Synthesis, Characterization, and Catalytic Hydrogenation Activity of New N-Acyl-Benzotriazole Rh(I) and Ru(III) Complexes in [bmim][BF4]
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Ligand and Complexes
2.1.1. Ligand Characterization
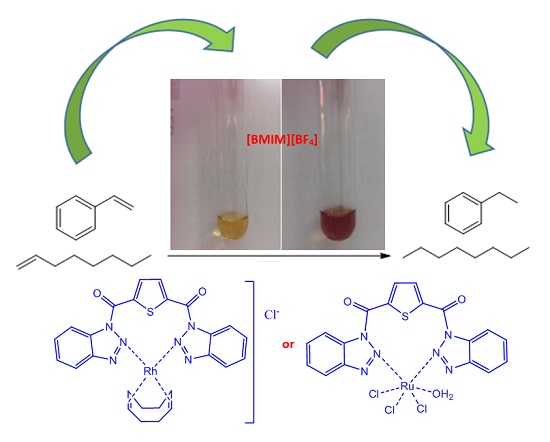
2.1.2. Rhodium Complex Characterization
2.1.3. Ruthenium Complex Characterization
2.2. Hydrogenation Activity of Complexes
2.2.1. Catalytic Activity of Rhodium Catalyst
2.2.2. Catalytic Activity of Ruthenium Catalyst
3. Materials and Methods
3.1. General
3.2. Synthesis of Ligand and Complexes
3.2.1. Ligand Synthesis
3.2.2. Synthesis of [Rh(COD)L]Cl Catalyst
3.2.3. Synthesis of [Ru(L)(H2O)Cl3]
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilkes, J.S. Properties of ionic liquid solvents for catalysis. J. Mol. Catal. A 2004, 214, 11–17. [Google Scholar] [CrossRef]
- Muskawar, P.; Thenmozhi, K.; Bhagat, P. Designing of thermally stable amide functionalized benzimidazolium perchlorate ionic liquid for transamidation of primary carboxamides. Appl. Catal. A 2015, 493, 158–167. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, D. Cycling performance of lithium polymer cells assembled by in situ polymerization of a non-flammable ionic liquid monomer. Electrochim. Acta 2013, 106, 460–464. [Google Scholar] [CrossRef]
- Li, X.; Cao, R. Selective oxidation of alcohols with H2O2 catalyzed by long chain multi-SO3H functionalized heteropolyanion-based ionic liquids under solvent-free conditions. Catal. Commun. 2015, 69, 5–10. [Google Scholar] [CrossRef]
- Lentini, S.; Galloni, P.; Bosch, I. Ionic liquids as reaction media in catalytic oxidations with manganese and iron pyridyl triazacyclononane complexes. Inorg. Chim. Acta 2014, 410, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Arras, J.; Ruppert, D. Supported ruthenium catalysed selective hydrogenation of citral in presence of [NTf2] based ionic liquids. Appl. Catal. A 2009, 371, 73–77. [Google Scholar] [CrossRef]
- Floris, T.; Kluson, P. Quaternary ammonium salts ionic liquids for immobilization of chiral Ru-BINAP complexes in asymmetric hydrogenation of β-ketoesters. Appl. Catal. A 2009, 366, 160–165. [Google Scholar] [CrossRef]
- Norinder, J.; Rodrigues, C. Tandem hydroformylation–acetalization with a ruthenium catalyst immobilized in ionic liquids. J. Mol. Catal. A 2014, 391, 139–143. [Google Scholar] [CrossRef]
- Wessercheid, P.; Waffenschmidt, H. Ionic liquids in regioselective platinum-catalysed hydroformylation. J. Mol. Catal. A 2000, 164, 61–67. [Google Scholar] [CrossRef]
