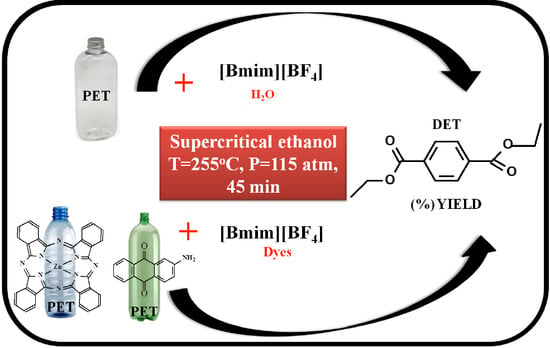
Poisoning Effects of Water and Dyes on the [Bmim][BF4] Catalysis of Poly(Ethylene Terephthalate) (PET) Depolymerization under Supercritical Ethanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Water on Depolymerization of PET under scEtOH
2.1.1. Through HPLC Analysis
2.1.2. 1H-NMR
2.1.3. FTIR Spectroscopy
2.1.4. Thermal Analysis
2.2. Effect of Dye on the Depolymerization of PET under scEtOH
2.2.1. HPLC Analysis
2.2.2. UV-Vis Analysis
3. Materials and Methods
3.1. Materials
3.2. Equipment and Procedures
3.2.1. Waste PET-Flakes Preparation and Characterization
3.2.2. Synthesis of 1-n-Butyl-3-Methylimidazolium Tetrafluoroborate ([Bmim][BF4])
3.2.3. Depolymerization of PET under scEtOH Catalyzed by [Bmim][BF4]
3.2.4. Characterization of PET and/or Products from PET Depolymerization
(i) Through HPLC
(ii) Through FTIR Spectroscopy
(iii) Through 1H-NMR Spectroscopy
(iv) Through Thermal Analysis (TGA and DSC)
(v) Use of UV-Vis for Evaluating the Interaction of [Bmim][BF4] and Zinc Phthalocyanine
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Imran, M.; Kim, B.K.; Han, M.; Cho, B.G.; Kim, D.H. Sub- and supercritical glycolysis of polyethylene terephthalate (pet) into the monomer bis(2-hydroxyethyl) terephthalate (bhet). Polym. Degrad. Stab. 2010, 95, 1686–1693. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Nikolaidis, A.K.; Sideridou, I.D.; Bikiaris, D.N.; Achilias, D.S. Chemical recycling of pet by glycolysis: Polymerization and characterization of the dimethacrylated glycolysate. Macromol. Mater. Eng. 2006, 291, 1338–1347. [Google Scholar] [CrossRef]
- Misra, E.; Basavarajaiah, S.; Kumar, K.R.; Ravi, P. Effect of recycling on the properties of poly(ethylene terephthalate) films. Polym. Int. 2000, 49, 1636–1640. [Google Scholar] [CrossRef]
- Buekens, A. Introduction to feedstock recycling of plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics; John Wiley & Sons, Ltd: Hoboken, NJ, 2006; pp. 1–41. [Google Scholar]
- Oromiehie, A.; Mamizadeh, A. Recycling pet beverage bottles and improving properties. Poly. Int. 2004, 53, 728–732. [Google Scholar] [CrossRef]
- Genta, M.; Iwaya, T.; Sasaki, M.; Goto, M. Supercritical methanol for polyethylene terephthalate depolymerization: Observation using simulator. Waste Manag. 2007, 27, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Lancaster, N.L.; Sethi, A.R.; Welton, T. The role of hydrogen bonding in controlling the selectivity of Diels-Alder reactions in room-temperature ionic liquids. Green Chem. 2002, 4, 517–520. [Google Scholar] [CrossRef]
- Zhang, C.M.; Xu, L.; Zhang, H.H.; Yang, J.L.; Du, J.M.; Liu, Z.Y. Determination of solid products from the de-polymerization of poly (trimethylene terephthalate) in supercritical methanol. J. Chromatogr. A 2004, 1055, 115–121. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.E.N.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Depolymerization of poly(ethylene terephthalate) wastes using ethanol and ethanol/water in suipercritical conditions. J. Appl. Polymer Sci. 2006, 101, 2009–2016. [Google Scholar] [CrossRef]
- Nunes, C.S.; da Silva, M.J.V.; da Silva, D.C.; Freitas, A.D.; Rosa, F.A.; Rubira, A.F.; Muniz, E.C. Pet depolymerisation in supercritical ethanol catalysed by [BMIM][BF4]. Rsc Adv. 2014, 4, 20308–20316. [Google Scholar] [CrossRef]
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Su, Y.; Brown, H.M.; Li, G.S.; Zhou, X.D.; Amonette, J.E.; Fulton, J.L.; Camaioni, D.M.; Zhang, Z.C. Accelerated cellulose depolymerization catalyzed by paired metal chlorides in ionic liquid solvent. Appl. Catal. A 2011, 391, 436–442. [Google Scholar] [CrossRef]
