Abstract
The catalytic organosolv pulping of sugar maple bark was performed adopting the concept of forest biorefinery in order to transform bark into several valuable products. Our organosolv process, consisting of pre-extracting the lignocellulosic material followed by pulping with ferric chloride as a catalyst, was applied to sugar maple bark. The pre-extraction step has yielded a mixture of phenolic extractives, applicable as antioxidants. The organosolv pulping of extractives-free sugar maple bark yielded a solid cellulosic pulp (42.3%) and a black liquor containing solubilized bark lignin (24.1%) and products of sugars transformation (22.9% of hemicelluloses), mainly represented by furfural (0.35%) and 5-hydroxymethyl furfural (HMF, 0.74%). The bark cellulosic pulp was determined to be mainly constituted of glucose, with a high residual lignin content, probably related to the protein content of the original bark (containing cambium tissue). The biorefinery approach to the transformation of a solid bark residue into valuable biopolymers (lignin and cellulose) along with phenolic antioxidants from pre-extraction and the HMF derivatives from black liquor (applicable for 2,5-diformylfuran production) is an example of a catalytic process reposing on sustainable engineering and green chemistry concepts.
1. Introduction
The complete valorization of forest biomass residues through integrated biorefinery offers an opportunity for the sustainable and eco-friendly transformation of renewable materials. Bark residues, produced in huge amounts by the forest industry, are available for transformation through an integrated biorefinery process, which could yield new products for interesting applications. Indeed, the structural complexity of bark is a key feature to take into account when anticipating its uses. However, most of the industrially available bark is simply incinerated to meet energy needs of the industry, which has increased the utilization of the biomass for energy generation by 47% in the period between 2000 and 2013 [1]. The high ash content of bark is one of the reasons explaining the lack of interest for other forms of its transformation, even though the high content of mineral nutrients (especially Ca and K) does make this tree tissue an interesting reserve of bio-elements [2]. Indeed, the ash content of bark is between 4 and 8 times higher than that of the corresponding wood [3]. In addition, tree barks are usually rich in phenolic extractives, which can be used as a source of natural antioxidants. Thus, we have studied Canadian hardwood tree barks [4,5,6] as potential sources of antioxidants, as has also been done in the study on the bioactive properties of the extractives of the bark of Q. acutissima Carruth as a potential source of natural antioxidants [7]. Our previous studies on red maple bark and wood extracts have demonstrated the high polyphenol content and radical scavenging activity of red maple bark extract [5]. Besides ash and extractive compounds, the structural components of bark cell walls, lignin, and polysaccharides could also be isolated from bark and valorized. Indeed, lignin content in bark was evaluated at 27.9% for Picea abies and at 33.7% for Pinus sylvestris [8], while Fagus crenata and Quercus mongolica bark were determined to contain 34.6% and 24.9% of total lignin, respectively [9]. A comparative study of different lignins from Eucalyptus globulus was performed to evaluate the relationship between the process of lignin isolation and its structure [10]. That study had revealed that 21% of lignin was recovered by kraft and 26% by organosolv process from eucalyptus wood. Lignin extraction by acidolysis yielded 31% from wood and 33% from bark of eucalyptus. Wood and bark lignin obtained in that study were determined to have similar structures, in particular for the organosolv lignins. The organosolv process has thus been demonstrated to be adequate for efficient lignin recovery. On the other hand, the strong association between polysaccharides and tannins in bark causes their incomplete hydrolysis and therefore imposes difficulties in the commercial applications of bark [11]. However, the relatively easy mechanical fibrillation of cellulose from bark makes it available for the production of cellulose nanofibers [12]. Water soluble products originating from hemicelluloses included levoglucosan, acetic acid, formic acid, and furfural, along with some aromatic compounds [13]. Furfural and HMF are available from the acid hydrolysis/dehydration of cellulose and hemicelluloses from bark [14]. An important potential application of hemicelluloses is 2,5-Diformylfuran (DFF) production from 5-hydroxymethyl furfural (HMF) oxidation. Indeed, DFF represents an important building block for the manufacture of new polymers that may replace existing materials derived from fossil fuel resources [15]. A comparative study of the different pulping processes of bark revealed that organosolv pulping with ethanol/water solvent was an efficient method for the fractionation of birch bark [16]. We have demonstrated in our previous study that a new catalytic orgaonsolv process is well suited to the isolation of high purity lignin from trembling aspen wood [17]. The catalytic lignocellulosic fractionation of lignocellulosics has been a focus of multiple studies in recent years, as well as the topic of a recent review [18]. Thus, we have applied in the present research the approach of forest biorefinery, applying the catalytic organosolv process to ferric chloride, in order to get access to a new organosolv lignin from bark, along with cellulosic pulp and phenolic extractives. Thus, adopting a biorefinery concept, we are proposing to valorize all components of the sugar maple bark, extractives, organosolv lignin, and cellulosic pulp, while adding a trial of HMF transformation into DFF by residual liquor treatment.
2. Results and Discussion
The organosolv process applied to extractives-free bark particles was previously described [17,19,20]. The catalyst choice for our organosolv process strategy was inspired by natural system of peroxidase enzyme. Indeed, peroxidases are enzymes which are capable of oxidizing the phenolic lignin substrates or anilines, along with a variety of non-phenolic lignin subunits [21]. Lignin peroxidases contain eight cysteine residues forming disulphide bridges [22]. Iron atom of the heme of lignin peroxidases ensures the coordination bond between histidine residues, stabilized by hydrogen bond. Thus, during the enzymatic activity of lignin peroxidase in the presence of H2O2 or Manganese (for manganese peroxidase), the iron from the heme site evolves from Fe (+III) to Fe (+IV). Thus, iron chloride III has been selected to mimic the catalytic activity of peroxidase.
Prior to organosolv pulping, the chemical composition of bark of sugar maple was determined following the standard procedures, as previously reported [20]. The results presented in the previous study indicated that the total carbohydrates (glucan and xylan) represented the main components of sugar maple bark followed by lignin, along with protein, estimated from nitrogen determination at 2.44% (0.39% of nitrogen content), which is related to living cells constituting cambium tissue in bark. Indeed, the chemical composition analyses of sugar maple bark [20] indicated a total lignin content of 29.6% while glucan and xylan represented 23.7% and 15.9%, respectively.
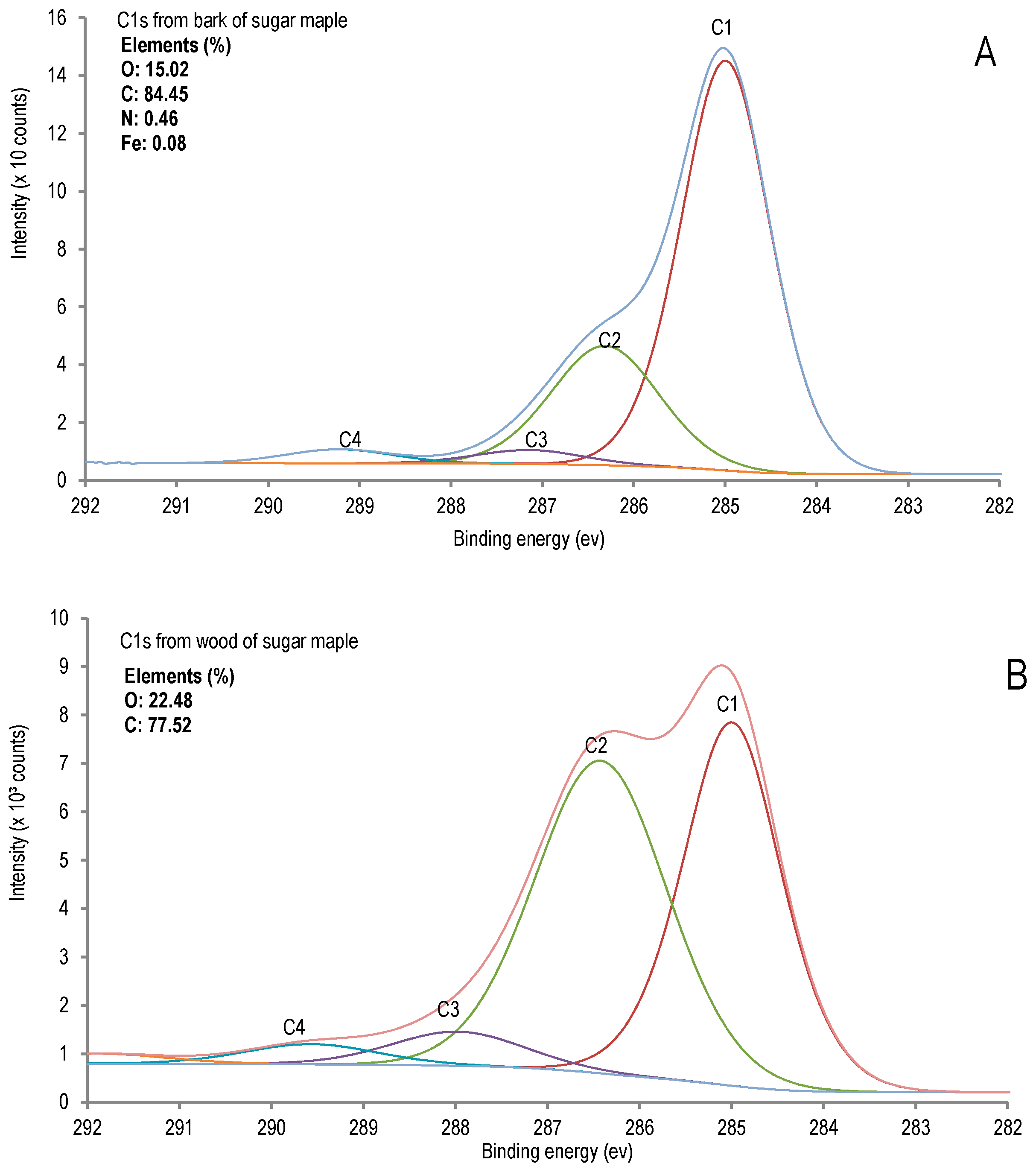
A comparative X-ray photoelectron spectroscopy (XPS) study was performed to evaluate the chemical differences between sugar maple bark and wood (Figure 1).
