Synthesis, Structure and 1,3-Butadiene Polymerization Behavior of Vanadium(III) Phosphine Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure of V(III)–Phosphine Complexes
2.2. Polymerization of 1,3-Butadiene
3. Materials and Methods
3.1. General Procedures and Materials
3.2. Synthesis of the V(III)–Phosphine Complexes
3.2.1. Trichlorobis(triphenylphosphine)vanadium(III) [VCl3(PPh3)2, 1a]
3.2.2. Trichlorobis(methyldiphenylphosphine)vanadium(III) [VCl3(PMePh2)2, 1b]
3.2.3. Trichlorobis(ethyldiphenylphosphine)vanadium(III) [VCl3(PEtPh2)2, 1c]
3.2.4. Trichlorobis(iso-propyldiphenylphosphine)vanadium(III) [VCl3(PiPrPh2)2, 1d]
3.2.5. Trichlorobis(cyclohexyldiphenylphosphine)vanadium(III) [VCl3(PCyPh2)2, 1e]
3.2.6. Trichlorobis(dimethyiphenylphosphine)vanadium(III) [VCl3(PMe2Ph)2, 1f]
3.2.7. Trichlorobis(diethylphenylphosphine)vanadium(III) [VCl3(PEt2Ph)2, 1g]
3.2.8. Trichlorobis(dicyclohexylphenylphosphine)vanadium(III) [VCl3(PCy2Ph)2, 1h]
3.2.9. Trichlorobis(tricyclopentylphosphine)vanadium(III) [VCl3(PCyp3)2, 2a]
3.2.10. Trichlorobis(tricyclohexylphosphine)vanadium(III) [VCl3(PCy3)2, 2b]
3.2.11. Trichlorobis(tri-normal-propylphosphine)vanadium(III) [VCl3(PnPr3)2, 2c]
3.2.12. Trichlorobis(tri-tert-butylphosphine)vanadium(III) [VCl3(PtBu3)2, 2d]
3.3. X-ray Crystallographic Studies
3.4. Computational Details
3.5. Polymerization of 1,3-Butadiene
3.6. Polymer Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Porri, L.; Giarrusso, A. Part II. In Conjugated Diene Polymerization in Comprehensive Polymer Science; Pergamon: Oxford, UK, 1989; Volume 4, pp. 53–108. [Google Scholar]
- Thiele, S.K.-H.; Wilson, D.R. Alternate Transition Metal Complex Based Diene Polymerization. J. Macromol. Sci. Part C Polym. Rev. 2003, C43, 581–628. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-Based Ziegler/Natta Catalysts and their Application in Diene Polymerization. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar]
- Natta, G.; Porri, L.; Corradini, P.; Morero, D. Polimerizzazioni stereospecifiche di diolefine coniugate. Chim. Ind. 1958, 40, 362–371. [Google Scholar]
- Natta, G.; Porri, L.; Carbonaro, A. Stereospecificity of homogeneous catalysts prepared from vanadium trichloride in the polymerization of conjugated diolefins. Atti Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1961, 31, 189, Chem. Abstr. 1962, 57, 4848. [Google Scholar]
- Porri, L.; Carbonaro, A.; Ciampelli, F. Copolymerization of 1,3-butadiene and 1,3-pentadiene with homogeneous Al (C2H5)2Cl-vanadium compounds catalyst systems. I. Preparation and properties of the copolymers. Makromol. Chem. 1963, 61, 90–103. [Google Scholar] [CrossRef]
- Ricci, G.; Italia, S.; Comitani, C.; Porri, L. Polymerization of conjugated dialkenes with transition metal catalysts. Influence of methylaluminoxane on catalyst activity and stereospecificity. Polym. Commun. 1991, 32, 514–517. [Google Scholar]
