Oligomerization of Butene Mixture over NiO/Mesoporous Aluminosilicate Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural Properties of Catalysts
2.2. Acid Properties of Catalysts
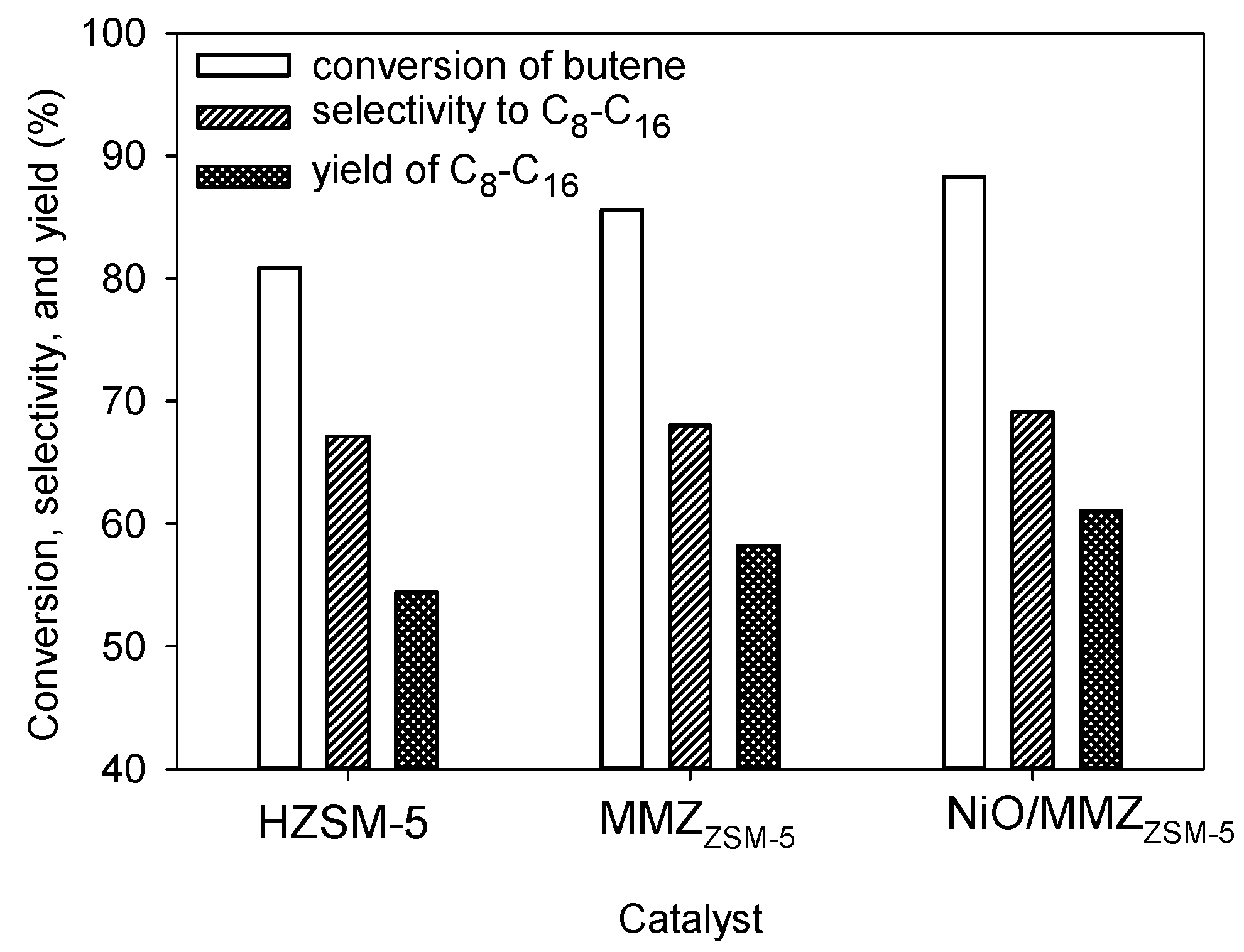
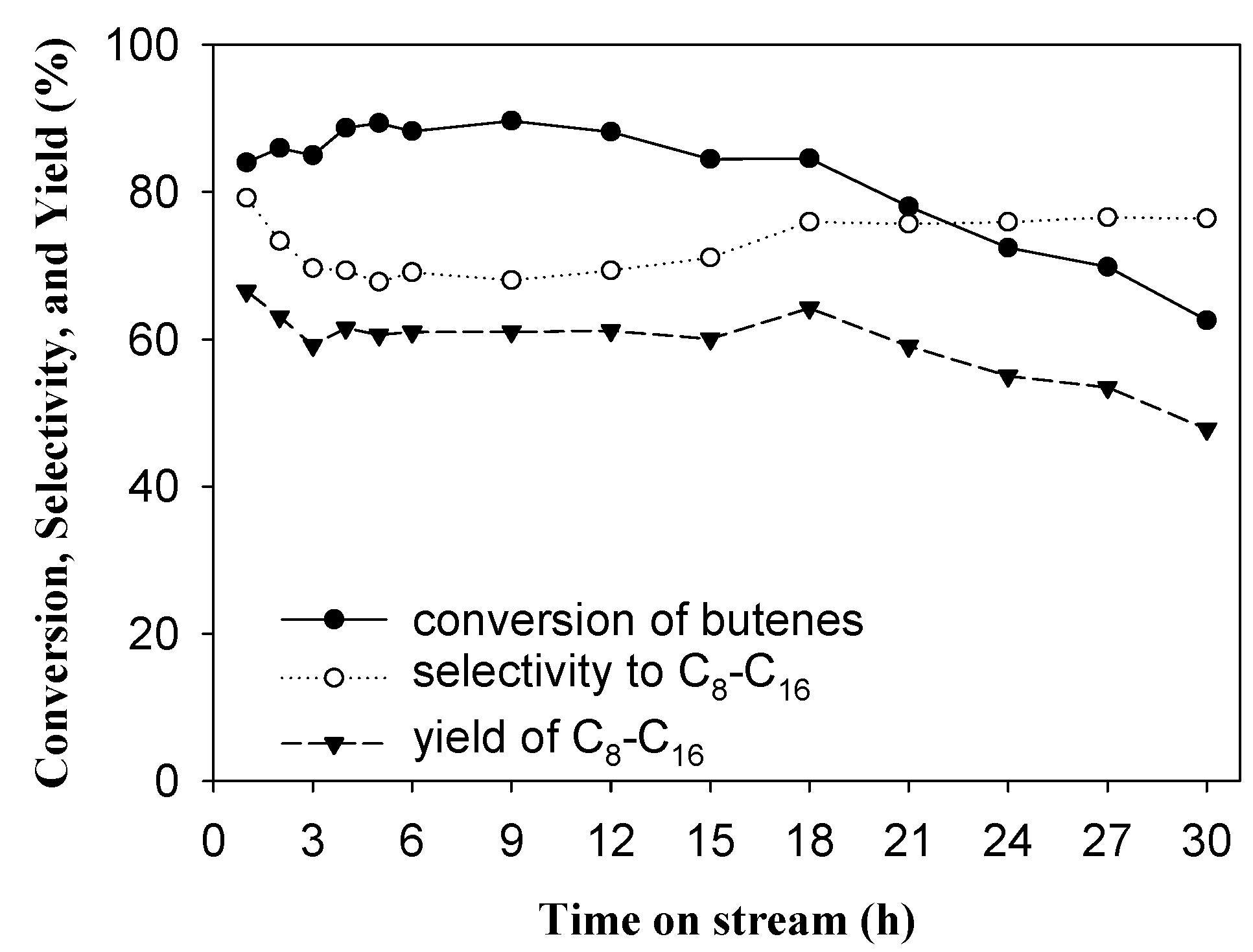
2.3. Oligomerization of Mixed-Butene
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization of Catalysts
3.3. Oligomerization of Butene Mixture
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fearnside, P.M. Global Warming and tropical land-use change: Greenhouse gas emissions from biomass burning, decomposition and soils in forest conversion, shifting cultivation and secondary vegetation. Clim. Chang. 2000, 46, 115–158. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Wang, W.C.; Tao, L. Bio-aviation fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef]
- Wright, M.E.; Harvey, B.G.; Quintana, R.L. Highly efficient zirconium-catalyzed batch conversion on 1-butene: A new route to aviation fuels. Energy Fuels 2008, 22, 3299–3302. [Google Scholar] [CrossRef]