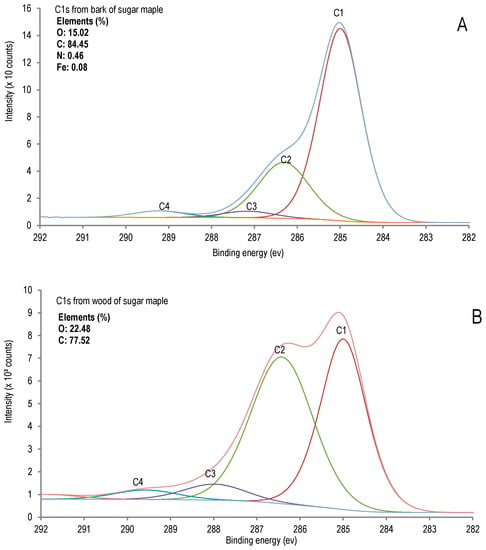
Figure 1.
X-ray photoelectron spectroscopy (XPS) analysis of sugar maple. (A) Bark; (B) wood.
The chemical composition of bark analysis confirmed the presence of nitrogen in original bark (0.39% of nitrogen in O.D bark, determined in this research), and it is also determined in organosolv pulp and lignin of bark, as presented in the Tables 3 and 4. Bark is much richer in C-C carbon than wood and contains nitrogen which is not detected in wood. The elemental analysis by XPS revealed nitrogen in bark contrary to wood in which it was not detected, along with a significant amount of iron (0.08%), (Figure 1). Regarding the C1s component (corresponding to C-C bonds) in bark, C1 bonds (69.91%) were more important than C2 (24.38%), whereas these contents were determined to be comparable in wood (Table 1).

Table 1.
Comparative XPS between bark and wood of sugar maple.
This result indicates that more condensed substructures are present in bark constituents such as, for example, the 5-5 units in lignin or in extractives (condensed tannins) in the case of bark. Indeed, high total extractives, determined by ethanol-toluene extraction followed by hot water extraction with 1% of sodium hydroxide solution [20], are a result comparable to that reported from our previous study on red maple bark [5]. As discussed further, condensed tannins are quantified in the studied bark extracts by spectrophotometry as proanthocyanidines (in terms of cyaniding chloride equivalents). Since the XPS data are related only to surface, in order to be able to get more information on the bulk composition additional analytical techniques would be required, such as CP/MAS NMR and ICP. Nevertheless, we considered that the XPS analyses provided interesting results for comparative purposes, particularly as complementary results to the other tests for the important finding of this nitrogen content of bark, which is also determined both in organosolv lignin and cellulosic pulp.
2.1. Bark Extractives from Ethanol–Water Pretreatment
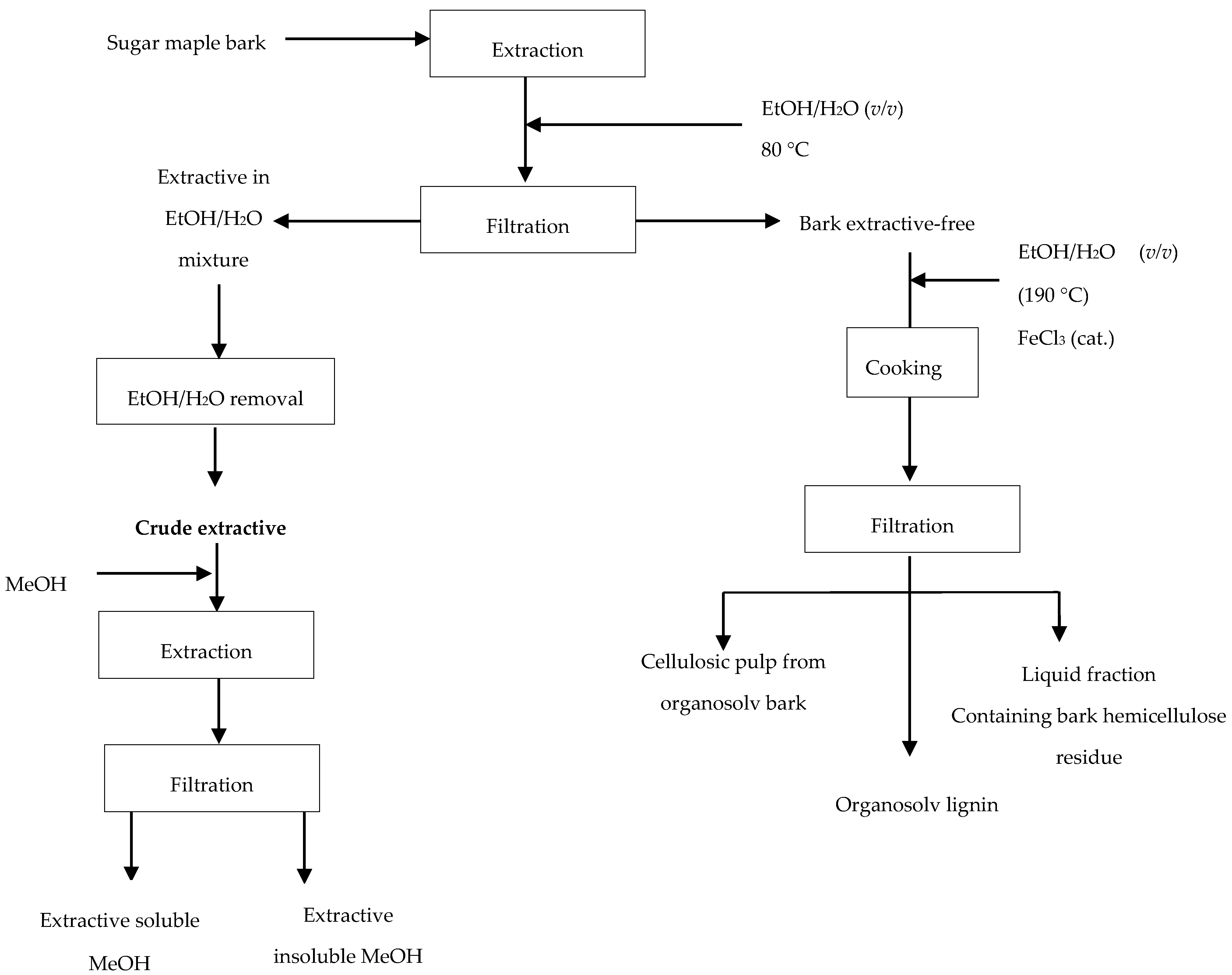
Our previous studies on catalytic organosolv pulping performed on extractive-free trembling aspen wood [17] have demonstrated access to a high purity lignin. Likewise, prior to organosolv process applied to sugar maple bark studied here, extractives were removed by extraction with ethanol–water mixture (1:1, v/v) at 80 °C for 6 h. The crude sugar maple bark extract obtained after solvent removal was fractionated into soluble and insoluble methanol fractions prior to chemical composition analyses being performed, as presented in Figure 2.