- Ricci, G.; Zetta, L.; Alberti, E.; Motta, T.; Canetti, M.; Bertini, F. Butadiene–isoprene copolymerization with V(acac)3-MAO. Crystalline and amorphous trans-1,4 copolymers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4635–4646. [Google Scholar] [CrossRef]
- Colamarco, E.; Milione, S.; Cuomo, C.; Grassi, A. Homo- and Copolymerization of Butadiene Catalyzed by an Bis(imino)pyridyl Vanadium Complex. Macromol. Rapid Commun. 2004, 25, 450–454. [Google Scholar] [CrossRef]
- Natta, G.; Porri, L.; Zanini, G.; Fiore, L. Stereospecific polymerization of conjugated diolefins. IV. Preparation of syndiotactic 1,2-polybutadiene. Chim. Ind. (Milan) 1959, 41, 526–532, Chem. Abstr. 1960, 54, 1258. [Google Scholar]
- Porri, L.; Ricci, G.; Giarrusso, A. Metalorganic Catalysts for Synthesis and Polymerization; Kaminsky, W., Ed.; Springer: Berlin/Heidelberg, German, 1999; p. 519. [Google Scholar]
- Porri, L.; Giarrusso, A.; Ricci, G. Metallocene-Based Polyolefins; Scheirs, J., Kaminsky, W., Eds.; John Wiley & Sons Ltd.: London, UK, 2000; p. 115. [Google Scholar]
- Ricci, G.; Panagia, A.; Porri, L. Polymerization of 1,3-dienes with catalysts based on mono-and bis-cyclopentadienyl derivatives of vanadium. Polymer 1996, 37, 363–365. [Google Scholar] [CrossRef]
- Bradley, S.; Camm, K.D.; Furtado, S.J.; Gott, A.L.; McGowan, P.C.; Podesta, T.J.; Thornton-Pett, M. Synthesis and Structure of Amino-Functionalized Cyclopentadienyl Vanadium Complexes and Evaluation of Their Butadiene Polymerization Behavior. Organometallics 2002, 21, 3443–3453. [Google Scholar] [CrossRef]
- Ricci, G.; Battistella, M.; Porri, L. Chemoselectivity and Stereospecificity of Chromium(II) Catalysts for 1,3-Diene Polymerization. Macromolecules 2001, 34, 5766–5769. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Sonzogni, M. New Chromium(II) Bidentate Phosphine Complexes: Synthesis, Characterization, and Behavior in the Polymerization of 1,3-Butadiene. Organometallics 2004, 23, 3727–3732. [Google Scholar] [CrossRef]
- Ricci, G.; Boglia, A.; Motta, T. Synthesis of new Cr(II) complexes with bidentate phosphine ligands and their behavior in the polymerization of butadiene. Influence of the phosphine bite angle on catalyst activity and stereoselectivity. J. Mol. Catal. A Chem. 2007, 267, 102–107. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Motta, T.; Zannoni, G.; Canetti, M.; Bertini, F. Synthesis and X-ray Structure of CoCl2(PiPrPh2)2. A New Highly Active and Stereospecific Catalyst for 1,2 Polymerization of Conjugated Dienes When Used in Association with MAO. Macromolecules 2005, 38, 1064–1070. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Motta, T. Synthesis, structure, and butadiene polymerization behavior of alkylphosphine cobalt(II) complexes. J. Mol. Catal. A Chem. 2005, 226, 235–241. [Google Scholar] [CrossRef]