- Wright, M.E.; Harvey, B.G.; Quintana, R.L. Diesel and Aviation Fuels Based on the oligomerization of butene. U.S. Patent US8395007B2, 12 March 2013. [Google Scholar]
- Matthew, W.P.; Taylor, J.D. Renewable Aviation Fuel Blendstock from Isobutanol. U.S. Patent US8373012B2, 12 February 2013. [Google Scholar]
- Wright, M.E. Process for the Dehydration of Aqueous Bio-Derived Terminal Alcohols to Terminal Alkenes. U.S. Patent US8912373B2, 16 December 2014. [Google Scholar]
- Zakaria, Z.Y.; Linnekoski, J.; Amin, N.A.S. Catalyst screening for conversion of glycerol to light olefins. Chem. Eng. J. 2012, 207–208, 803–813. [Google Scholar] [CrossRef]
- Korstanje, T.J.; Jastrzebski, J.T.B.H.; Kleingebbink, R.J.M. Mechanistic insights into the rhenium-catalyzed alcohol-to-olefin dehydration reaction. Chem. A Eur. J. 2013, 19, 13224–13234. [Google Scholar] [CrossRef] [PubMed]
- Kamiguchi, S.; Nagashima, S.; Komori, K.I.; Kodomari, M.; Chihara, T. Thermal activation of molecular tungsten halide clusters with the retention of an octahedral metal framework and the catalytic dehydration of alcohols to olefins as a solid acid catalyst. J. Clust. Sci. 2007, 18, 414–430. [Google Scholar] [CrossRef]