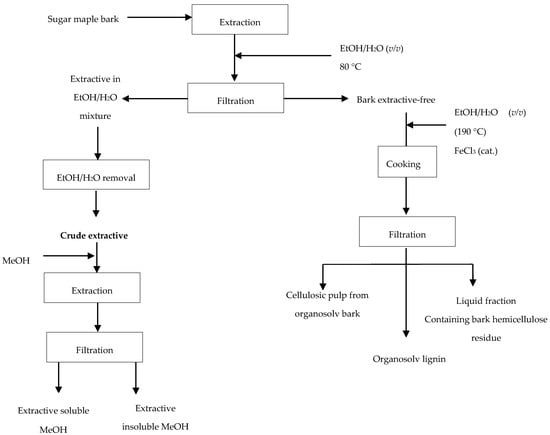
Figure 2.
Diagram of biorefinery strategy.
After fractionation, the methanol-insoluble fraction was poured into a mixture of methanol–water (9/1); v/v), which revealed the presence of crystals. These crystals were analysed by XRD analysis and determined to be halite crystal from NaCl (see Figure S1 in Supplementary Materials for XRD spectrum and MEB). Extraction yield, total phenols, and proanthocyanidin contents of sugar maple bark are presented in Table 2.

Table 2.
Chemical composition of sugar maple bark extracts.
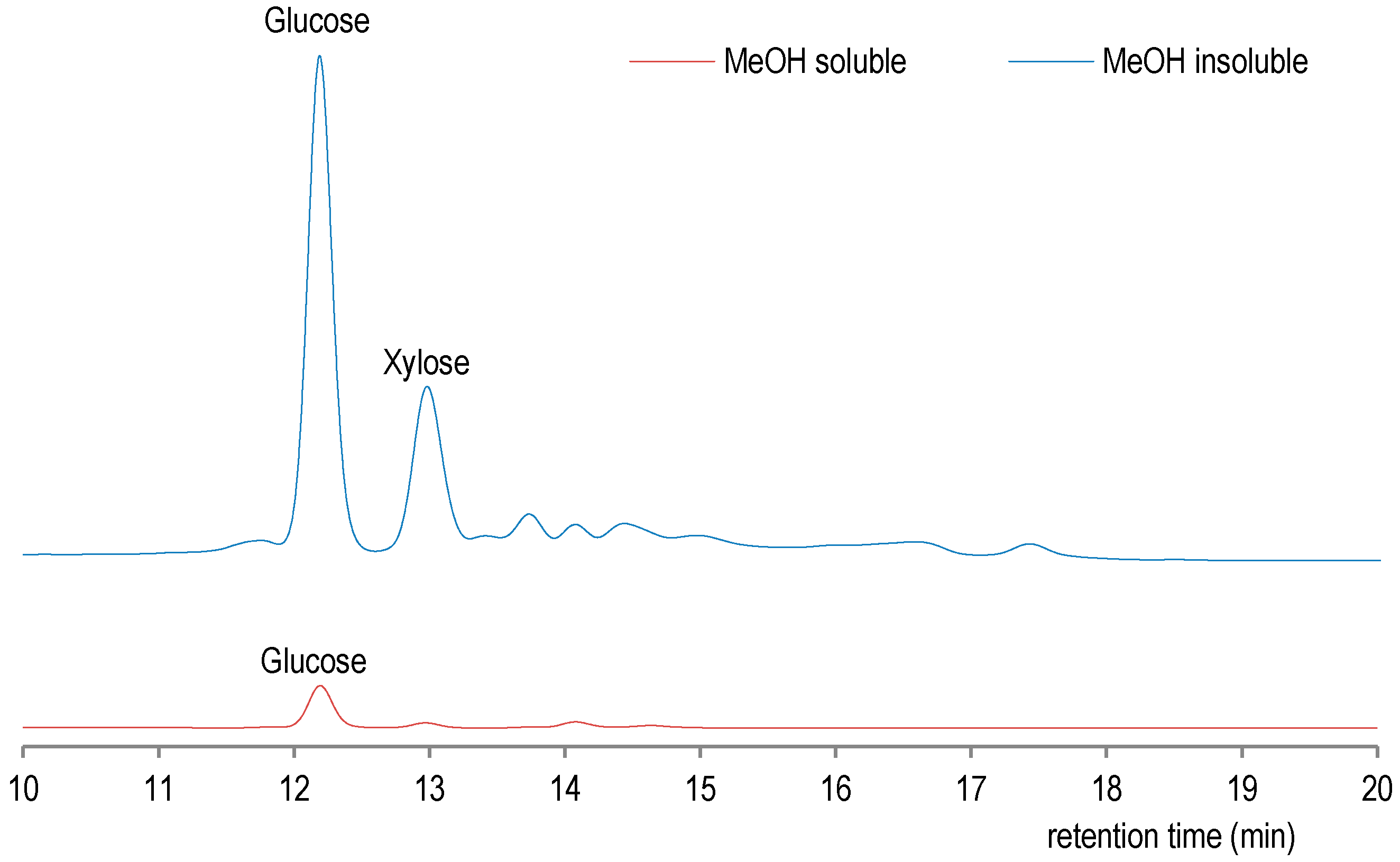
The results on total phenols and proanthocyanidin content of methanol fractions of sugar maple bark ethanol–water extract indicate that methanol-soluble fraction (50.6 mg CChE/g of dry extract) is rich in proanthocyanidines (condensed tannins). Indeed, in our previous study on ethanol–water extraction of sugar maple bark, the total phenols content was determined at 170 of gallic acid equivalents/g extract, while for proanthocyanidines at 35 mg, CChE/g of dry extract has been determined [6]. The acid hydrolysis of methanol-soluble and methanol-insoluble fraction followed by high performance liquid chromatography (HPLC) (Figure 3) analysis has demonstrated the presence of sugar residues, which are particularly important in methanol-insoluble fraction of the crude ethanol–water sugar maple bark extract.
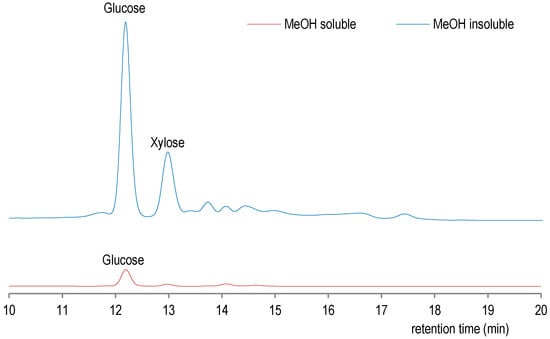
Figure 3.
Comparative HPLC analysis for sugar content in methanol and water soluble fraction.
As shown in Figure 3, methanol-soluble fraction contains a few sugars with glucose as major monosaccharide, while in methanol-insoluble fraction there are glucose and xylose which are detected in higher concentrations. On the other hand, the higher level of total phenol determined by spectrophotometry in methanol-soluble fraction (Table 2) was also confirmed by GC-MS analysis of silylated methanol-soluble fraction (See Figure S2 Supplementary Materials for GC-MS analysis), thus confirming the well-known affinity of polyphenols for methanol as solvent [23]. Thus, methanol-soluble fraction could be used for its antioxidant activity, which is usually correlated with total phenols content [24]. Indeed in GC-MS analysis, methanol-soluble fraction of ethanol–water extract was determined to contain more constituents, which include phenolic, acid, and protein derivatives. Indeed, the phenolic amines, such as tyramine derivative, have already been reported for root bark of Lycium chinense [25]. In methanol-insoluble fraction, only phenolic acids were detected (see Figure S2 in Supplementary Materials). These findings are consistent with somewhat lower total phenols determined in methanol-insoluble fraction, as presented in Table 2.