- Ricci, G.; Forni, A.; Boglia, A.; Sommazzi, A.; Masi, F. Synthesis, structure and butadiene polymerization behavior of CoCl2(PRxPh3−x)2 (R = methyl, ethyl, propyl, allyl, isopropyl, cyclohexyl; x = 1, 2). Influence of the phosphorous ligand on polymerization stereoselectivity. J. Organomet. Chem. 2005, 690, 1845–1854. [Google Scholar] [CrossRef]
- Ricci, G.; Motta, T.; Boglia, A.; Alberti, E.; Zetta, L.; Bertini, F.; Arosio, P.; Famulari, A.; Meille, S.V. Synthesis, Characterization, and Crystalline Structure of Syndiotactic 1,2-Polypentadiene: The Trans Polymer. Macromolecules 2005, 38, 8345–8352. [Google Scholar] [CrossRef]
- Ricci, G.; Boglia, A.; Motta, T.; Bertini, F.; Boccia, A.C.; Zetta, L.; Alberti, E.; Famulari, A.; Arosio, P.; Meille, S.V. Synthesis and structural characterization of syndiotactic trans-1,2 and cis-1,2 polyhexadienes J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5339–5353. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Boccia, A.C.; Zetta, L. cis-1,4-alt-3,4 Polyisoprene: Synthesis and Characterization. Macromolecules 2009, 42, 9263–9267. [Google Scholar] [CrossRef]
- Boccia, A.C.; Leone, G.; Boglia, A.; Ricci, G. Novel stereoregular cis-1,4 and trans-1,2 poly(diene)s: Synthesis, characterization, and mechanistic considerations. Polymer 2013, 54, 3492–3503. [Google Scholar] [CrossRef]
- Bansemer, R.L.; Huffman, J.C.; Caulton, K.G. Synthesis and characterization of VCl3(PMePh2)2. Inorg. Chem. 1985, 24, 3003–3006. [Google Scholar] [CrossRef]
- Holt, D.G.L.; Larkworthy, L.F.; Povey, D.C.; Smith, G.W.; Leigh, G.J. Synthesis and structural studies of complexes of vanadium(II) and vanadium(III) halides with tertiary phosphines. Inorg. Chim. Acta 1993, 207, 11–19. [Google Scholar] [CrossRef]
- Bultitude, J.; Larkworthy, L.F.; Povey, D.C.; Smith, G.W.; Dilworth, J.R. The preparation and crystal and molecular structures of trichlorobis(methyldiphenylphosphine)vanadium(III) and its acetonitrile adduct. J. Chem. Soc. Dalton Trans. 1986, 2253–2258. [Google Scholar] [CrossRef]
- Nieman, J.; Teuben, J.H.; Huffman, J.C.; Caulton, K.G. Preparation and characterization of mono-cyclopentadienylvanadium dihalide bis-phosphine complexes—Crystal-structure of (eta-5-C5H5)VCl2(PMe3)2. J. Org. Chem. 1983, 255, 193–204. [Google Scholar] [CrossRef]
- Issleib, V.K.; Bohn, V. Phosphin- und Phosphinoxydkomplexe des 3wertigen Vanadins. Z. Anorg. Allg. Chem. 1959, 301, 188–196. [Google Scholar] [CrossRef]
- Leone, G.; Pierro, I.; Zanchin, G.; Forni, A.; Bertini, F.; Rapallo, A.; Ricci, G. Vanadium(III)–catalyzed copolymerization of ethylene with norbornene: Microstructure at tetrad level and reactivity ratios. J. Mol. Catal. A Chem. 2016, 424, 220–231. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Sommazzi, A.; Forni, A.; Masi, F. Phosphinic Vanadium Complex, Catalytic System Comprising Said Phosphinic Vanadium Complex and Process for the (co)polymerization of Conjugated Dienes. U.S. Patent Application 20,170,275,312, 18 August 2017. [Google Scholar]
