, M.; Bensch, W. Decomposition of tetraalkylammonium thiotungstates characterized by thermoanalysis, mass spectrometry, X-ray diffractometry and scanning electron microscopy. Thermochim. Acta 2007, 453, 42–51. [Google Scholar] [CrossRef]
- Minaev, P.P.; Nikulshin, P.A.; Kulikova, M.S.; Pimerzin, A.A.; Kogan, V.M. NiWS/Al2O3 hydrotreating catalysts prepared with 12-tungstophosphoric heteropolyacid and nickel citrate: Effect of Ni/W ratio. Appl. Catal. A Gen. 2015, 505, 456–466. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Nyberg, M.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; Besenbacher, F. Hydrodesulfurization reaction pathways on MoS2 nanoclusters revealed by scanning tunneling microscopy. J. Catal. 2004, 224, 94–106. [Google Scholar] [CrossRef]
- Vázquez, P.; Pizzio, L.; Blanco, M.; Cáceres, C.; Thomas, H.; Arriagada, R.; Bendezú, S.; Cid, R.; García, R. NiMo(W)-based hydrotreatment catalysts supported on peach stones activated carbon. Appl. Catal. A Gen. 1999, 184, 303–313. [Google Scholar] [CrossRef]
- Vissers, J.P.R.; Scheffer, B.; de Beer, V.H.J.; Moulijn, J.A.; Prins, R. Effect of the support on the structure of Mo-based hydrodesulfurization catalysts: Activated carbon versus alumina. J. Catal. 1987, 105, 277–284. [Google Scholar] [CrossRef]
- Topsøie, H.; Candia, R.; Topsøe, N.-Y.; Clausen, B.S.; Topsøe, H. On the state of the Co-Mo-S model. Bull. Soc. Chim. Belg. 1984, 93, 783–806. [Google Scholar] [CrossRef]
- Hinnemann, B.; Nørskov, J.K.; Topsøe, H. A density functional study of the chemical differences between type I and type II MoS2-based structures in hydrotreating catalysts. J. Phys. Chem. B 2005, 109, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating catalysis. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1996; pp. 1–269. [Google Scholar]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, F.; Ren, H.; Jing, X.; Wang, W.; Ma, H.; Zhao, H.; Zhu, G. Targeted synthesis of a porous aromatic framework with a high adsorption capacity for organic molecules. J. Mater. Chem. 2011, 21, 13498–13502. [Google Scholar] [CrossRef]