- Lee, I.C.; Clair, J.G.S.; Gamson, A.S. Catalytic Oxidative Dehydration of Butanol Isomers: 1-Butanol, 2-Butanol, and Isobutanol; Army Research Laboratory: Adelphi, MD, USA, 2011. [Google Scholar]
- Choi, H.; Bae, J.H.; Kim, D.H.; Park, Y.K.; Jeon, J.K. Butanol dehydration over V2O5-TiO2/MCM-41 catalysts prepared via liquid phase atomic layer deposition. Materials 2013, 6, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, H.; Bae, J.H.; Kim, D.H.; Peden, C.H.F.; Park, Y.K.; Jeon, J.K. Synthesis of butenes through 2-butanol dehydration over mesoporous materials produced from ferrierite. Catal. Today 2012, 185, 191–197. [Google Scholar] [CrossRef]
- Coelho, A.; Caeiro, G.; Lemos, M.; Lemos, F.; Ribeiro, F.R. 1-butene oligomerization over ZSM-5 zeolite: Part 1–Effect of reaction conditions. Fuel 2013, 111, 449–460. [Google Scholar] [CrossRef]
- Golombok, M.; de Bruijn, J. Dimerization of n-butenes for high octane gasoline components. Ind. Eng. Chem. Res. 2000, 39, 267–271. [Google Scholar] [CrossRef]