- Cotton, F.A.; Lu, J. EPR and Crystallographic Studies of Some Reaction Products of VCl4, NbCl4, and TaCl4 with Trialkyl- and Triarylphosphines. Inorg. Chem. 1995, 34, 2639–2644. [Google Scholar] [CrossRef]
- Ernst, R.D.; Freeman, J.W.; Stahl, L.; Wilson, D.R.; Arif, A.M.; Nuber, B.; Ziegler, M.L. Longer but Stronger Bonds: Structures of PF3, P(OEt)3, and PMe3 Adducts of an Open Titanocene. J. Am. Chem. Soc. 1995, 117, 5075–5081. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Evans, T.A.; Seddon, K.R.; Pálinkó, I. The C–H···Cl hydrogen bond: Does it exist? New J. Chem. 1999, 23, 145–152. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well Defined Transition Metal Complexes with Phosphorus and Nitrogen Ligands for 1,3-Dienes Polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Christman, D.L. Preparation of polyethylene in solution. J. Polym. Sci. Part A1 1972, 10, 471–487. [Google Scholar] [CrossRef]
- Gumboldt, V.A.; Helberg, J.; Schleitzer, G. Makromol. Chem. Über die reaktivierung der bei der äuthylen/propylen-copolymerisation verwendeten vanadium-katalysatoren. Macromol. Chem. Phys. 1967, 101, 229–245. [Google Scholar] [CrossRef]
- Tait, P.J.T.; Watkins, N.D. Monoalkene Polymerization: Mechanisms in: Comprehensive Polymer Science; Eastmond, G.C., Ledwith, A., Russo, S., Sigwalt, P., Eds.; Pergamon Press Ltd.: Oxford, UK, 1989; Volume 4, Part II; p. 27. [Google Scholar]
- Tolman, C.A. Electron donor-acceptor properties of phosphorus ligands. Substituent additivity. J. Am. Chem. Soc. 1970, 92, 2953–2956. [Google Scholar] [CrossRef]
- Tolman, C.A. Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev. 1977, 77, 313–348. [Google Scholar] [CrossRef]
- Bruker. SMART, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]


| Bond Lengths and Angle | VCl3(PMePh2)2 (1b) | VCl3(PEtPh2)2 (1c) | VCl3(PEt2Ph)2 (1g) | VCl3(PCyp3)2 (2a) |
|---|---|---|---|---|
| V–Cl | 2.2287(8) | 2.2408(6) | 2.246(10) | 2.247(10) |
| 2.243 | 2.243 | 2.245 | 2.251 | |
| 2.255 | 2.255 | 2.258 | 2.261 | |
| V–P | 2.5280(6) | 2.5465(6) | 2.5196(11) | 2.5696(10) |
| 2.537 | 2.551 | 2.549 | 2.587 | |
| 2.607 | 2.617 | 2.613 | 2.660 | |
| P–Car | 1.820(2) | 1.8251(19) | 1.813(4) | - |
| 1.825 | 1.826 | 1.828 | - | |
| 1.840 | 1.841 | 1.841 | - | |
| P–Caliph | 1.822(2) | 1.8332(19) | 1.828(4) | 1.847(3) |
| 1.828 | 1.844 | 1.842 | 1.850 | |
| 1.843 | 1.861 | 1.860 | 1.869 | |
| Cl–V–Cl | 119.98(3) | 119.99(2) | 114.6(2) | 120.00(4) |
| 120.00 | 120.00 | 119.98 | 120.00 | |
| 120.00 | 120.00 | 119.98 | 120.00 | |
| P–V–P | 169.02(2) | 177.87(2) | 167.24(4) | 170.48(3) |
| 174.74 | 177.79 | 174.06 | 174.66 | |
| 175.06 | 177.78 | 174.25 | 174.77 | |