- Maximov, A.; Zolotukhina, A.; Murzin, V.; Karakhanov, E.; Rosenberg, E. Ruthenium nanoparticles stabilized in cross-linked dendrimer matrices: Hydrogenation of phenols in aqueous media. ChemCatChem 2015, 7, 1197–1210. [Google Scholar] [CrossRef]
- Berhault, G.; Cota Araiza, L.; Duarte Moller, A.; Mehta, A.; Chianelli, R.R. Modifications of unpromoted and cobalt-promoted MoS2 during thermal treatment by dimethylsulfide. Catal. Lett. 2002, 78, 81–90. [Google Scholar] [CrossRef]
- Karakhanov, E.; Boronoev, M.; Ignatyeva, V.; Maximov, A.; Filippova, T.; Kardasheva, Y. Hydroprocessing of aromatics using sulfide catalysts supported on ordered mesoporous phenol-formaldehyde polymers. J. Inorg. Organomet. Polym. Mater. 2016, 1–6. [Google Scholar] [CrossRef]
- Ishutenko, D.; Nikulshin, P.; Pimerzin, A. Relation between composition and morphology of K(Co)MoS active phase species and their performances in hydrotreating of model FCC gasoline. Catal. Today 2016, 271, 16–27. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Jiang, F.; Chu, Y.; Nie, H. The relation between morphology of (Co)MoS2 phases and selective hydrodesulfurization for CoMo catalysts. Catal. Today 2010, 149, 35–39. [Google Scholar] [CrossRef]
- Lemberton, J.L.; Baudon, A.; Guisnet, M.; Marchal, N.; Mignard, S. Hydrocracking of C10 hydrocarbons over a sulfided NiMo/Y zeolite catalyst. In Studies in Surface Science and Catalysis; Froment, G.F., Delmon, B., Grange, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 106, pp. 129–136. [Google Scholar]
- Liu, C.; Wang, S.; An, C.; Yan, F.; He, J.; Wang, Z.; Zhang, Q.-H. Oil-soluble Ni-Mo sulfide nanoparticles and their hydrogenation catalytic properties. Pet. Sci. 2013, 10, 571–576. [Google Scholar] [CrossRef]
- Maximov, A.; Zolotukhina, A.; Kulikov, L.; Kardasheva, Y.; Karakhanov, E. Ruthenium catalysts based on mesoporous aromatic frameworks for the hydrogenation of arenes. React. Kinet. Mech. Catal. 2016, 117, 729–743. [Google Scholar] [CrossRef]
