Synthesis of Magnetic Carbon Supported Manganese Catalysts for Phenol Oxidation by Activation of Peroxymonosulfate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Fe3O4/C/Mn Hybrids
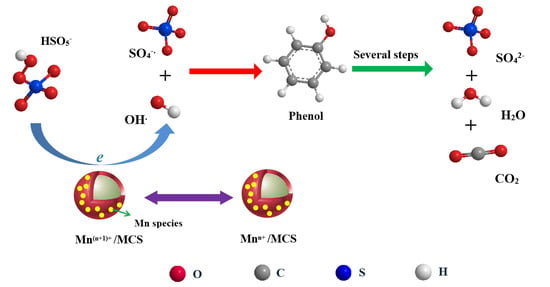
2.2. Catalytic Oxidation of Phenol
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Manganese Loaded Magnetic Carbon Spheres (Fe3O4/C/Mn)
3.3. Characterization of Materials
3.4. Catalytic Activity Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Gupta, V.K.; Srivastava, S.K.; Tyagi, R. Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res. 2000, 34, 1543–1550. [Google Scholar] [CrossRef]
- Heng, S.; Yeung, K.L.; Julbe, A.; Ayral, A.; Schrotter, J.-C. Preparation of composite zeolite membrane separator/contactor for ozone water treatment. Microporous Mesoporous Mater. 2008, 115, 137–146. [Google Scholar] [CrossRef]
- Tušar, N.N.; Maučec, D.; Rangus, M.; Arčon, I.; Mazaj, M.; Cotman, M.; Pintar, A.; Kaučič, V. Manganese functionalized silicate nanoparticles as a fenton-type catalyst for water purification by advanced oxidation processes (AOP). Adv. Funct. Mater. 2012, 22, 820–826. [Google Scholar] [CrossRef]
- Wang, S. A comparative study of fenton and fenton-like reaction kinetics in decolourisation of wastewater. Dyes Pigm. 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Watts, R.; Sarasa, J.; Loge, F.; Teel, A. Oxidative and reductive pathways in manganese-catalyzed fenton’s reactions. J. Environ. Eng. 2005, 131, 158–164. [Google Scholar] [CrossRef]
- Hu, L.; Yang, X.; Dang, S. An easily recyclable Co/SBA-15 catalyst: Heterogeneous activation of peroxymonosulfate for the degradation of phenol in water. Appl. Catal. B 2011, 102, 19–26. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ. Sci. Technol. 2005, 40, 1000–1007. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; MosbÆK, H.; Siegrist, R.L.; Bjerg, P.L. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit. Rev. Env. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tadé, M.O. Nanosized Co3O4/SiO2 for heterogeneous oxidation of phenolic contaminants in waste water. Sep. Purif. Technol. 2011, 77, 230–236. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. A comparative study of spinel structured Mn3O4, Co3O4 and Fe3O4 nanoparticles in catalytic oxidation of phenolic contaminants in aqueous solutions. J. Colloid Interface Sci. 2013, 407, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tadé, M.O.; Wang, S.B. Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environ. Sci. Technol. 2013, 47, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, S.; Zhou, G.; Ang, H.M.; Tadé, M.O.; Wang, S. Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants. ACS Appl. Mater. Interfaces 2012, 4, 5466–5471. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, Z.; Sun, H.; Wang, S. Hydrothermal synthesis of Co3O4-graphene for heterogeneous activation of peroxymonosulfate for decomposition of phenol. Ind. Eng. Chem. Res. 2012, 51, 14958–14965. [Google Scholar] [CrossRef]
- Gong, J.-L.; Wang, B.; Zeng, G.-M.; Yang, C.-P.; Niu, C.-G.; Niu, Q.-Y.; Zhou, W.-J.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhan, Y.; Cai, B.; Hao, C.; Wang, J.; Liu, C.; Meng, Z.; Yin, Z.; Chen, Q. Shape-controlled synthesis of Mn3O4 nanocrystals and their catalysis of the degradation of methylene blue. Nano Res. 2010, 3, 235–243. [Google Scholar] [CrossRef]
- Kaminski, M.D.; Nuñez, L. Extractant-coated magnetic particles for cobalt and nickel recovery from acidic solution. J. Magn. Magn. Mater. 1999, 194, 31–36. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Shao, D.; Li, J.; Wang, X. Adsorption behavior of multiwall carbon nanotube/iron oxide magnetic composites for Ni(II) and Sr(II). J. Hazard. Mater. 2009, 164, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.J.; Miao, S.D.; Liu, S.Z.; Ma, L.P.; Sun, H.Q.; Wang, S.B. Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, J.; Song, X.; Wang, G.; Yang, W. Facile synthesis of Fe3O4/SiO2 composite nanoparticles from primary silica particles. Colloids Interface A 2008, 317, 450–456. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, R.; Wang, W.; Guo, Y.; Cui, P.; Song, W. Controllable synthesis and magnetic properties of Fe3O4 and Fe3O4@SiO2 microspheres. J. Mater. Sci 2010, 45, 5347–5352. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Magnetic Fe3O4/carbon sphere/cobalt composites for catalytic oxidation of phenol solutions with sulfate radicals. Chem. Eng. J. 2014, 245, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Synthesis of magnetic core/shell carbon nanosphere supported manganese catalysts for oxidation of organics in water by peroxymonosulfate. J. Colloid Interface Sci. 2014, 433, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Mukherjee, N.; Ahammed, S.; Ranu, B.C. Highly selective reduction of nitroarenes by iron(0) nanoparticles in water. Chem. Commun. 2012, 48, 7982–7984. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, D.; Lou, X.W. Shape-controlled synthesis of MnO2 nanostructures with enhanced electrocatalytic activity for oxygen reduction. J. Phys. Chem. C 2009, 114, 1694–1700. [Google Scholar] [CrossRef]