2.2. Organosolv Fractionation
The effects of organosolv pulping with ferric chloride as catalyst of extractives-free sugar maple bark were evaluated in terms of organosolv pulp yield, analysis of residual liquor, and properties of organosolv lignin precipitated from residual liquor. Typically, the extractives-free bark was treated using ethanol–water mixture (1:1, v/v; 0.5 L of final volume for 50 g of bark) in Parr reactor series 4842 (2 L) using ferric chloride as a catalyst at 190 °C for 90 min (9 mmol of FeCl3·6H2O). After cooking, the reaction mixture was cooled. The obtained organosolv pulp was separated by filtration and washed with ethanol (3 × 200 mL) to recover lignin. After removal of ethanol by evaporation, the organosolv lignin was precipitated from spent liquor by acidification with 2 M HCl to pH = 1.5. The precipitated organosolv lignin was filtered and dried, as described previously [20].
2.2.1. Organosolv Lignin
Organosolv lignin was obtained in form of a solid brown powder. The results of chemical analyses performed on organosolv lignin from sugar maple bark are summarized in Table 3, as previously reported, except for the nitrogen content which was determined in this research [20].

Table 3.
Chemical properties of sugar maple bark lignin obtained by organosolv pulping of pre extracted bark.
Organosolv lignin was determined to contain a high Klason lignin (94.4% including protein content), indicating a good recovery of high purity organosolv lignin, with a relatively higher level of acid-soluble lignin (3.5%). The residual sugars are also present in important amount (1.5% of glucan). For high level of acid-soluble lignin, the explanation could be proposed by suberin depolymerization during organosolv pulping. Indeed, suberin and lignin were determined to be the two major phenolic components of bark [26]. Suberin depolymerization could promote the release of organic acids such as phenolic and fatty acids, which could interfere with acid-soluble lignin determination. This result, along with somewhat higher residual sugars, is also completed by high level of residual ferric chloride, as detected by ICP analysis (Table S1 in Supplementary Materials). Indeed, this high level of ferric chloride from catalyst could form a complex with phenol, which absorbs also in UV, as measured by spectrophotometry. This kind of complex could also be measured as acid-soluble lignin, which uses UV spectrophotometry for quantification.
2.2.2. Cellulosic Pulp from Sugar Maple Bark
The cellulosic pulp obtained by organosolv pulping from sugar maple bark was separated from spent liquor by filtration following previously described procedure [17]. The averages of three measurements of properties of sugar maple bark organosolv pulp are presented in Table 4.

Table 4.
Constituents of cellulosic pulp obtained from bark of sugar maple.
The organosolv pulp obtained from sugar maple bark contained low glucan content (43.2%), corresponding to cellulose, with high residual lignin and ash contents and no detected xylan. It is interesting to remark that calcium oxalate was isolated at high yield (10.3%) upon washing the sugar maple bark pulp with water (See Figure S3 in Supplementary Materials for MEB image). Indeed, there are other reports on presence of calcium oxalate in hardwood barks, as it was described for bark phloem of teak [2]. The chemical composition of ashes from sugar maple bark (5.2%) before pulping [20] and cellulosic pulp were analysed by ICP analysis, and calcium was determined as major element (Table S2 in Supplementary Materials). Indeed in ashes from bark, calcium content (87,391 mg/kg) seems to be concentrated in cellulosic pulp (52,702 mg/kg) after organosolv process. This result can be explained by the presence of calcium oxalate crystals, as indicated by their precipitation upon washing of the cellulosic pulp from sugar maple bark. On the other hand, the high residual lignin content in pulp (Klason lignin of pulp at 31.1% and when corrected for protein at 27.5%) could be attributed to condensed structure of bark lignin with 5-5 and other C-C linked substructures which could have survived pulping reactions. The higher nitrogen content of bark could be related to an important presence of meristematic tissues in bark [27]. Proteins are likely to condense with phenolic lignin structures during pulping and/or Klason lignin determination, and thus influence the results on Klason lignin. With 3.5% attributed to protein content in pulp (Table 5), Klason lignin in organosolv pulp of bark had to be estimated by correction for protein content [28]. After acid hydrolysis of residual pulp for sugar quantification, only glucose was detected in hydrolysate as major compound (See Figure S4 in Supplementary Materials). In our previous study [17], the use of sulfuric acid as catalyst has caused the exhaustive hydrolysis of xylan, as evidenced by furfural production from xylose in acid conditions. In addition to ferric chloride as acid source (the pulping conditions applied in this study correspond to pH = 2.4), other organic acids (oxalic, ferulic, etc.) could also promote furfural production during organosolv pulping of sugar maple bark, as it has been shown that it contained important concentration of calcium oxalate, which could be a source of oxalic acid. It is also important to note that hydrolysis of other hardwood barks, such as eucalyptus, yielded little xylose, while glucose was identified as the most important product [29].

Table 5.
Analysis of liquid fraction obtained after removal of precipitated lignin from residual liquor of bark organosolv pulping.
2.2.3. Water Soluble Fraction of Residual Liquor
After delignification, organosolv pulp was separated from residual liquor by filtration. Ethanol was removed by vacuum evaporation, thus facilitating the lignin precipitation after slight acidification of the liquid fraction, which was separated by filtration. The yield of liquid fraction (the mass corresponding to wood constituents found in liquid fraction) was calculated according to procedure presented in our previous study [17], as presented in Table 5.
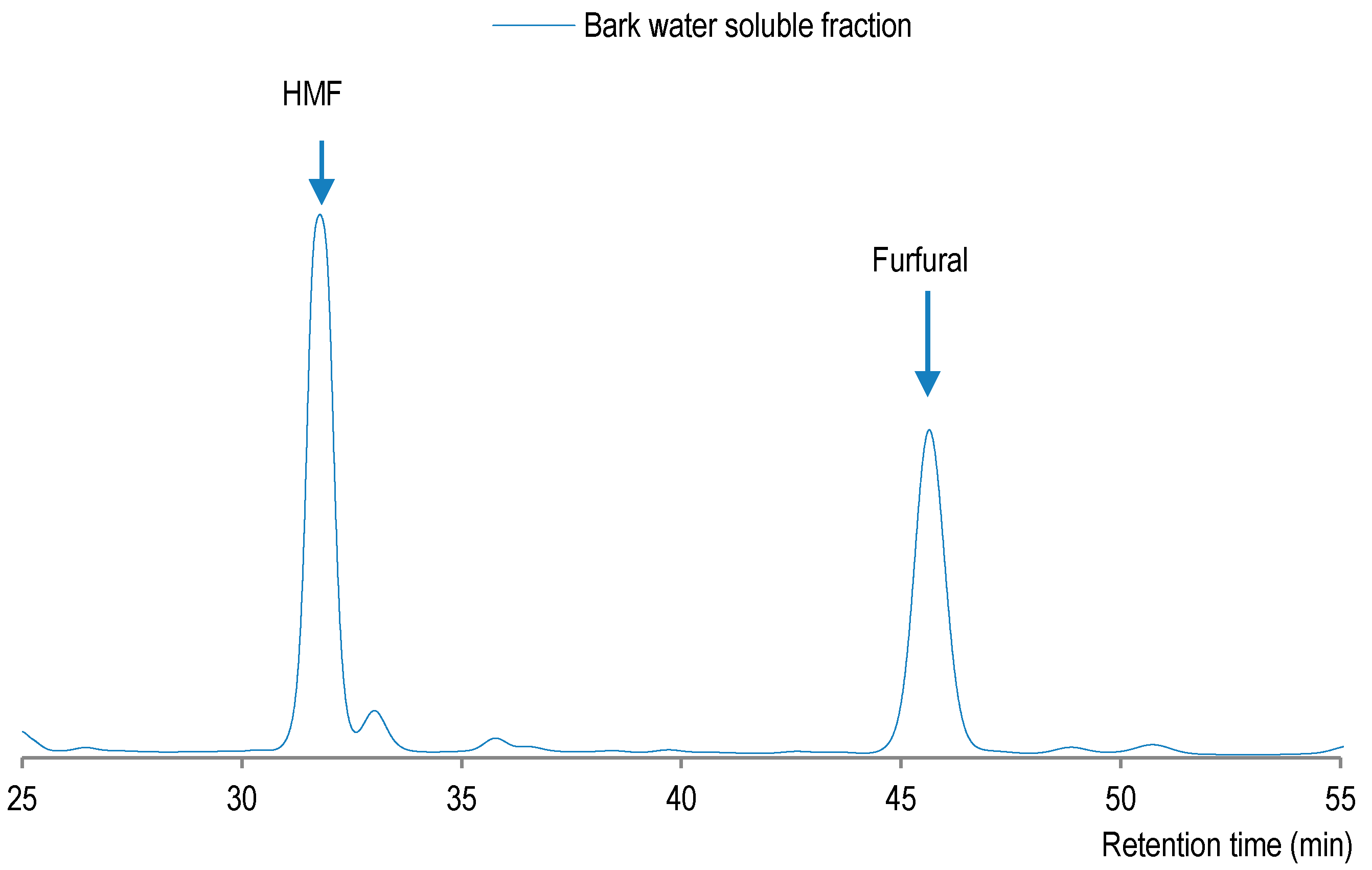
The pulping of pre-extracted sugar maple bark has yielded a water-soluble fraction with a higher xylose content than glucose (Table 5), which could indicate a good preservation of cellulose originally present in bark. Under such conditions, the hydrolysis reaction of xylan seems to yield xylose, which is transformed into furfural, while glucose fragments are dehydrated into hydroxymethyl-furfural (HMF). On the other hand, HMF was determined to be more abundant (0.74%) than furfural (0.35%) in water-soluble fraction. Indeed, due to high temperature of organosolv pulping (190 °C), the furfural level decreased while the production of HMF increased (Figure 4). One should note that glucose could also be produced by hydrolysis of glucan with β-1,3 glycosidic bonds, also known to be present in bark [30], which is easier to hydrolyse and thus it could have also contributed to release of glucose, which was then converted into HMF.