- Azizov, A.G.; Kalbalieva, E.S. Oligomerization of hexene-1 in the presence of catalytic dystems on the basis of ionic liquids. Petroleum Chem. 2010, 50, 57–65. [Google Scholar] [CrossRef]
- Stenzel, O.; Brüll, R. Oligomerization of olefins in a chloroaluminate ionic liquid. J. Mol. Catal. A 2003, 192, 217–222. [Google Scholar] [CrossRef]
- Dupont, J.; de Souza, R.F.; Suarez, P.A. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [PubMed]
- Chauvin, Y.; Mussmann, L.; Olivier, H. A novel class of versatile solvents for two-phase catalysis: Hydrogenation, isomerization, and hydroformylation of alkenes catalyzed by rhodium complexes in liquid 1,3-dialkylimidazoliurn salts. Angew. Chem. Int. Ed. 1995, 34, 23–24. [Google Scholar] [CrossRef]
- Verma, A.; Jha, R.; Chaudhary, R. 2-(1-Benzotriazolyl)pyridine: A robust bidentate ligand for the palladium catalyzes C–C (Suzuki, Heck, Fujiwara-Moritani, Sonogashira), C–N and C–S coupling reactions. Adv. Synth. Catal. 2013, 355, 421–438. [Google Scholar]
- Das, D.; Singh, P.; Singh, A.K. Palladium and half sandwich ruthenium(II) complexes of selenated and tellurated benzotriazoles: Synthesis, structural aspects and catalytic applications. J. Organomet. Chem. 2010, 695, 955–962. [Google Scholar] [CrossRef]
- Richardson, C.; Stell, P.J. Benzotriazole as a structural component in chelating and bridging heterocyclic ligands; ruthenium, palladium, copper and silver complexes. Dalton Trans. 2003. [Google Scholar] [CrossRef]
- Christiansen, A.; Selent, D.; Spannenberg, A. Reaction of secondary phosphine oxides with rhodium(I). Organometallics 2010, 29, 3139–3145. [Google Scholar] [CrossRef]
- Takahashi, Y. Synthesis of various cationic ruthenium-aqua complexes with hydrotris(3,5-diisopropylpyrazolyl)borate ligand, TpiPrRu(OH2)n(L)3-n·(CF3SO3)(THF)x, and their structures supported by hydrogen-bonding networks. Inorg. Chem. 1998, 37, 3186–3194. [Google Scholar] [CrossRef]




| Entry | T (K) | PH2 (bar) | t (h) | ns/nc | Total Conversion (%) | TON | TOF |
|---|---|---|---|---|---|---|---|
| 1 | 343 | 10 | 1 | 106 | 18.3 | 20 | 20 |
| 2 | 353 | 10 | 1 | 106 | 19.2 | 21 | 21 |
| 3 | 363 | 10 | 1 | 106 | 24.4 | 26 | 26 |
| 4 | 373 | 10 | 1 | 106 | 24.6 | 26 | 26 |
| 5 | 383 | 10 | 1 | 106 | 35.8 | 38 | 38 |
| 6 | 393 | 10 | 1 | 106 | 39.7 | 43 | 43 |
| 7 | 393 | 10 | 6 | 106 | 100 | 107 | 18 |
| 8 b | 393 | 10 | 6 | 106 | 22.9 | 25 | 4 |
| 9 c | 393 | 10 | 6 | 106 | 100 | 107 | 18 |
| 10 | 343 | 30 | 1 | 106 | 41.6 | 45 | 45 |
| 11 | 343 | 60 | 1 | 106 | 63.4 | 68 | 68 |
| 12 | 393 | 10 | 6 | 531 | 43.5 | 233 | 39 |
| 13 | 393 | 10 | 6 | 177 | 82.4 | 148 | 25 |
| 14 d | 393 | 10 | 6 | 106 | 78.6 | 85 | 14 |
| Entry | T (K) | Total Conversion (%) | Products (%) | TON | TOF | ||