- Zhang, L.; Ke, M.; Song, Z.; Liu, Y.; Shan, W.; Wang, Q.; Xia, C.; Li, C.; He, C. Improvement of the catalytic efficiency of butene oligomerization using alkali metal hydroxide-modified hierarchical ZSM-5 Catalysts. Catalysts 2018, 8, 298. [Google Scholar] [CrossRef]
- Kocaman, E.; Akarçay, Ö.; Bağlar, N.; Çelebi, S.; Uzun, A. Isobutene oligomerization on MCM-41-supported tungstophosphoric acid. Mol. Catal. 2018, 457, 41–50. [Google Scholar] [CrossRef]
- Nadolny, F.; Hannebauer, B.; Alscher, F.; Peitz, S.; Reschetilowski, W.; Franke, R. Experimental and theoretical investigation of heterogeneous catalyzed oligomerization of a mixed C4 stream over modified amorphous aluminosilicates. J. Catal. 2018, 367, 81–94. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Zhu, S.; Liu, G.; Wu, J. Research on butene oligomerization reaction over the hemicellulose modified HZSM-5. J. Fuel Chem. Technol. 2017, 45, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.W.; Chang, J.S.; Lee, H.; Kim, T.J.; Jhung, S.H. Trimerization of isobutene over a zeolite beta catalyst. J. Catal. 2007, 245, 253–256. [Google Scholar] [CrossRef]