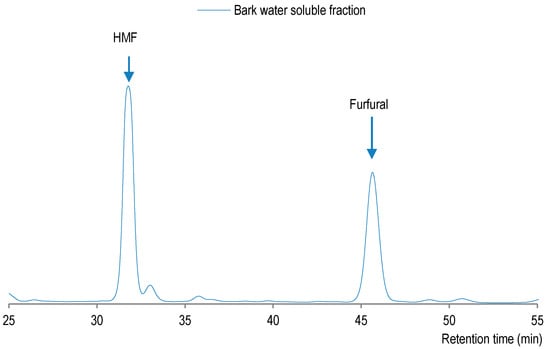
Figure 4.
HPLC profile of water-soluble fraction from organosolv pulping of pre-extracted sugar maple bark.
On the other hand, the products of hemicelluloses degradation products such as formic and acetic acid [31] were not detected with the method applied in this study, but it could also indicate a different substitution pattern of bark hemicelluloses. Other degradation products of hemicelluloses such as levulinic acid were difficult to quantify by HPLC method applied in this research [32]. The mass balance, expressed in %, is calculated on basis of O.D. bark. We have estimated the hemicelluloses in residual liquor by subtracting the sum of: extractives (5.2%), lignin (24.1%), cellulose (42.3%), and ash content of sugar maple before pulping (5.2%) [20], from 100%. That gave the estimate of hemicelluloses in residual liquor presented in Table 5. Indeed, the efficiency of this organosolv process might be improved with catalyst recycling. However, one should note that one part of the catalyst is remaining in the products recovered from pulping, cellulosic pulp, and lignin, as revealed by ICP analysis (See Table S2 in Supplementary Materials). As HMF was major compound of hemicellulose degradation, its valorisation was studied for transformation into added-value products.
2.2.4. Hemicellulose Valorisation Pathway from HMF Transformation
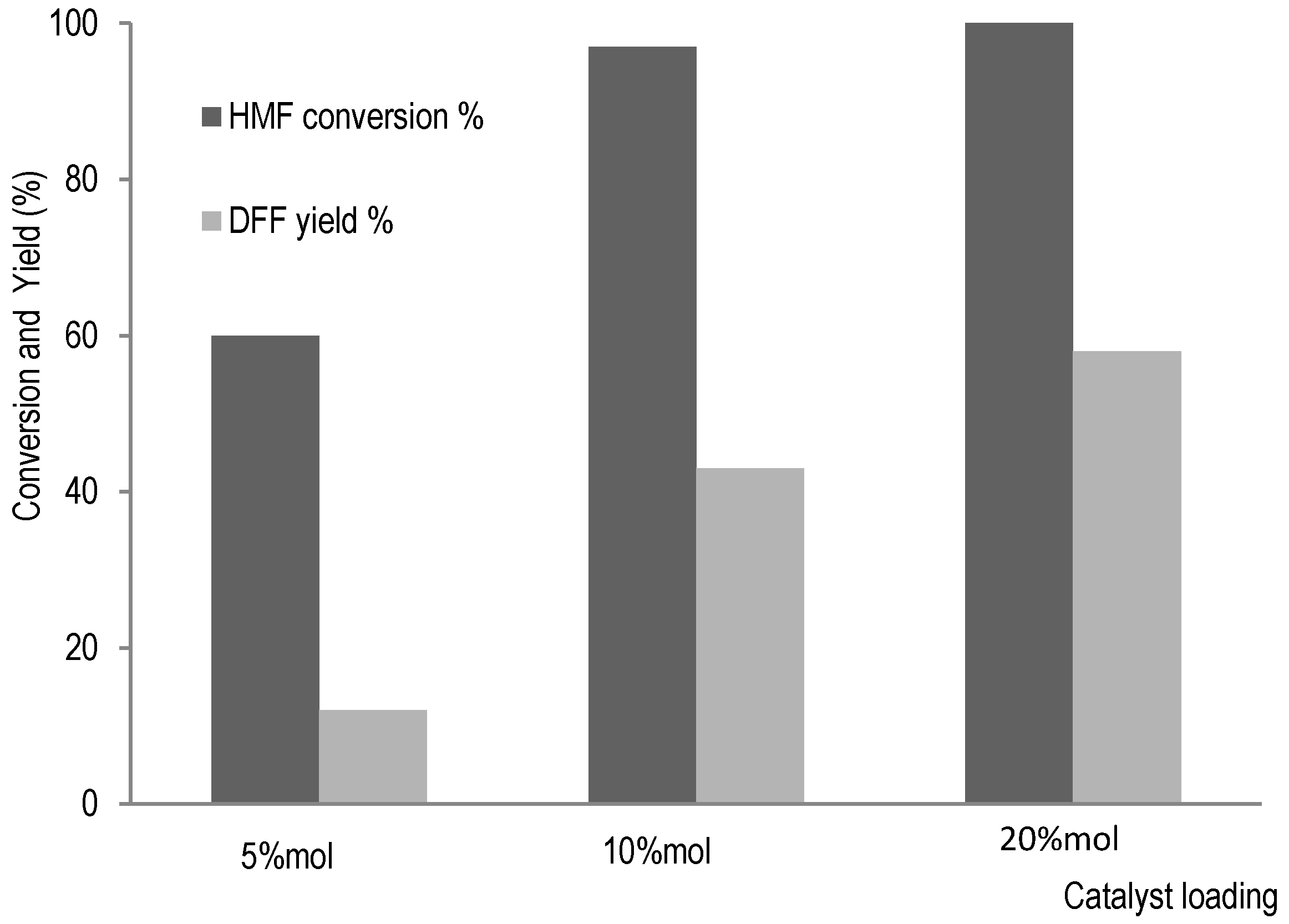
2,5-diformylfuran (DFF) is a possible compound which can be used as a precursor for synthesis of various polymers and commodities such as terephthalic acid used for resins production [33]. Thus, with HMF as major compound from hemicellulose degradation in residual liquor from bark pulping, oxidative catalysis can allow the obtaining of DFF. In our previous study [17], furfural production was more important than HMF when wood tissue was subjected to organosolv process with a smaller amount of ferric chloride as catalyst. In bark tissue, HMF was major product, probably due to an important amount of catalyst required for bark pulping, which also promotes glucose isomerisation to fructose. The hemicellulose valorisation could thus be achieved by production of 2,5-diformylfuran which can be obtained from HMF oxidation with sodium bromide in combination with phosphotungstic acid H3PW12O40, a polyoxometalate (POM), as activating agent for NaBr transformation into HBr and H2O2, and for transforming HBr into HOBr, the oxidizing agent here. For 2,5-diformylfuran production, catalytic system was directly applied to oxidative reaction of HMF. The conversion reaction of HMF was monitored by HPLC (See Figure S5 in Supplementary Materials) and the results are summarized in Figure 5.