- Xu, J.; Chu, W.; Luo, S. Synthesis and characterization of mesoporous V-MCM-41 molecular sieves with good hydrothermal and thermal stability. J. Mol. Catal. A: Chem. 2006, 256, 48–56. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Replication and coating of silica templates by hydrothermal carbonization. Adv. Funct. Mater. 2007, 17, 1010–1018. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Ai, Z.; Deng, K.; Wan, Q.; Zhang, L.; Lee, S. Facile microwave-assisted synthesis and magnetic and gas sensing properties of Fe3O4 nanoroses. J. Phys. Chem. C 2010, 114, 6237–6242. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Z.; Jia, D.; Guo, Z.; Cho, W.I. Preparation and characterization of core-shell structure Fe3O4/C nanoparticles with unique stability and high electrochemical performance for lithium-ion battery anode material. Electrochim. Acta 2011, 56, 9233–9239. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liang, H.; Zhou, G.; Wang, S. Supported cobalt catalysts by one-pot aqueous combustion synthesis for catalytic phenol degradation. J. Colloid Interface Sci. 2013, 394, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Bao, H.; Yao, Y.; Lu, W.; Chen, W. Novel green activation processes and mechanism of peroxymonosulfate based on supported cobalt phthalocyanine catalyst. Appl. Catal. B 2014, 154–155, 36–43. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Facile synthesis of hierarchically structured magnetic MnO2/ZnFe2O4 hybrid materials and their performance in heterogeneous activation of peroxymonosulfate. ACS Appl. Mater. Interfaces 2014, 6, 19914–19923. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Shape-controlled activation of peroxymonosulfate by single crystal α-Mn2O3 for catalytic phenol degradation in aqueous solution. Appl. Catal. B 2014, 154–155, 246–251. [Google Scholar] [CrossRef]
- Sun, H.; Tian, H.; Hardjono, Y.; Buckley, C.E.; Wang, S. Preparation of cobalt/carbon-xerogel for heterogeneous oxidation of phenol. Catal. Today 2012, 186, 63–68. [Google Scholar] [CrossRef]
- Shukla, P.R.; Wang, S.; Sun, H.; Ang, H.M.; Tadé, M. Activated carbon supported cobalt catalysts for advanced oxidation of organic contaminants in aqueous solution. Appl. Catal. B 2010, 100, 529–534. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Red mud and fly ash supported Co catalysts for phenol oxidation. Catal. Today 2012, 190, 68–72. [Google Scholar] [CrossRef]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tadé, M.O. Co-SBA-15 for heterogeneous oxidation of phenol with sulfate radical for wastewater treatment. Catal. Today 2011, 175, 380–385. [Google Scholar] [CrossRef]













| Catalyst | Surface Area (SBET m2/g) | Pore Volume (cm3/g) | First-Order Rate Constant (min−1) | R2 |
|---|---|---|---|---|
| Mn/MCS-R | 53.8 | 0.11 | 0.074 | 0.996 |
| Mn/MCS-I | 74.6 | 0.14 | 0.052 | 0.989 |
| Mn/MCS-H | 26.6 | 0.058 | 0.056 | 0.991 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, Y.; Chen, C.; Duan, X.; Sun, H.; Wang, S. Synthesis of Magnetic Carbon Supported Manganese Catalysts for Phenol Oxidation by Activation of Peroxymonosulfate. Catalysts 2017, 7, 3. https://doi.org/10.3390/catal7010003
Wang Y, Xie Y, Chen C, Duan X, Sun H, Wang S. Synthesis of Magnetic Carbon Supported Manganese Catalysts for Phenol Oxidation by Activation of Peroxymonosulfate. Catalysts. 2017; 7(1):3. https://doi.org/10.3390/catal7010003
Chicago/Turabian StyleWang, Yuxian, Yongbing Xie, Chunmao Chen, Xiaoguang Duan, Hongqi Sun, and Shaobin Wang. 2017. "Synthesis of Magnetic Carbon Supported Manganese Catalysts for Phenol Oxidation by Activation of Peroxymonosulfate" Catalysts 7, no. 1: 3. https://doi.org/10.3390/catal7010003
APA StyleWang, Y., Xie, Y., Chen, C., Duan, X., Sun, H., & Wang, S. (2017). Synthesis of Magnetic Carbon Supported Manganese Catalysts for Phenol Oxidation by Activation of Peroxymonosulfate. Catalysts, 7(1), 3. https://doi.org/10.3390/catal7010003