- Yoon, J.W.; Chang, J.S.; Lee, H.D.; Kim, T.J.; Jhung, S.H. Trimerization of isobutene over cation exchange resins: Effect of physical properties of the resins and reaction conditions. J. Mol. Catal. A Chem. 2006, 260, 181–186. [Google Scholar] [CrossRef]
- Yoon, J.W.; Jhung, S.H.; Chang, J.S. Trimerization of isobutene over solid acid catalysts: Comparison between cation-exchange resin and zeolite catalysts. Bull. Korean Chem. Soc. 2008, 29, 339–341. [Google Scholar]
- Mantilla, A.; Ferrat, G.; López-Ortega, A.; Romero, E.; Tzompantzi, F.; Torres, M.; Ortíz-Islas, E.; Gómez, R. Catalytic behavior of sulfated TiO2 in light olefins oligomerization. J. Mol. Catal. A Chem. 2005, 228, 333–338. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, J.; Wang, J.; Gao, J.; Liu, A. Trimerization of butene over Ni-doped zeolite catalyst: Effect of textural and acidic properties. Catal. Lett. 2008, 126, 388–395. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, J.; Wang, J.; Zhang, L.; Gao, J.; Liu, A. Catalytic performance and characterization of Ni-doped HZSM-5 catalysts for selective trimerization of n-butene. Fuel Process. Technol. 