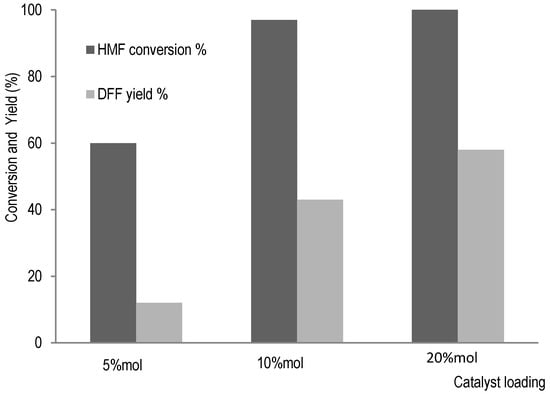
Figure 5.
HMF oxidation to 2,5-Diformylfuran (DFF) catalysed by NaBr/POM/H2O2.
Without POM, no conversion of HMF was detected (see Figure S5A in Supplementary Materials). In addition to DFF synthesis from HMF oxidation with POM, the effects of catalyst amount on HMF conversion were evaluated. Indeed, with increase of catalyst amount, synthesis of DFF was promoted, as shown in Figure 5. An efficient synthesis of DFF from HMF is presented with POM as an activating agent, with a possible mechanism proposed in Scheme 1.
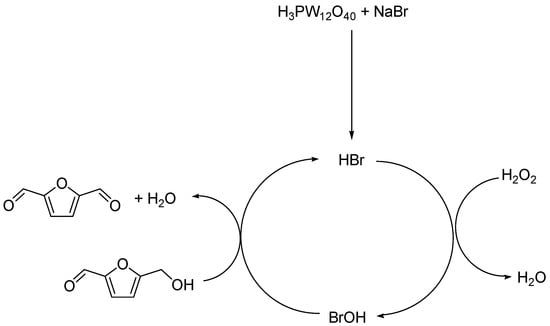
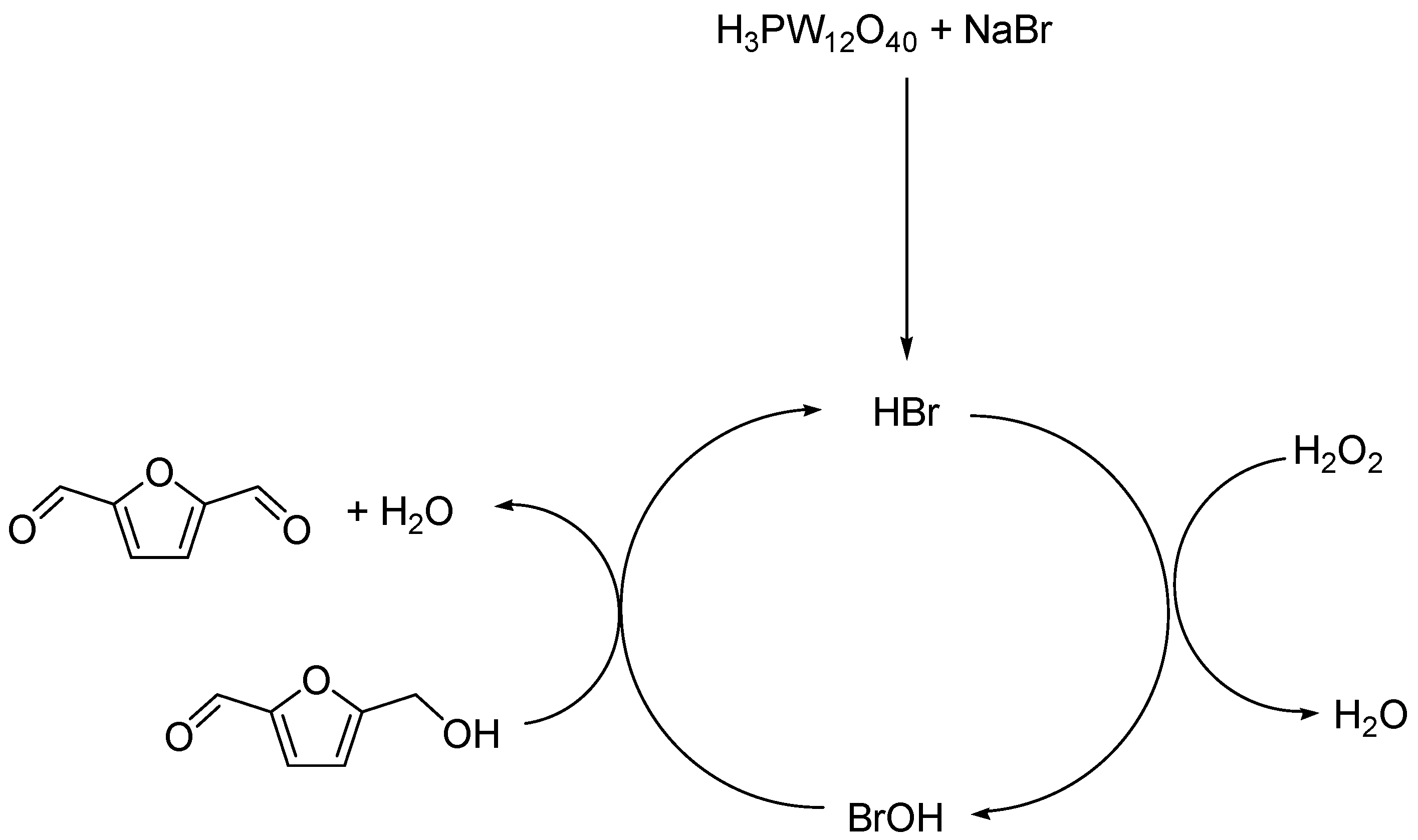
Scheme 1.
Proposed mechanism of HMF oxidation to DFF.
Reaction conditions: HMF (0.1 g, 0.79 mmol), DMSO (9 mL)/water (1 mL). NaBr (0.016 g, 0.16 mmol). H3PW12O40 (from 3.95 to 15.8 mmol). H2O2 (aqueous 30%, 120 μL). 130 °C for 12 h.
After activation reaction between phosphotungstic acid sodium bromide, HBr thus produced was oxidised into BrOH by H2O2. The BrOH catalyses then the oxidation of HMF, as presented in Scheme 1. Indeed, the BrOH production using NaBr, in combination with hydrogen peroxide, was already applied for aromatic alcohols oxidation with sodium bromate NaBrO3 as catalyst [34]. In our case, H3PW12O40 was used as an activating agent for transformation of NaBr into HBr. Other oxidant system such as boric acid/NaBr/formic acid was applied for HMF oxidation for DFF production [35] or with Rh/C catalyst under conditions of compressed carbon dioxide (scCO2) [36] and with vanadium derivative as catalyst [37].
3. Materials and Methods
3.1. Material
Chemical Composition of Sugar Maple Wood and Bark
Sugar maple (Acer saccharum) bark was provided by Decacer Inc. (Lévis, QC, Canada). It was ground to particle size between 40 and 60 meshes prior to chemical analyses. After drying at 80 °C for 24 h, bark material was used for organosolv pulping experiments. Furfural, 5-Hydroxymethyl Furfural (HMF), 2,5-diformylfuran (DFF), Ferric chloride, and sulfuric acid (95–97%) were used as purchased from Sigma Aldrich (Sigma-Aldrich Canada Co. Oakville, ON, Canada).
3.2. Methods
All analytical methods applied for bark composition determination were as reported in our previous study [17,20].
3.2.1. Pre-Extraction Procedure
Prior to delignification, the bark particles were pretreated with ethanol–water mixture (1:1, v/v; 1 L of final volume mixture for 100 g of bark) at 80 °C for 6 h in a Soxhlet extractor in order to remove phenolic and other extractive compounds, along with volatile materials, which could interfere with delignification. After filtration, extractive-free bark particles were dried at 80 °C for 24 h prior to pulping. The crude extracts obtained after elimination of solvent, were dried in vacuum at 45 °C for 24 h prior to analyze.