| Car–P–Car | 103.73(10) | 103.85(8) | - | - |
| 103.64 | 104.39 | - | - | |
| 103.74 | 104.29 | - | - | |
| Car–P–Caliph | 105.20(11) | 105.42(9) | 105.1(2) | - |
| 104.03 | 104.31 | 104.20 | - | |
| 104.09 | 104.53 | 104.17 | - | |
| Caliph–P–Caliph | - | - | 103.7(2) | 105.5(2) |
| - | - | 102.79 | 104.09 | |
| - | - | 102.87 | 104.99 |
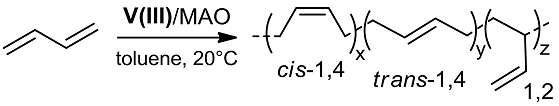 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Phosphine | Yield (%) | N d (h−1) | cis:trans:1,2 e (mol %) | Mw f (×103) | Mw/Mn f | ||
| (Type) | υCO b (cm−1) | θ c (°) | |||||||
| 1 | 1a/dMAO | PPh3 | 2068.9 | 145 | 57 | 739 | 33:31:36 | 275 | 1.9 |
| 2 * | 1a/dMAO | PPh3 | 2068.9 | 145 | 100 | 123 | 30:34:36 | 290 | 1.6 |
| 3 * | 1a/sMAO | PPh3 | 2068.9 | 145 | 51 | 63 | 65:19:16 | 177 | 4.2 |
| 4 | 1b/dMAO | PMePh2 | 2067.0 | 136 | 46 | 593 | 29:37:34 | 270 | 2.1 |
| 5 | 1b/sMAO | PMePh2 | 2067.0 | 136 | 7 | 93 | 69:17:14 | 164 | 1.9 |
| 6 | 1c/dMAO | PEtPh2 | 2066.7 | 140 | 42 | 360 | 30:38:32 | 83 | 2.1 |
| 7 | 1d/dMAO | PiPrPh2 | 2065.7 | 150 | 50 | 648 | 28:33:39 | 112 | 3.5 |
| 8 | 1d/sMAO | PiPrPh2 | 2065.7 | 150 | 17 | 222 | 66:18:16 | 102 | 2.6 |
| 9 | 1e/dMAO | PCyPh2 | 2064.8 | 153 | 46 | 593 | 37:28:35 | 116 | 3.0 |
| 10 * | 1e/sMAO | PCyPh2 | 2064.8 | 153 | 43 | 46 | 67:15:18 | 100 | 2.9 |
| 11 | 1f/dMAO | PMe2Ph | 2065.3 | 122 | 43 | 556 | 41:20:39 | 75 | 3.8 |
| 12 | 1g/dMAO | PEt2Ph | 2063.7 | 136 | 37 | 480 | 36:24:38 | 97 | 2.7 |
| 13 | 1h/dMAO | PCy2Ph | 2060.6 | 159 | 25 | 324 | 14:73:13 | 274 | 2.8 |
| 14 | 2a/dMAO | PCyp3 | 36 | 472 | 46:33:21 | 183 | 3.5 | ||
| 15 | 2b/dMAO | PCy3 | 2056.4 | 170 | 21 | 269 | 34:46:20 | 193 | 3.3 |
| 16 | 2c/dMAO | PnPr3 | 2059.1 | 132 | 26 | 333 | 36:21:43 | 296 | 1.8 |
| 17 | 2d/dMAO | PtBu3 | 2056.1 | 182 | 13 | 167 | 45:34:21 | 253 | 3.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, G.; Zanchin, G.; Pierro, I.; Sommazzi, A.; Forni, A.; Ricci, G. Synthesis, Structure and 1,3-Butadiene Polymerization Behavior of Vanadium(III) Phosphine Complexes. Catalysts 2017, 7, 369. https://doi.org/10.3390/catal7120369
Leone G, Zanchin G, Pierro I, Sommazzi A, Forni A, Ricci G. Synthesis, Structure and 1,3-Butadiene Polymerization Behavior of Vanadium(III) Phosphine Complexes. Catalysts. 2017; 7(12):369. https://doi.org/10.3390/catal7120369
Chicago/Turabian StyleLeone, Giuseppe, Giorgia Zanchin, Ivana Pierro, Anna Sommazzi, Alessandra Forni, and Giovanni Ricci. 2017. "Synthesis, Structure and 1,3-Butadiene Polymerization Behavior of Vanadium(III) Phosphine Complexes" Catalysts 7, no. 12: 369. https://doi.org/10.3390/catal7120369
APA StyleLeone, G., Zanchin, G., Pierro, I., Sommazzi, A., Forni, A., & Ricci, G. (2017). Synthesis, Structure and 1,3-Butadiene Polymerization Behavior of Vanadium(III) Phosphine Complexes. Catalysts, 7(12), 369. https://doi.org/10.3390/catal7120369