2009, 90, 863–870. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Park, Y.K.; Jeon, J.K. Synthesis of aviation fuel through the oligomerization of butenes on zeolite catalysts. Res. Chem. Intermed. 2018, 44, 3823–3833. [Google Scholar] [CrossRef]
- Lee, H.I.; Park, H.J.; Park, Y.K.; Hur, J.Y.; Jeon, J.K.; Kim, J.M. Synthesis of highly stable mesoporous aluminosilicates from commercially available zeolites and their application to the pyrolysis of woody biomass. Catal. Today 2008, 132, 68–74. [Google Scholar] [CrossRef]
- Bagshaw, S.A.; Baxter, N.I.; Brew, D.R.M.; Hosie, C.F.; Yuntong, N.; Jaenicke, S.; Khuan, C.G. Highly ordered mesoporous MSU-SBEA/zeolite Beta composite material. J. Mater. Chem. 2006, 16, 2235–2244. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Lee, H.I.; Kim, J.M.; Lee, J.Y.; Park, Y.K.; Jeon, J.K.; Yim, J.H.; Park, S.H.; Lee, K.J.; Kim, S.S.; Jeong, K.E. Catalytic conversion of 1,2-dichlorobenzene over mesoporous materials from zeolite. J. Nanosci. Nanotechnol. 