3.2.2. Determination of Total Phenol Content of the Bark Extracts
The total phenol (TP) content of the extracts was quantified according to Folin-Ciocalteu’s method, such as previously reported [4]. Typically, a total of 0.5 mL of methanol solution of sample at 0.2 mg/mL was mixed with 2.5 mL of the Folin-Ciocalteu reagent (diluted 10 times by distilled water) and 2.0 mL of an aqueous sodium carbonate solution (75 mg/mL). The final mixture was heated at 50 °C for 10 min, after which the absorbance was read at 760 nm against a blank (solution with no extract added). Gallic acid was used as standard and TP content was expressed as milligrams of gallic acid equivalents (GAE) per gram of dry extract sample (mg GAE/g of dry extract).
3.2.3. Determination of Proanthocyanidin Content of the Bark Extracts
Proanthocyanidin were determined through cyanidin chloride formation after treatment in a hydrochloric medium with ferric ammonium sulphate as a catalyst, following the procedure previously reported [5]. The proanthocyanidin content (PAs) was expressed as milligrams of cyanidin chloride equivalents (CChE) per gram of dry extract (mg CChE/g of dry extract) using calibration curve traced for cyaniding chloride.
3.2.4. GC-MS Analyses
Between 1 and 5 mg of dried extractives sample were dissolved in 100 μL of dichloromethane and then silylated with 50 μL of BSTFA (N,O-bis (trimethylsilyl) trifluoroacetamide) and 50 μL of TMCS (trimethylchlorosilane) prior to GC-MS analysis of the constituents of extracts. The analyses were performed using a Varian GC-MS system (GC model CP-3800; MS model Saturn 2200 MS/MS) equipped with a Varian Factor Four capillary column (Zorbax SB-C18, 4.6 × 250 mm 5 micron) (Agilent Technologies Inc., Wilmington, DE, USA). The oven temperature was programmed from 80 °C (initial hold time of 1 min) to 150 °C at a rate of 5 °C min−1 and held for 1 min. Then, it was programmed from 150 to 230 °C at a rate of 20 °C min−1 and held for 1 min. The final temperature reached at rate of 5 °C min−1, from 230 to 280 °C, was maintained for 1 min. Total ion chromatograms (TICs) were recorded and treated with Workstation Toolbar software (scan range from m/z 33 to m/z 500 at 1.05 scan s−1). Helium as a gas flow through the column was kept at 1 mL/min. The mass spectrometer (Varian Canada Inc., Mississauga, ON, Canada) was operated in the electron positive-mode ionization (EI), with electron energy at 70 eV. Ion source temperature was 235 °C. The identification of compounds was performed by comparison of the spectral properties with NIST 2.0 library, which was incorporated in the software.
3.2.5. XPS Analyses
XPS analysis was performed with an AXIS-ULTRA instrument (Kratos Analytical Limited, Manchester, UK). It has 3 communicating chambers: the analysis chamber comprising the electron spectroscopy for chemical analysis (ESCA) analyzer, the preparation chamber, and the introduction chamber. Base pressure in the analysis chamber was 5 × 10−10 Torr. The X-ray source was a monochromatic Al source operated at 300 watts. The analyzer was run in the constant pass energy mode, with the lens system in the “hybrid” configuration and the electrostatic lens aperture in the “slot” position. This assured the highest sensitivity with an analyzed spot approximately 800 microns × 400 microns in size, which is the size of the monochromatic X-ray beam. Electron counting was performed with an 8 channel detector. The electrostatic charge appearing on electrically insulating samples under X-ray irradiation was neutralized with an integrated, very low energy electron flood gun, whose parameters were calibrated against standard reference samples:
Au4f7/2: 83.95 eV Ag3d5/2: 368.2 eV Cu2p3/2: 932.6 eV.
These parameters were set to optimize energy resolution and counting rate. Survey scans were recorded with a pass energy of 160 eV and a step size of 1 eV. Detailed high resolution spectra were recorded at 10 eV, 20 eV, or 40 eV pass energy with a step size of 0.025, 0.05 eV, or 0.1 eV, depending on the amount of the element.
3.2.6. Nitrogen Analysis
Nitrogen content of original sugar maple bark, as well as of organosolv lignin and organosolv cellulosic pulp obtained by organosolv process, has been determined. Each sample (60 to 80 mg) placed in a tin capsule was analysed using a PerkinElmer (PerkinElmer Precisely Series II Nitrogen analyser 2410, Woodbridge, ON, Canada). The standard vial (EDTA) was placed in a stainless tube for calibration. All analyses were performed on the PE2410 Series II Nitrogen Analyzer, (Perkin Elmer, Inc., Woodbridge, ON, Canada).
3.2.7. DFF Synthesis from HMF
Typically, to a solution containing HMF (0.1 g, 0.79 mmol) in DMSO (9 mL) was added water (1 mL) containing sodium bromide (0.016 g, 0.16 mmol) and appropriate amount of phosphotungstic acid (respectively 3.95, 7.9, and 15.8 mmol). The reaction starts with addition of aqueous 30% H2O2 (120 μL). The reaction mixture was stirred at 130 °C for 12 h and monitored by HPLC using an Agilent Technologies 1100 Series (Agilent Technologies Inc., Wilmington, DE, USA) with a Lichrospher RP 18, 250 × 4.6 mm, 10 μm column, with UV-DAD detector at 254 nm. Acetonitrile/water (10/90) with 1% formic acid in water was used as eluent at 1 mL/min. The identification and quantification of products was performed by retention time with comparison using commercial standards grade.
4. Conclusions
We have demonstrated in this study that residual bark from wood transformation of sugar maple could represent an interesting renewable source for biopolymer and small molecules production through a biorefinery concept. Even though the residues of wood transformation will continue to be applied as bioenergy sources, the unexplored potential of bark merits to be further studied. The biopolymers separated from sugar maple bark by the new catalytic organosolv process could find applications in developments of new composite materials containing lignin and cellulose but also as a source of HMF for DFF production, while phenolic extractives are applicable as antioxidants or in other specialty chemicals applications. The application of catalytic processes is demonstrated here both at the stage of organosolv pulping with ferric chloride as catalyst, and by residual liquor transformation. The residual liquor after removal of precipitated organosolv lignin was examined for catalytic conversion of HMF into 2,5-diformylfuran (DFF), an important intermediate in terephtalic acid production, using polyoxometalate (POM)/NaBr/H2O2 system as catalyst for this conversion. It is noteworthy, however, that the important concentration of HMF that we detected in the residual liquor could represent an interesting starting-material for enzymatic conversions into platform chemicals. Namely, very interesting results have been reported on trials of the application of an enzymatic toolbox for the selective oxidation of HMF into DFF, or on the fungal aryl alcohol oxidase oxidation of HMF into yet another platform chemical, 2,5-furan-dicarboxylic acid (FDCA). Thus, one could anticipate the addition of an enzymatic pathways to finalize the complete conversion of this residual lignocellulosic material (sugar maple bark) into biopolymers and platform chemicals. This research offers an example of solid bark waste transformation into several valuable products, while complying with sustainable engineering and green chemistry principles.
Supplementary Materials
The following are available online at www.mdpi.com/2073-4344/7/10/294/s1, Figure S1: XRD spectrum and MEB image of halite crystal, Figure S2: Comparative GC-MS analysis of sugar maple extractives present in methanol soluble fraction (red) and methanol insoluble fraction (blue)- after silylation, Figure S3: XRD spectrum and MEB image for Calcium oxalate, Figure S4: HPLC analysis for celullosic pulp after complete hydrolysis, Figure S5: HPLC analysis of HMF oxidation reaction with POM as activating agent. (A) activating agent loading effect on DFF production after 12 hat 130 °C; (B) Kinetic of DFF production according to time reaction, Table S1: Elemental ICP analysis of organosolv lignin from bark, Table S2: Comparative elemental ICP composition in ash from bark and cellulosic pulp.
Acknowledgments
The authors gratefully acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Décacer and Levaco Inc., No RDCPJ-452658-13. The authors are grateful to C. Levasseur of Levaco Inc. for his personal involvement and generous supply of sugar maple wood and bark.