- Gordon, C.M. New developments in catalysis using ionic liquids. Appl. Catal. A 2001, 222, 101–117. [Google Scholar] [CrossRef]
- Cammarata, L.; Kazarian, S.G.; Salter, P.A.; Welton, T. Molecular states of water in room temperature ionic liquids. Phys. Chem. Chem. Phys. 2001, 3, 5192–5200. [Google Scholar] [CrossRef] [Green Version]
- Kazarian, S.G.; Briscoe, B.J.; Welton, T. Combining ionic liquids and supercritical fluids: In situ ATR-IR study of CO2 dissolved in two ionic liquids at high pressures. Chem. Commun. 2000, 2047–2048. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Allin, S.M.; Siskin, M. Aquathermolysis: Reactions of organic compounds with superheated water. Acc. Chem. Res. 1996, 29, 399–406. [Google Scholar] [CrossRef]
- Siskin, M.; Katritzky, A.R. Reactivity of organic-compounds in hot water-geochemical and technological implications. Science 1991, 254, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Siskin, M.; Katritzky, A.R. A review of the reactivity of organic compounds with oxygen-containing functionality in superheated water. J. Anal. Appl. Pyrolysis 2000, 54, 193–214. [Google Scholar] [CrossRef]
- Akdemir, N.; Gumrukcuoglu, I.E.; Agar, E. Synthesis and characterization of novel phthalocyanines containing N-(n-octyl)mercapto acetamide substituents. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2005, 35, 819–824. [Google Scholar] [CrossRef]
- Kalkan, A.; Bayir, Z.A. Phthalocyanines with rigid carboxylic acid containing pendant arms. Polyhedron 2006, 25, 39–42. [Google Scholar] [CrossRef]
- Kulac, D.; Bulut, M.; Altindal, A.; Ozkaya, A.R.; Salih, B.; Bekaroglu, O. Synthesis and characterization of novel 4-nitro-2-(octyloxy)phenoxy substituted symmetrical and unsymmetrical Zn(ii), Co(ii) and Lu(iii) phthalocyanines. Polyhedron 2007, 26, 5432–5440. [Google Scholar] [CrossRef]
- Takahashi, C.; Shirai, T.; Fuji, M. Observation of interactions between hydrophilic ionic liquid and water on wet agar gels by FE-SEM and its mechanism. Mater. Chem. Phys. 2012, 133, 565–572. [Google Scholar] [CrossRef]
- Liu, W.W.; Zhao, T.Y.; Zhang, Y.M.; Wang, H.P.; Yu, M.F. The physical properties of aqueous solutions of the ionic liquid [BMIM][BF4]. J. Solut. Chem. 2006, 35, 1337–1346. [Google Scholar] [CrossRef]
- Wamser, C.A. Hydrolysis of fluoboric acid in aqueous solution. J. Am. Chem. Soc. 1948, 70, 1209–1215. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Khupse, N.D.; Kumar, A. Ionic liquids: New materials with wide applications. Indian J. Chem. Sect. A 2010, 49, 635–648. [Google Scholar]
- Donald, L.; Pavia, G.M.L.; Kriz, G.S.; Vyvyan, J.R. Introdução á Espectroscopia, 4th ed.; Norte-Americana ed.; CENGAGE Learning: São Paulo, Brazil, 2010. [Google Scholar]
- Wang, H.; Liu, Y.Q.; Li, Z.X.; Zhang, X.P.; Zhang, S.J.; Zhang, Y.Q. Glycolysis of poly(ethylene terephthalate) catalyzed by ionic liquids. Eur. Polym. J. 2009, 45, 1535–1544. [Google Scholar] [CrossRef]
- Chen, C.H. Study of glycolysis of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. Iii. Further investigation. J. Appl. Polym. Sci. 2003, 87, 2004–2010. [Google Scholar] [CrossRef]
- Ghaemy, M.; Mossaddegh, K. Depolymerisation of poly(ethylene terephthalate) fibre wastes using ethylene glycol. Polym. Degrad. Stab. 2005, 90, 570–576. [Google Scholar] [CrossRef]