2010, 10, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.M.; Starace, A.K.; Mukarakate, C.; Crow, A.M.; Leshnov, M.A.; Magrini, K.A. Biomass catalytic pyrolysis on Ni/ZSM-5: Effects of nickel pretreatment and loading. Energy Fuels 2016, 30, 5259–5268. [Google Scholar] [CrossRef]
- Chakraborty, B.; Viswanathan, B. Surface acidity of MCM-41 by in situ IR studies of pyridine adsorption. Catal. Today 1999, 49, 253–260. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Al-Sagheer, F.A.; Pasupulety, L. In situ FTIR spectra of pyridine adsorbed on SiO2-Al2O3, TiO2, ZrO2 and CeO2: General considerations for the identification of acid sites on surfaces of finely divided metal oxides. Colloids Surf. A Physicochem. Eng. Asp. 2001, 190, 261–274. [Google Scholar] [CrossRef]
- Popov, A.G.; Pavlov, V.S.; Ivanova, I.I. Effect of crystal size on butenes oligomerization over MFI catalysts. J. Catal. 2016, 335, 155–164. [Google Scholar] [CrossRef]
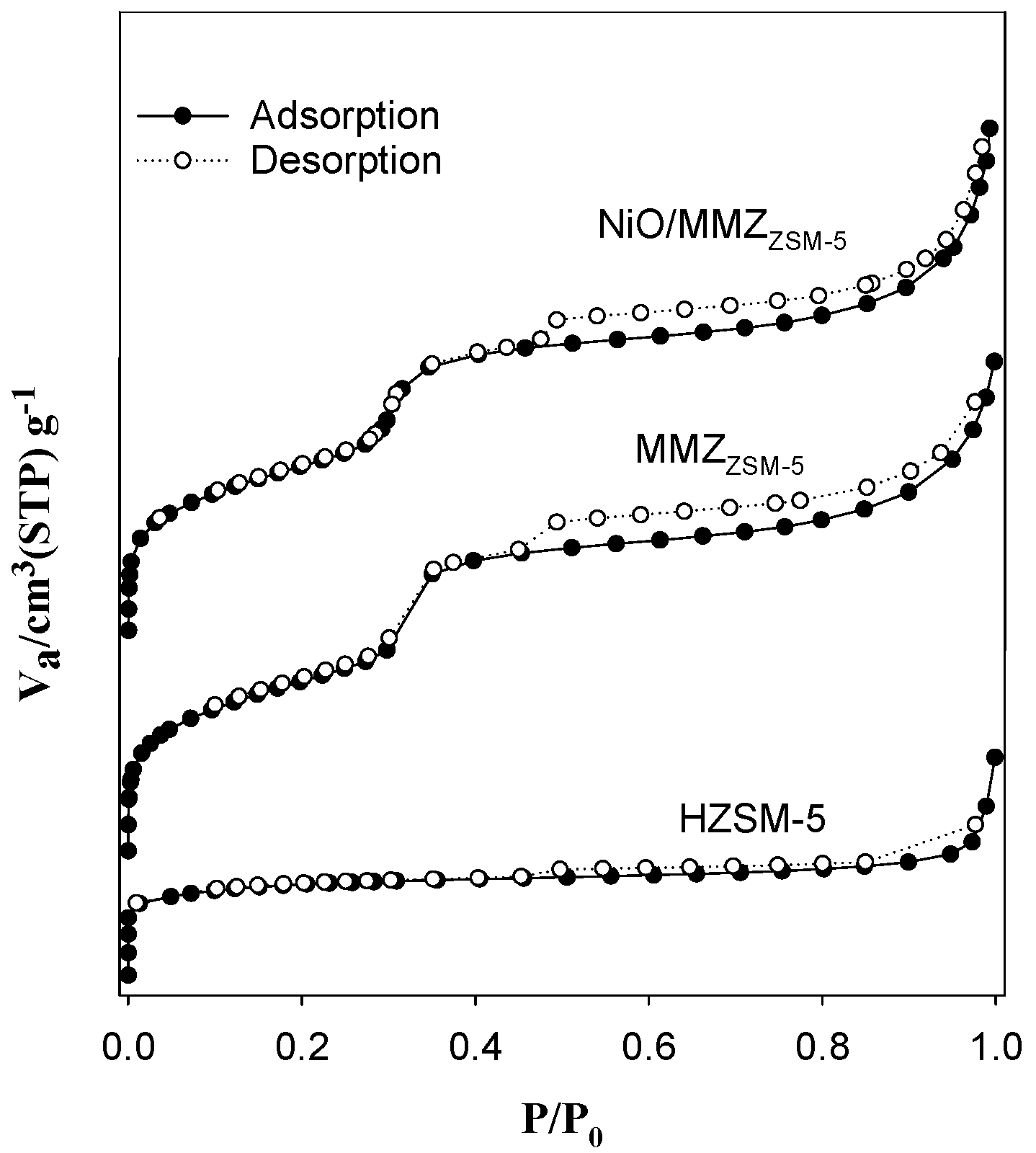
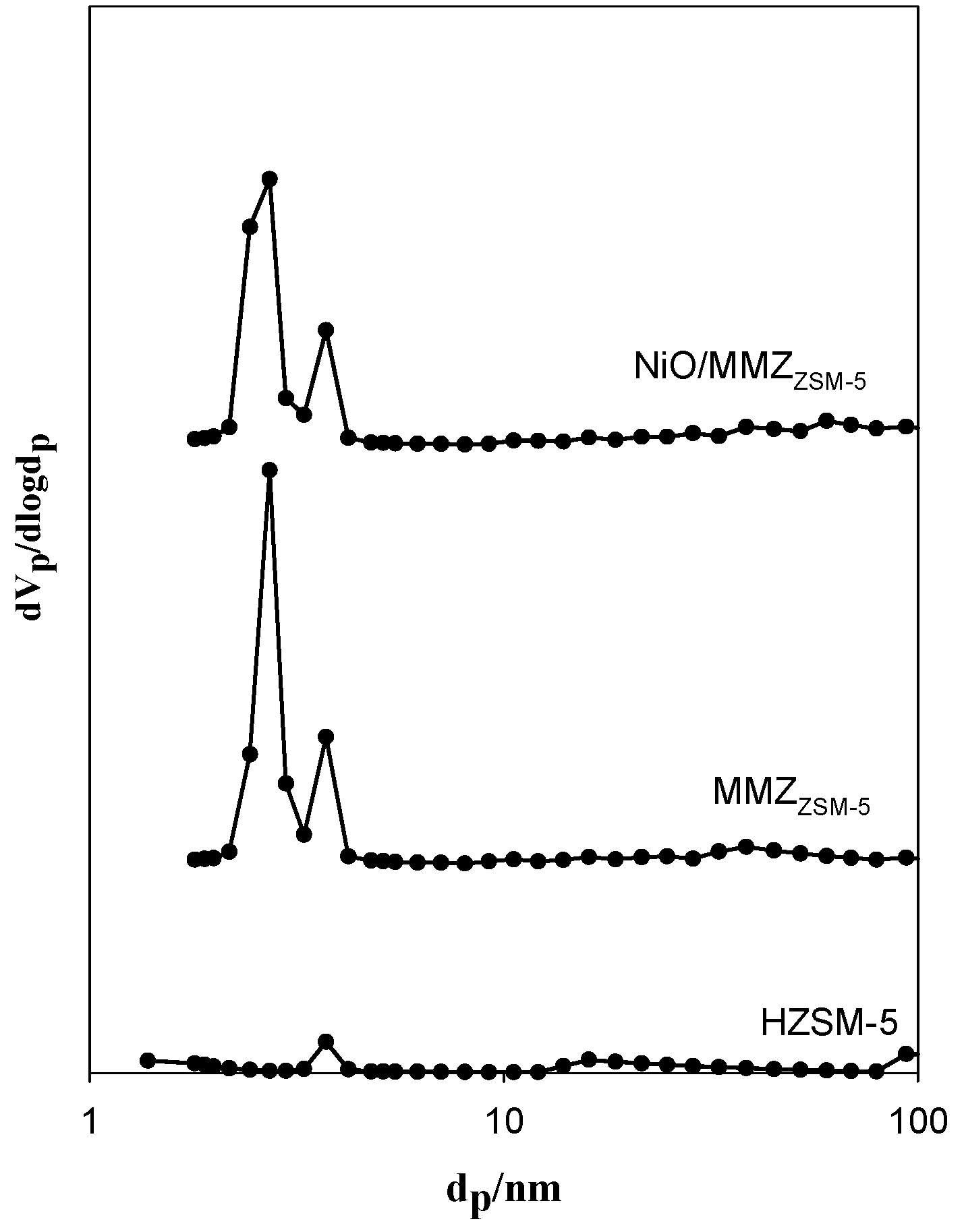
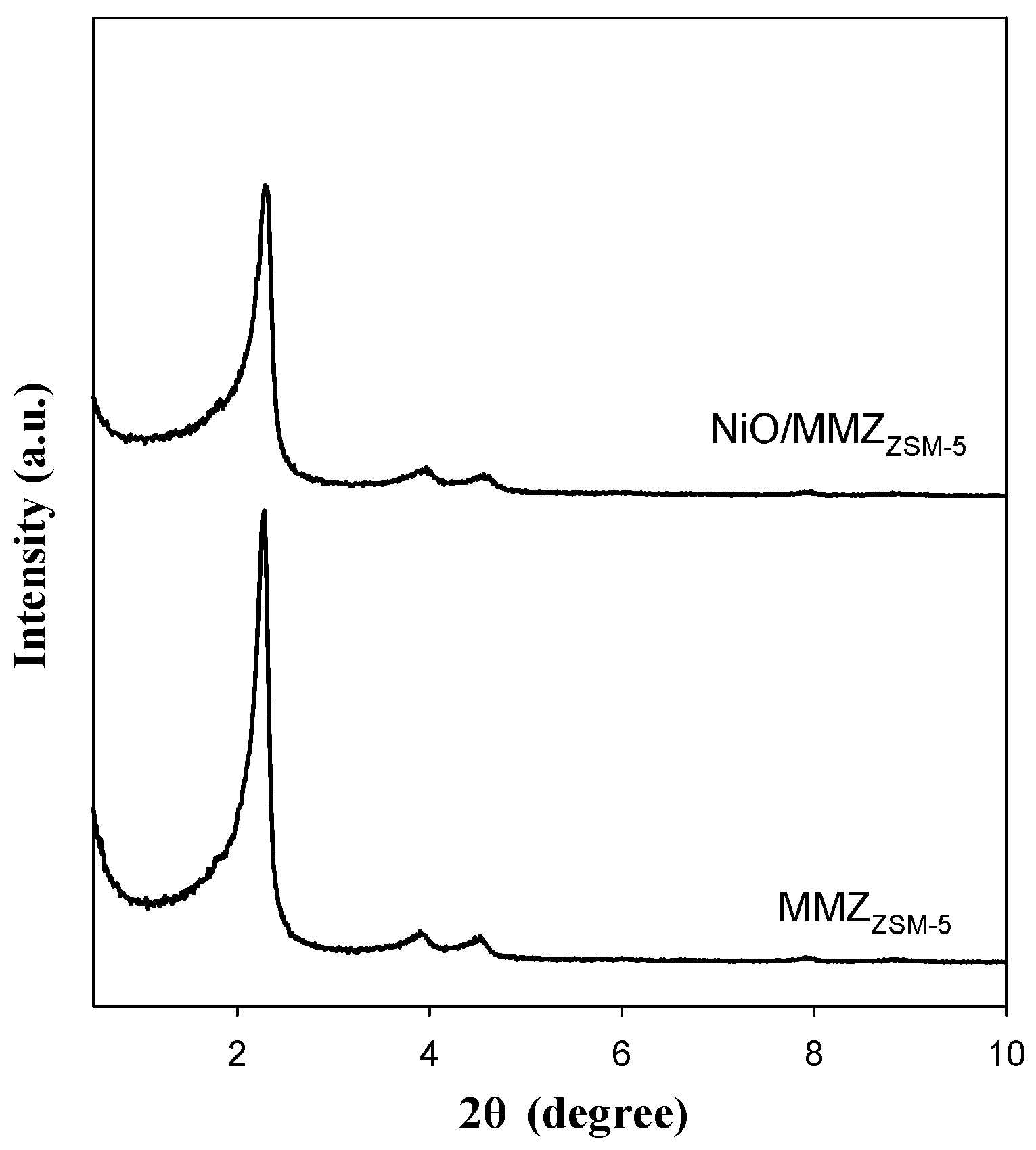
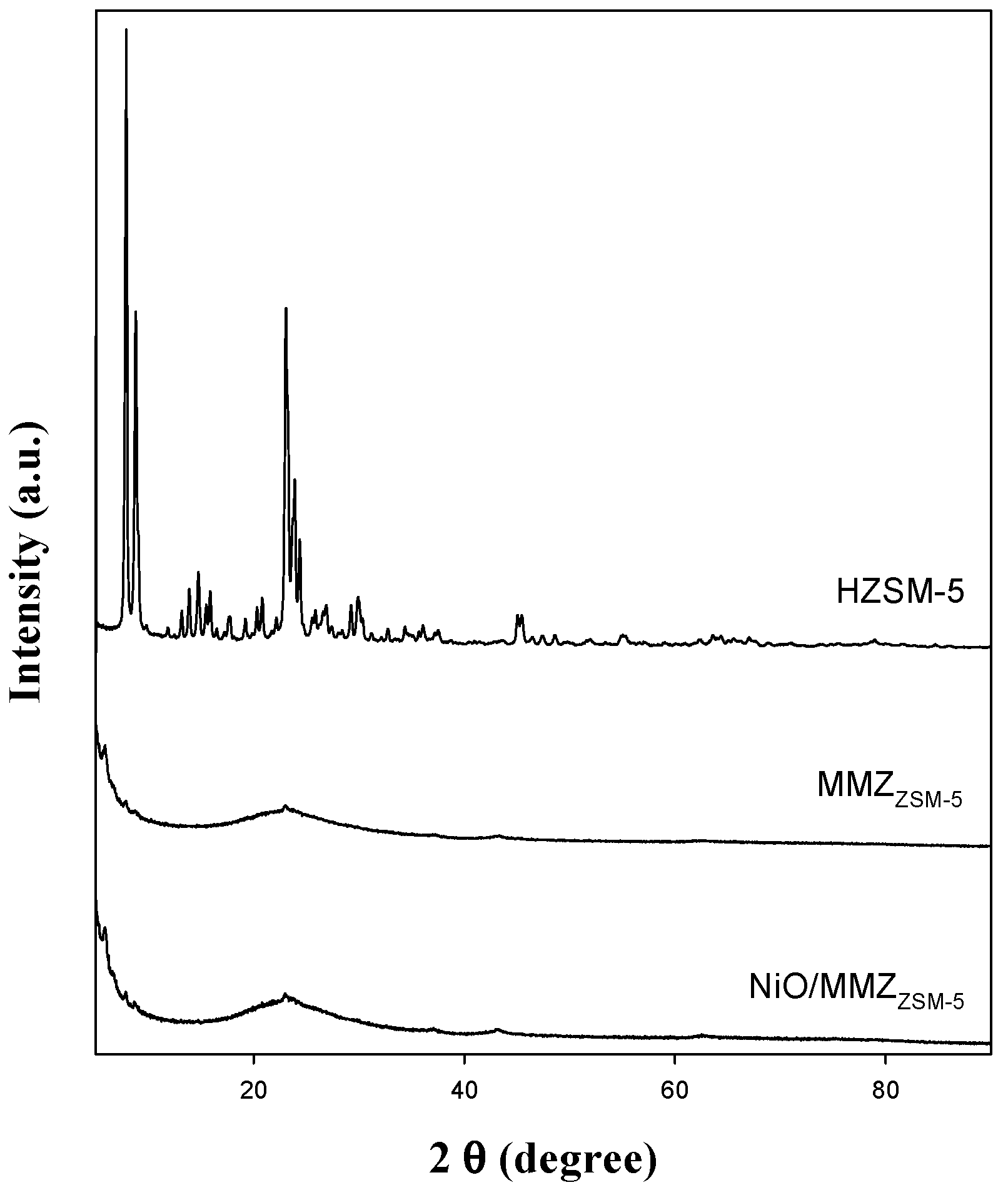
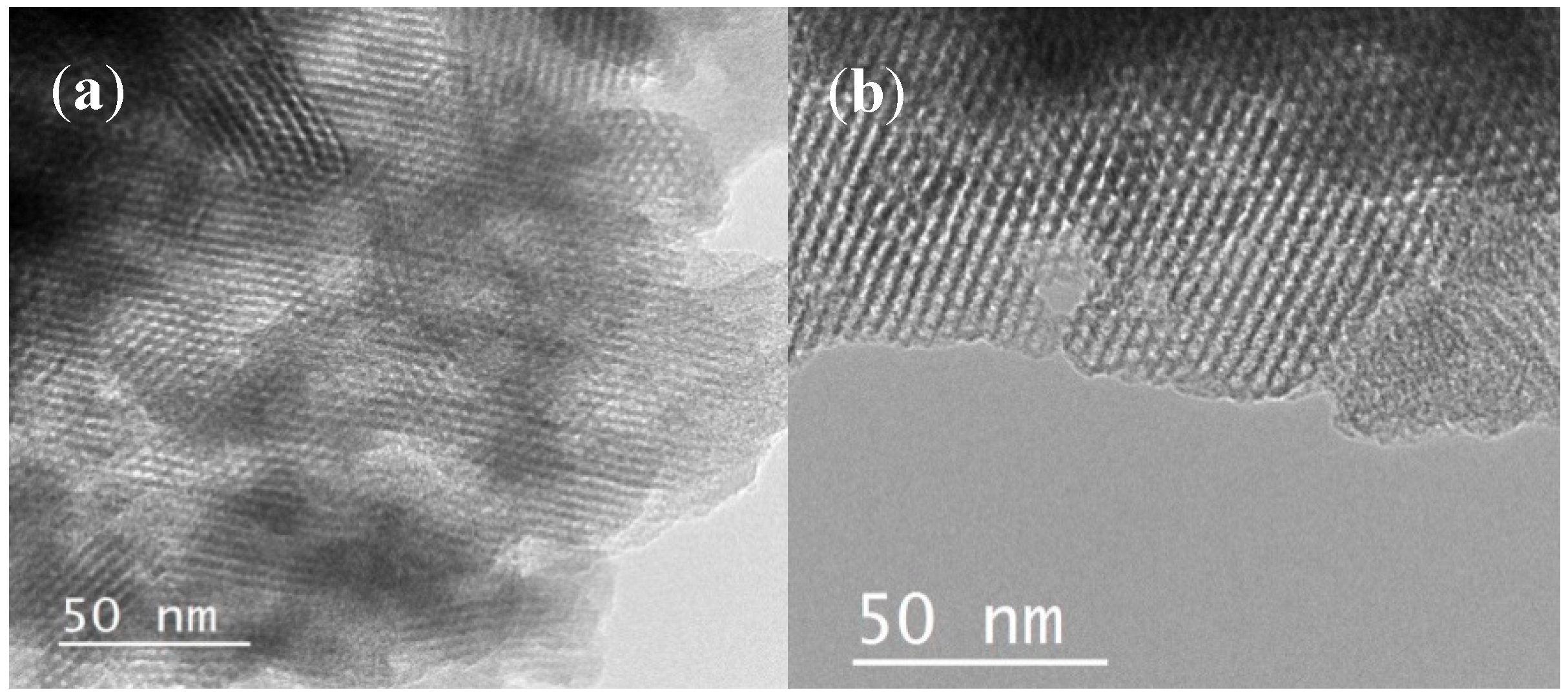
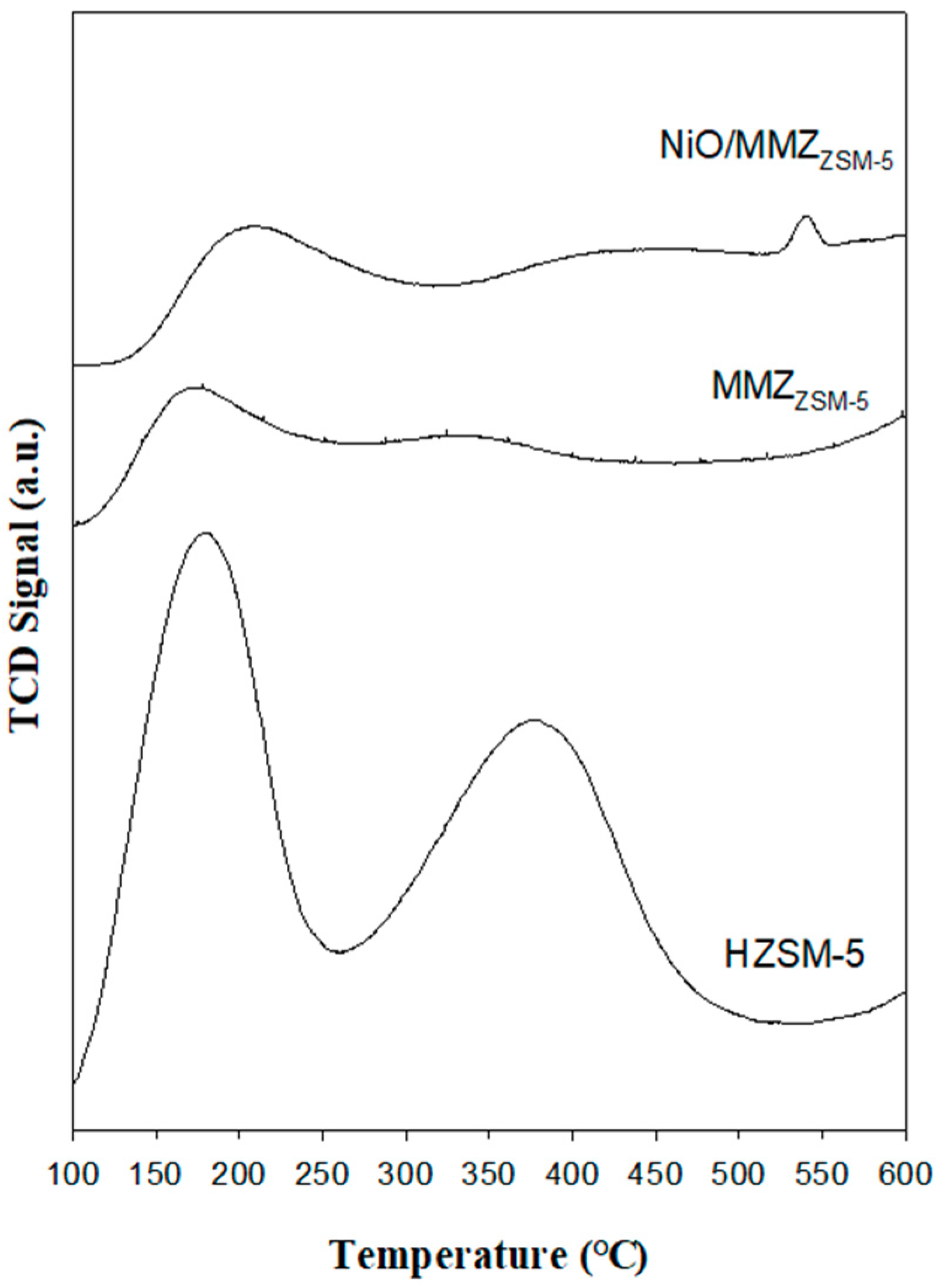