|---|---|---|---|---|---|---|---|
| (n-Octane) | (2-Octene) | (3-Octene) | |||||
| 1 | 323 | 10.5 | 3.7 | 4.2 | 2.5 | 83 | 83 |
| 2 | 333 | 20.8 | 5.9 | 9.1 | 5.8 | 16 | 16 |
| 3 | 343 | 28.9 | 15.2 | 7.7 | 6.0 | 23 | 23 |
| 4 | 353 | 36.8 | 18.2 | 11.1 | 7.5 | 29 | 29 |
| 5 | 363 | 65.1 | 30.7 | 20.4 | 14.1 | 51 | 51 |
| 6 | 373 | 86.7 | 46.0 | 24.3 | 16.4 | 68 | 68 |
| 7 | 383 | 100 | 62.5 | 26.6 | 10.9 | 79 | 79 |
| 8 b | 383 | 55.2 | 27.2 | 17.7 | 10.3 | 43 | 43 |
| 9 c | 383 | 81.3 | 52.3 | 18.4 | 10.6 | 64 | 64 |
| 10 d | 383 | 62.9 | 31.3 | 18.2 | 13.4 | 49 | 49 |
| Entry | T (K) | PH2 (bar) | t (h) | ns/nc | Total Conversion (%) | TON | TOF |
|---|---|---|---|---|---|---|---|
| 1 | 363 | 10 | 1 | 103 | 12.6 | 13 | 13 |
| 2 | 373 | 10 | 1 | 103 | 22.2 | 23 | 23 |
| 3 | 383 | 10 | 1 | 103 | 22.9 | 24 | 24 |
| 4 | 393 | 10 | 1 | 103 | 34.8 | 36 | 36 |
| 5 | 393 | 10 | 6 | 103 | 84.3 | 88 | 15 |
| 6 b | 393 | 10 | 6 | 103 | 19.1 | 20 | 3 |
| 7 c | 393 | 10 | 6 | 103 | 43.1 | 45 | 7 |
| 8 | 373 | 30 | 1 | 103 | 95.7 | 100 | 100 |
| 9 | 373 | 60 | 1 | 103 | 100 | 104 | 104 |
| 10 | 393 | 10 | 6 | 515 | 47.2 | 246 | 41 |
| 11 | 393 | 10 | 6 | 172 | 54.9 | 95 | 16 |
| 12 d | 393 | 10 | 6 | 103 | 71.6 | 75 | 12 |
| Entry | T (K) | Total Conversion (%) | Products (%) | TON | TOF | ||
|---|---|---|---|---|---|---|---|
| (n-Octane) | (2-Octene) | (3-Octene) | |||||
| 1 | 373 | 2.5 | 2.5 | 0 | 0 | 2 | 0.3 |
| 2 | 383 | 3.2 | 3.2 | 0 | 0 | 2 | 0.4 |
| 3 | 393 | 4.4 | 4.4 | 0 | 0 | 3 | 0.6 |
| 4 b | 393 | 6 | 1.8 | 0 | 0 | 5 | 0.8 |
| 5 c | 393 | 53.7 | 11.5 | 28.4 | 13.8 | 41 | 7 |
| 6 d | 393 | 75.5 | 65.8 | 7.2 | 2.5 | 58 | 10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ünver, H.; Yılmaz, F. Synthesis, Characterization, and Catalytic Hydrogenation Activity of New N-Acyl-Benzotriazole Rh(I) and Ru(III) Complexes in [bmim][BF4]. Catalysts 2016, 6, 147. https://doi.org/10.3390/catal6090147
Ünver H, Yılmaz F. Synthesis, Characterization, and Catalytic Hydrogenation Activity of New N-Acyl-Benzotriazole Rh(I) and Ru(III) Complexes in [bmim][BF4]. Catalysts. 2016; 6(9):147. https://doi.org/10.3390/catal6090147
Chicago/Turabian StyleÜnver, Hakan, and Filiz Yılmaz. 2016. "Synthesis, Characterization, and Catalytic Hydrogenation Activity of New N-Acyl-Benzotriazole Rh(I) and Ru(III) Complexes in [bmim][BF4]" Catalysts 6, no. 9: 147. https://doi.org/10.3390/catal6090147
APA StyleÜnver, H., & Yılmaz, F. (2016). Synthesis, Characterization, and Catalytic Hydrogenation Activity of New N-Acyl-Benzotriazole Rh(I) and Ru(III) Complexes in [bmim][BF4]. Catalysts, 6(9), 147. https://doi.org/10.3390/catal6090147