- McGonigle, E.A.; Daly, J.H.; Gallagher, S.; Jenkins, S.D.; Liggat, J.J.; Olsson, I.; Pethrick, R.A. Physical ageing in poly(ethylene terephthalate)—its influence on cold crystallisation. Polymer 1999, 40, 4977–4982. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, J.; Li, C.X.; Gu, M.L.; Zhao, D.L.; Fan, Q.R. Study of the amorphous phase in semicrystalline poly(ethylene terephthalate) via dynamic mechanical thermal analysis. Polym. Bull. 2002, 49, 197–203. [Google Scholar] [CrossRef]
- Safavi, A.; Abdollahi, H.; Maleki, N.; Zeinali, S. Interaction of anionic dyes and cationic surfactants with ionic liquid character. J. Colloid Interface Sci. 2008, 322, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, A.J.; Seddon, K.R. Polarity study of some 1-alkyl-3-methylimidazolium ambient-temperature ionic liquids with the solvatochromic dye, nile red. J. Phys. Org. Chem. 2000, 13, 591–595. [Google Scholar] [CrossRef]
- Muldoon, M.J.; Gordon, C.M.; Dunkin, I.R. Investigations of solvent-solute interactions in room temperature ionic liquids using solvatochromic dyes. J. Chem. Soc.-Perkin Trans. 2 2001, 433–435. [Google Scholar] [CrossRef]
- Odabas, Z.; Altindal, A.; Bulut, M. Synthesis, characterization and electrical properties of novel metal-free, Co(ii), Cu(ii), Fe(ii), Mn(ii), Sn(ii) phthalocyanines peripherally tetra-substituted with 2,3-dihydro-1H-inden-5-yloxy moiety. Synth. Met. 2011, 161, 1742–1752. [Google Scholar] [CrossRef]
- Li, Z.; Sun, X.; Liu, J. Ionic liquids: Applications and perspectives. In Ionic Liquid as Novel Solvent for Extraction and Separation in Analytical Chemistry; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011; Volume 2, pp. 154–156. [Google Scholar]
- Dupont, J.; Consorti, C.S.; Suarez, P.A.Z.; de Souza, R.F. Preparation of 1-butyl-3-methyl imidazolium-based room temperature ionic liquids. Org. Synth. 2002, 79, 236. [Google Scholar] [CrossRef]
- Viana, M.E.; Riul, A.; Carvalho, G.M.; Rubira, A.F.; Muniz, E.C. Chemical recycling of pet by catalyzed glycolysis: Kinetics of the heterogeneous reaction. Chem. Eng. J. 2011, 173, 210–219. [Google Scholar] [CrossRef]










| Experimental Set | Ethanol/Water (v/v%) | PET Type | DET Yield (wt %) |
|---|---|---|---|
| exp1 | 100/0 | colourless | 98.0 |
| exp2 | 99/1 | colourless | 28.4 |
| exp3 | 98/2 | colourless | 13.8 |
| exp4 | 96/4 | colourless | 13.8 |
| exp5 | 100/0 | blue | 21.0 |
| exp6 | 100/0 | green | 66.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, C.S.; Souza, P.R.; Freitas, A.R.; Silva, M.J.V.d.; Rosa, F.A.; Muniz, E.C. Poisoning Effects of Water and Dyes on the [Bmim][BF4] Catalysis of Poly(Ethylene Terephthalate) (PET) Depolymerization under Supercritical Ethanol. Catalysts 2017, 7, 43. https://doi.org/10.3390/catal7020043
Nunes CS, Souza PR, Freitas AR, Silva MJVd, Rosa FA, Muniz EC. Poisoning Effects of Water and Dyes on the [Bmim][BF4] Catalysis of Poly(Ethylene Terephthalate) (PET) Depolymerization under Supercritical Ethanol. Catalysts. 2017; 7(2):43. https://doi.org/10.3390/catal7020043
Chicago/Turabian StyleNunes, Cátia S., Paulo R. Souza, Adonilson R. Freitas, Michael Jackson Vieira da Silva, Fernanda A. Rosa, and Edvani C. Muniz. 2017. "Poisoning Effects of Water and Dyes on the [Bmim][BF4] Catalysis of Poly(Ethylene Terephthalate) (PET) Depolymerization under Supercritical Ethanol" Catalysts 7, no. 2: 43. https://doi.org/10.3390/catal7020043
APA StyleNunes, C. S., Souza, P. R., Freitas, A. R., Silva, M. J. V. d., Rosa, F. A., & Muniz, E. C. (2017). Poisoning Effects of Water and Dyes on the [Bmim][BF4] Catalysis of Poly(Ethylene Terephthalate) (PET) Depolymerization under Supercritical Ethanol. Catalysts, 7(2), 43. https://doi.org/10.3390/catal7020043