Author Contributions
Tatjana Stevanovic conceived the experiments, Georges Koumba-Yoya designed and performed the experiments, Tatjana Stevanovic analyzed the data, Georges Koumba-Yoya and Tatjana Stevanovic wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- State of Canada’s Forests: Annual Report for 2016; Natural Resources Canada: Library and Archives Canada Cataloguing in Publication. Cat. No. Fo1-6E-PDF (Online); Library and Archives Canada: Gatineau, QC, Canada, 2016.
- Baptista, I.; Miranda, I.; Quilhó, T.; Gominho, J.; Pereira, H. Characterisation and fractioning of Tectona grandis bark in view of its valorisation as a biorefinery raw-material. Ind. Crop. Prod. 2013, 50, 166–175. [Google Scholar] [CrossRef]
- Ruiz-Aquinoa, F.; González-Pena, M.M.; Valdez-Hernández, J.I.; Revilla, U.S.; Romero-Manzanares, A. Chemical characterization and fuel properties of wood and bark of two oaks from Oaxaca, Mexico. Ind. Crop. Prod. 2015, 65, 90–95. [Google Scholar] [CrossRef]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Antioxidant properties and polyphenol contents of trembling aspen bark extracts. Wood Sci. Technol. 2009, 43, 457–470. [Google Scholar] [CrossRef]
- Royer, M.; Diouf, P.N.; Stevanovic, T. Polyphenol contents and radical scavenging capacities of red maple (Acer rubrum L.) extracts. Food Chem. Toxicol. 2011, 49, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, F.; Achim, A.; Stevanovic, T. Composition of ethanolic extracts of wood and bark from Acer saccharum and Betula alleghaniensis trees of different vigor classes. Ind. Crop. Prod. 2013, 41, 179–187. [Google Scholar] [CrossRef]
- Zeng, X.-L.; Fu, G.M.; Tian, K.; Sun, J.X.; Xiong, H.B.; Huang, X.Z.; Jiang, Z.Y. Acutissimanide, a new lignan with antioxidant activity isolated from the bark of Quercus acutissima Carruth. Nat. Prod. Res. 2014, 28, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind. Crop. Prod. 2012, 36, 395–400. [Google Scholar] [CrossRef]
- Kofujita, H.; Ettyu, K.; Ota, M. Characterization of the major components in bark from five Japanese tree species for chemical utilization. Wood Sci. Technol. 1999, 33, 223–228. [Google Scholar] [CrossRef]
- Costa, E.C.A.; Pinto, P.C.R.; Rodrigues, A.E. Evaluation of chemical processing impact on E. globulus wood lignin and comparison with bark lignin. Ind. Crop. Prod. 2014, 61, 479–491. [Google Scholar] [CrossRef]
- Churms, S.C.; Stephen, A.M. Chromatographic separation and examination of carbohydrate and phenolic components of the non-tannin fraction of black wattle (Acacia mearnsii) bark extract. J. Chromatogr. 1991, 550, 519–537. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Bark derived submicron-sized and nano-sized cellulose fibers: Fromindustrial waste to high performance materials. Carbohyd. Polym. 2015, 134, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Vispute, T.P.; Huber, G.W. Production of hydrogen, alkanes and polyols by aqueous phase processing of wood-derived pyrolysis oils. Green Chem. 2009, 11, 1433–1445. [Google Scholar] [CrossRef]
- Lima, M.A.; Lavorente, G.B.; da Silva, H.K.P.; Bragatto, J.; Rezende, C.A.; Bernardinelli, O.D.; de Azevedo, E.R.; Gomez, L.D.; McQueen-Mason, S.J.; Labate, C.A.; et al. Effects of pretreatment on morphology, chemical composition and enzymatic digestibility of eucalyptus bark: A potentially valuable source of fermentable sugars for biofuel production—Part 1. Biotechnol. Biofuels 2013, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.I.; Coelho, J.A.S.; Simeonov, S.P.; Lazarova, H.I.; Popova, M.D.; Afonso, C.A.M. Oxidation of 5-Chloromethylfurfural (CMF) to 2,5-Diformylfuran (DFF). Molecules 2017, 22, 329. [Google Scholar] [CrossRef] [PubMed]
- Karnaouri, A.; Rova, U.; Christakopoulos, P. Effect of Different Pretreatment Methods on Birch Outer Bark: New Biorefinery Routes. Molecules 2016, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Koumba-Yoya, G.; Stevanovic, T. New biorefinery strategy for high purity lignin production. Chem. Select 2016, 1, 6562–6570. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S.M. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, T.; Koumba-Yoya, G. Organosolv Process for the Extraction of Highly Pure Lignin and Products Comprising the Same. WO 2016197233 A1, 15 December 2016. [Google Scholar]
- Koumba-Yoya, G.; Stevanovic, T. Study of Organosolv Lignins as Adhesives in Wood Panel Production. Polymers 2017, 9, 46. [Google Scholar] [CrossRef]
- Kirk, T.K.; Farrell, R.L. Enzymatic Combustion: The Microbial-Degradation of Lignin. Annu. Rev. Microbiol. 1987, 41, 465–505. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar] [PubMed]
- Jayasekara, T.K.; Stevenson, P.C.; Belmain, S.R.; Farman, D.I.; Hall, D.R. Identification of methyl salicylate as the principal volatile component in the methanol extract of root bark of Securidaca longepedunculata Fers. J. Mass. Spectrom. 2002, 37, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crop. Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, Y.; Kim, M.R.; Jung, H.J.; Seu, Y.B.; Hahm, K.; Woo, E.R. Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol. Lett. 2004, 26, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Pasztory, Z.; Mohacsine, I.R.; Gorbacheva, G.; Borcsok, Z. The utilization of tree bark. Bioresources 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood Formation in Trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Ralph, J.; Marques, G.; Gutiérrez, A.; Martínez, A.T.; Del Río, J.C. Structural Characterization of Lignin Isolated from Coconut (Cocos nucifera) Coir Fibers. J. Agric. Food Chem. 2013, 61, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Lopes, O.R.; Colodette, J.L.; Porto, A.O.; Rieumont, J.; Chaussy, D.; Belgacem, M.N.; Silva, G.G. Characterization of three non-product materials from a bleached eucalyptus kraft pulp mill, in view of valorising them as a source of cellulose fibres. Ind. Crop. Prod. 2008, 27, 288–295. [Google Scholar] [CrossRef]
- Higuchi, T. Biochemistry and Molecular Biology of Wood; Springer: Berlin/Heidelberg, Germany, 2012; p. 94. [Google Scholar]
- Hu, R.F.; Lin, L.; Liu, T.J.; Liu, S.J. Dilute sulfuric acid hydrolysis of sugar maple wood extract at atmospheric pressure. Bioresour. Technol. 2010, 101, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Tu, M.; Wu, Y.; Adhikari, S. Improvement in HPLC separation of acetic acid and levulinic acid in the profiling of biomass hydrolysate. Bioresour. Technol. 2011, 102, 4938–4942. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qi, W.; Su, R.; He, Z. Selective synthesis of 2,5-diformylfuran and 2,5-Furandicarboxylic acid from 5-Hydroxymethylfurfural and fructose catalyzed by magnetically separable catalysts. Energy Fuels 2017, 31, 533–541. [Google Scholar] [CrossRef]
- Joshi, G.; Patil, R.D.; Adimurthy, S. Green bromine: In situ generated catalyst for the selective oxidation of alcohols using H2O2 as a benign oxidant. RSC Adv. 2012, 2, 2235–2239. [Google Scholar] [CrossRef]
- Girka, Q.; Estrine, B.; Hoffmann, N.; Le Bras, J.; Marinković, S.; Muzart, J. Simple efficient one-pot synthesis of 5-hydroxymethylfurfural and 2,5-diformylfuran from carbohydrates. React. Chem. Eng. 2016, 1, 176–182. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Chatterjee, A.; Kawanami, H. Dehydrogenation of 5-hydroxymethylfurfural to diformylfuran in compressed carbon dioxide: An oxidant free approach. Green Chem. 2017, 19, 1315–1326. [Google Scholar] [CrossRef]
- Halliday, G.A.; Young, R.J., Jr.; Grushin, V.V. One-pot, two-step, practical catalytic synthesis of 2,5-diformylfuran from fructose. Org. Lett. 2003, 5, 2003–2005. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).