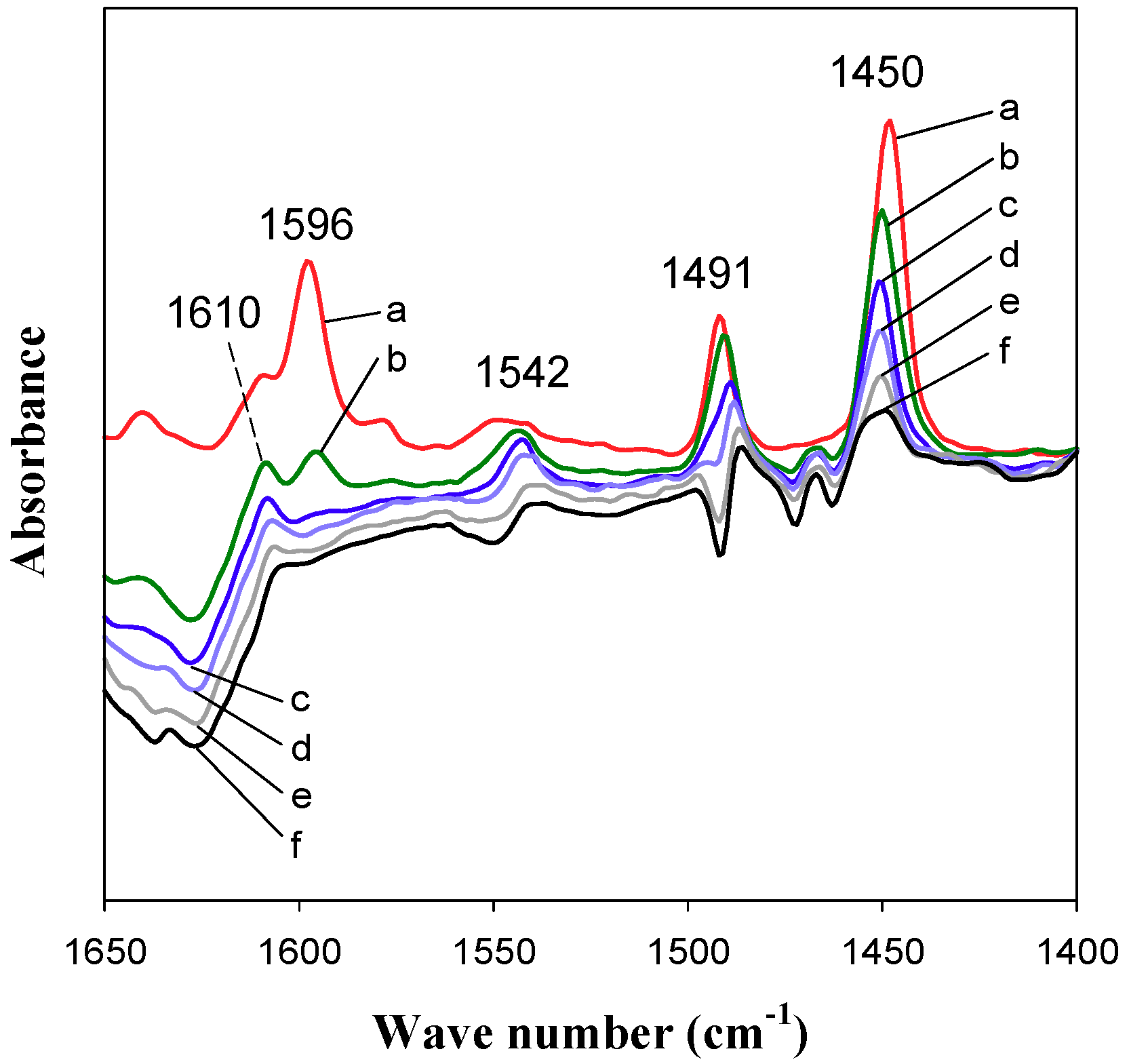
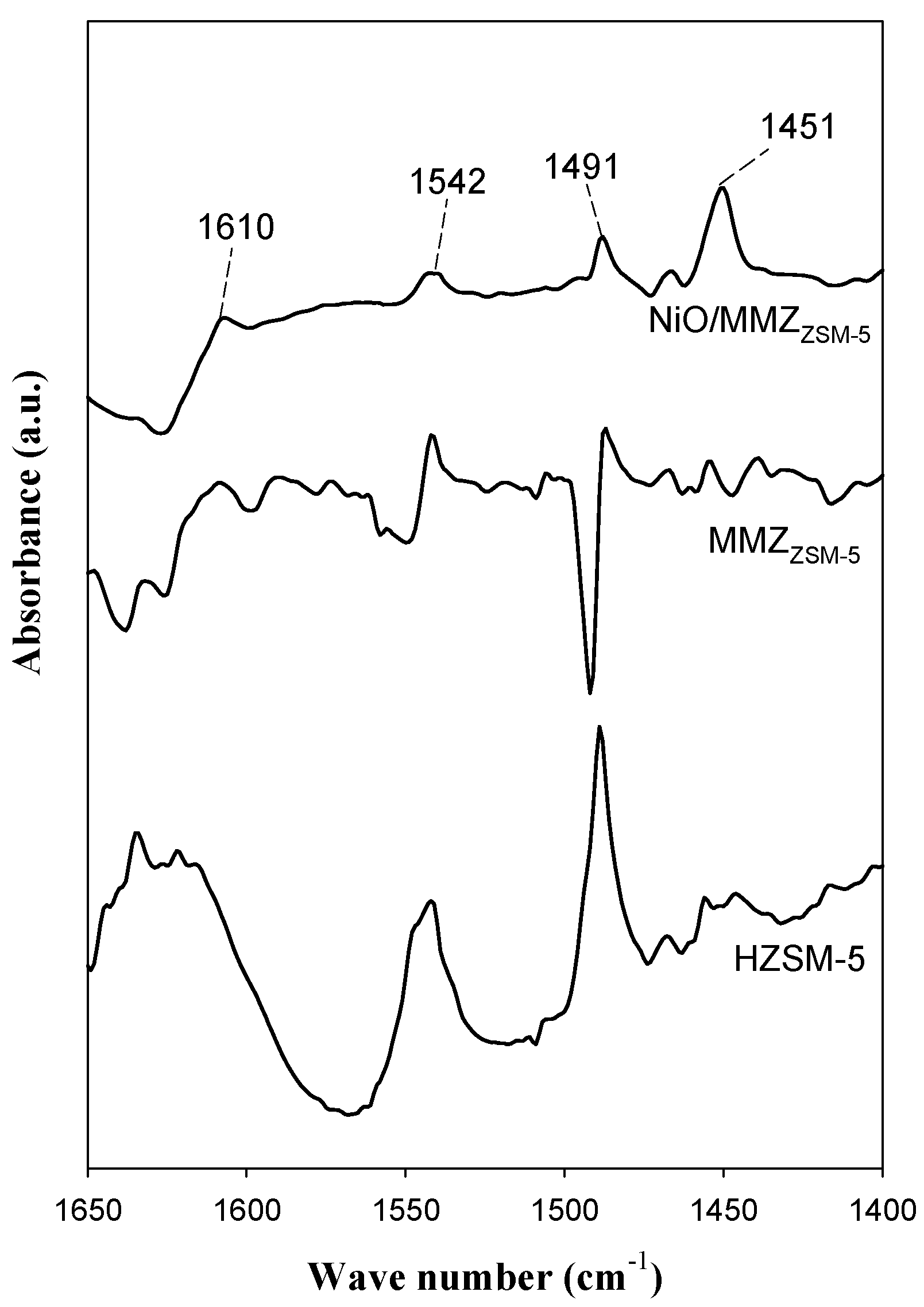



| Zeolite Type | SBET (m2 g−1) | VP (cm3 g−1) a | DP (nm) b |
|---|---|---|---|
| HZSM-5 | 448 | 0.19 | - |
| MMZZSM-5 | 745 | 0.47 | 2.7 |
| NiO/MMZZSM-5 | 726 | 0.43 | 2.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, H.; Park, Y.-K.; Jeon, J.-K. Oligomerization of Butene Mixture over NiO/Mesoporous Aluminosilicate Catalyst. Catalysts 2018, 8, 456. https://doi.org/10.3390/catal8100456
Lee D, Kim H, Park Y-K, Jeon J-K. Oligomerization of Butene Mixture over NiO/Mesoporous Aluminosilicate Catalyst. Catalysts. 2018; 8(10):456. https://doi.org/10.3390/catal8100456
Chicago/Turabian StyleLee, Donggun, Hyeona Kim, Young-Kwon Park, and Jong-Ki Jeon. 2018. "Oligomerization of Butene Mixture over NiO/Mesoporous Aluminosilicate Catalyst" Catalysts 8, no. 10: 456. https://doi.org/10.3390/catal8100456
APA StyleLee, D., Kim, H., Park, Y.-K., & Jeon, J.-K. (2018). Oligomerization of Butene Mixture over NiO/Mesoporous Aluminosilicate Catalyst. Catalysts, 8(10), 456. https://doi.org/10.3390/catal8100456