- McDonald, J.W.; Friesen, G.D.; Rosenhein, L.D.; Newton, W.E. Syntheses and characterization of ammonium and tetraalkylammonium thiomolybdates and thiotungstates. Inorg. Chim. Acta 1983, 72, 205–210. [Google Scholar] [CrossRef]



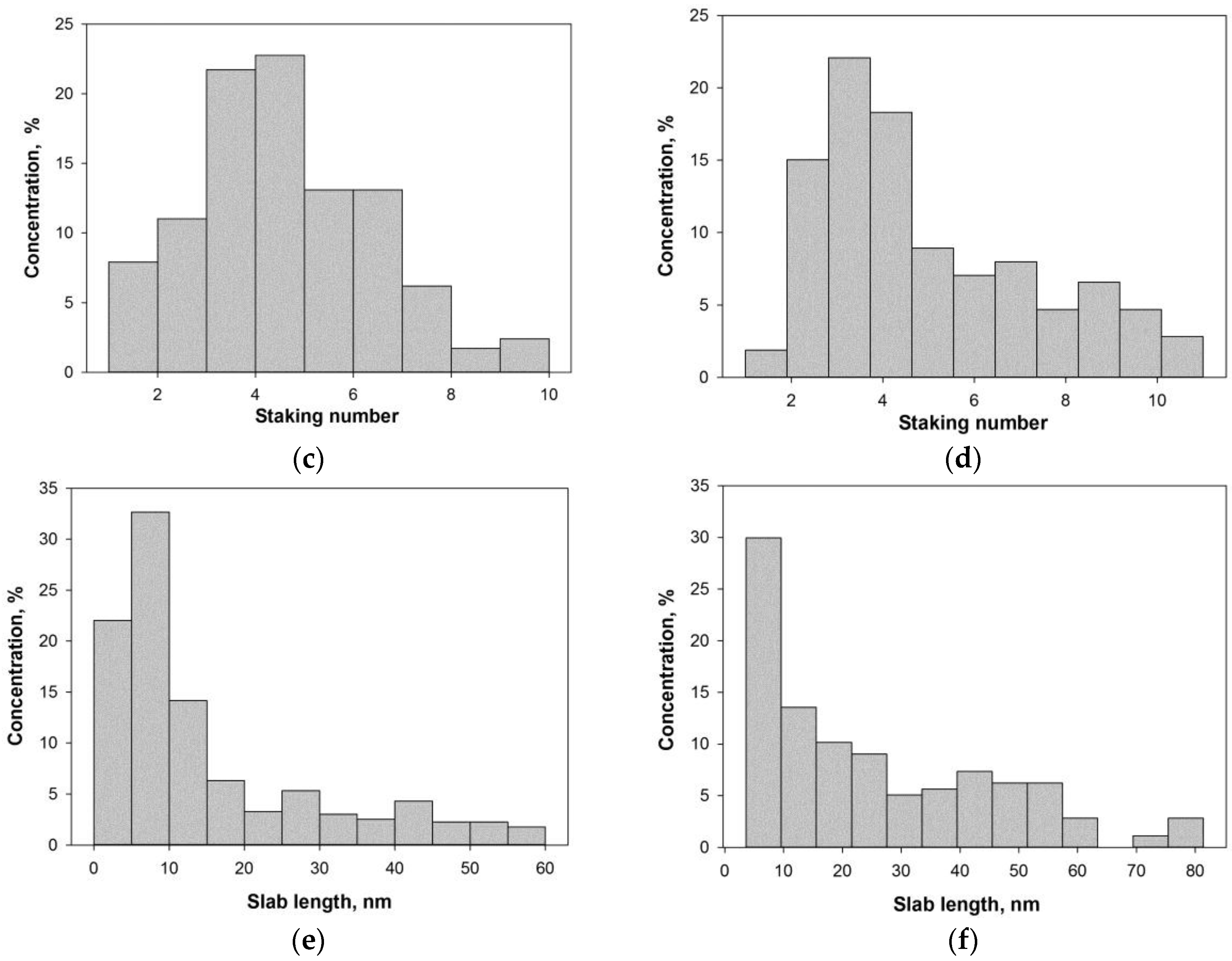





| Catalyst | , nm | D | fe/fc | |
|---|---|---|---|---|
| PAF-NiMoS | 25.5 ± 16.9 | 4.9 | 0.031 | 38.3 |
| PAF-NiWS | 15.2 ± 11.3 | 4.4 | 0.037 | 22.3 |
| Catalyst | , nm | MeSx | MeS2 | MeSxOy | Me6+ |
|---|---|---|---|---|---|
| PAF-20-NiWS | Binding energy (eV) | - | 32.6 4f7/2 | 33.5 4f7/2 | 36.0 4f7/2 |
| 34.2 4f5/2 | 35.3 4f5/2 | 38.1 4f5/2 | |||
| Weight fraction (%) | 3% | 3.5% | 93.5% | ||
| PAF-20-NiWS + S | Binding energy (eV) | 30.7 4f7/2 | 32.5 4f7/2 | 33.5 4f7/2 | 35.9 4f7/2 |
| 32.6 4f5/2 | 34.4 4f5/2 | 35.5 4f5/2 | 37.8 4f5/2 | ||
| Weight fraction (%) | 12.7% | 40.3% | 7.0% | 40.0% | |
| PAF-20-NiMoS + S | Binding energy (eV) | - | 229.0 4f7/2 | 230.0 4f7/2 | 232.8 4f7/2 |
| 232.1 4f5/2 | 233.6 4f5/2 | 235.7 4f5/2 | |||
| Weight fraction (%) | 55% | 26.2% | 18.8% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakhanov, E.; Kardasheva, Y.; Kulikov, L.; Maximov, A.; Zolotukhina, A.; Vinnikova, M.; Ivanov, A. Sulfide Catalysts Supported on Porous Aromatic Frameworks for Naphthalene Hydroprocessing. Catalysts 2016, 6, 122. https://doi.org/10.3390/catal6080122
Karakhanov E, Kardasheva Y, Kulikov L, Maximov A, Zolotukhina A, Vinnikova M, Ivanov A. Sulfide Catalysts Supported on Porous Aromatic Frameworks for Naphthalene Hydroprocessing. Catalysts. 2016; 6(8):122. https://doi.org/10.3390/catal6080122
Chicago/Turabian StyleKarakhanov, Eduard, Yulia Kardasheva, Leonid Kulikov, Anton Maximov, Anna Zolotukhina, Maria Vinnikova, and Andrey Ivanov. 2016. "Sulfide Catalysts Supported on Porous Aromatic Frameworks for Naphthalene Hydroprocessing" Catalysts 6, no. 8: 122. https://doi.org/10.3390/catal6080122
APA StyleKarakhanov, E., Kardasheva, Y., Kulikov, L., Maximov, A., Zolotukhina, A., Vinnikova, M., & Ivanov, A. (2016). Sulfide Catalysts Supported on Porous Aromatic Frameworks for Naphthalene Hydroprocessing. Catalysts, 6(8), 122. https://doi.org/10.3390/catal6080122