Comparative Study Between Aluminum Monolith and Foam as Carriers for The Intensification of The CO Water Gas Shift Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization Results
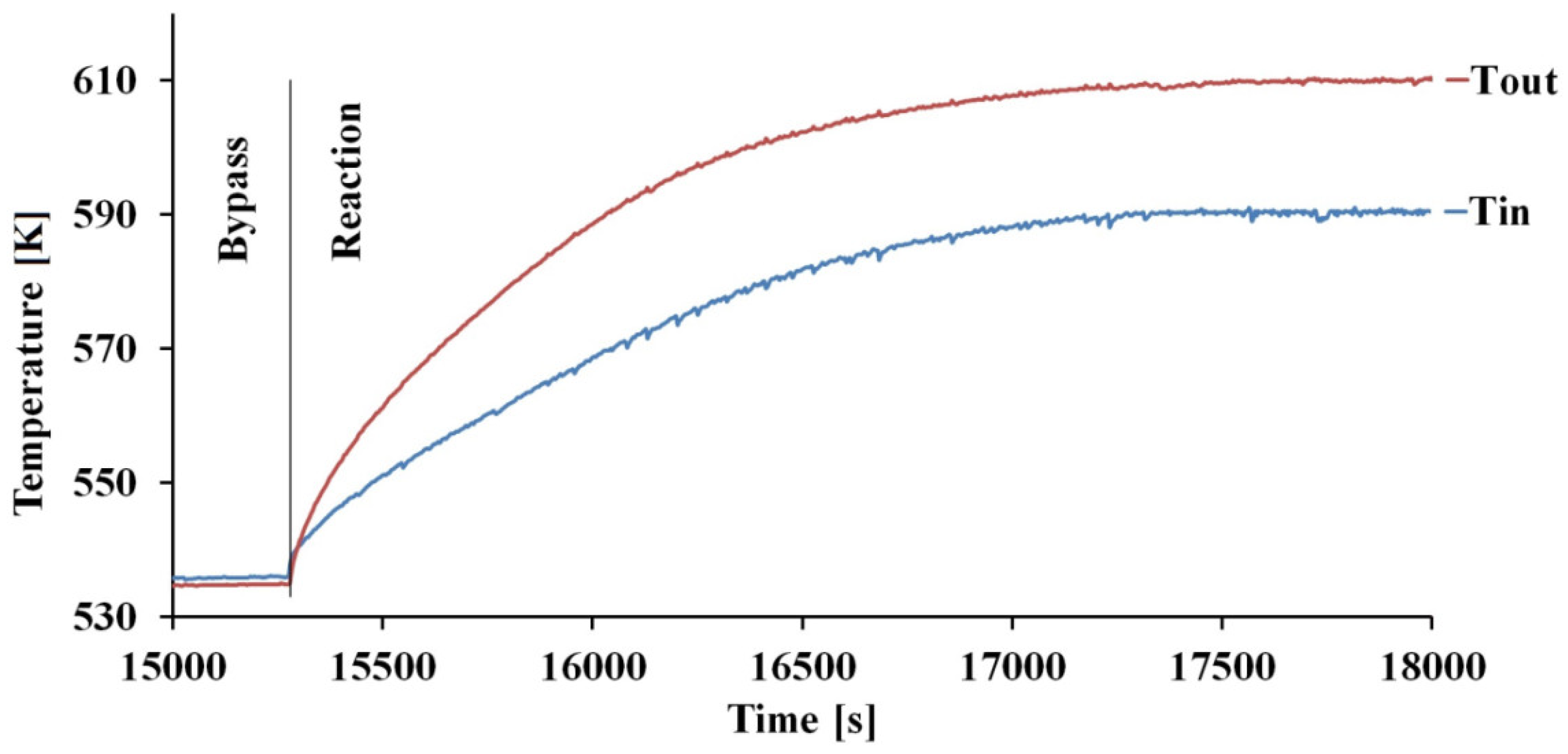
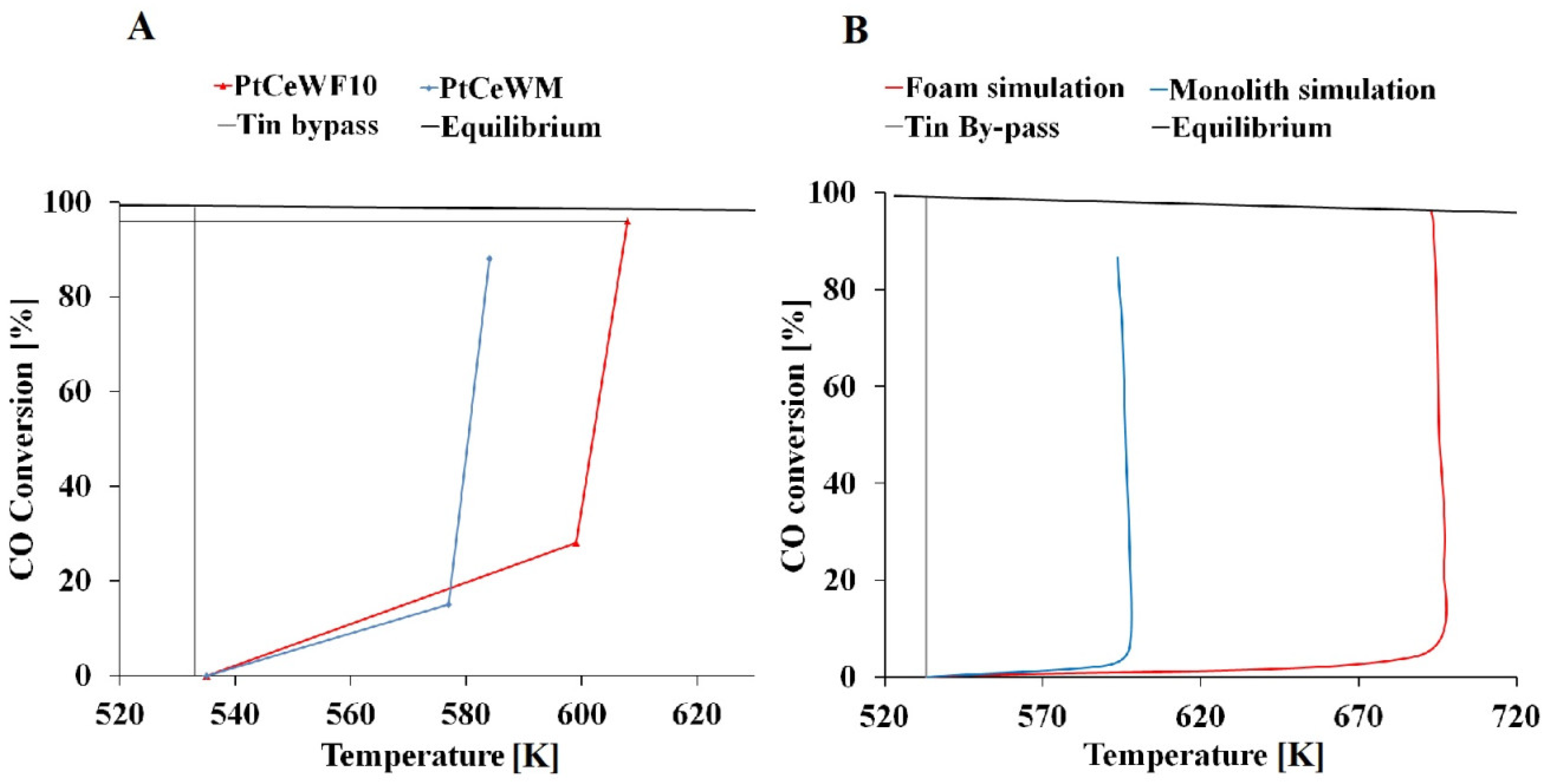
2.2. Activity Tests
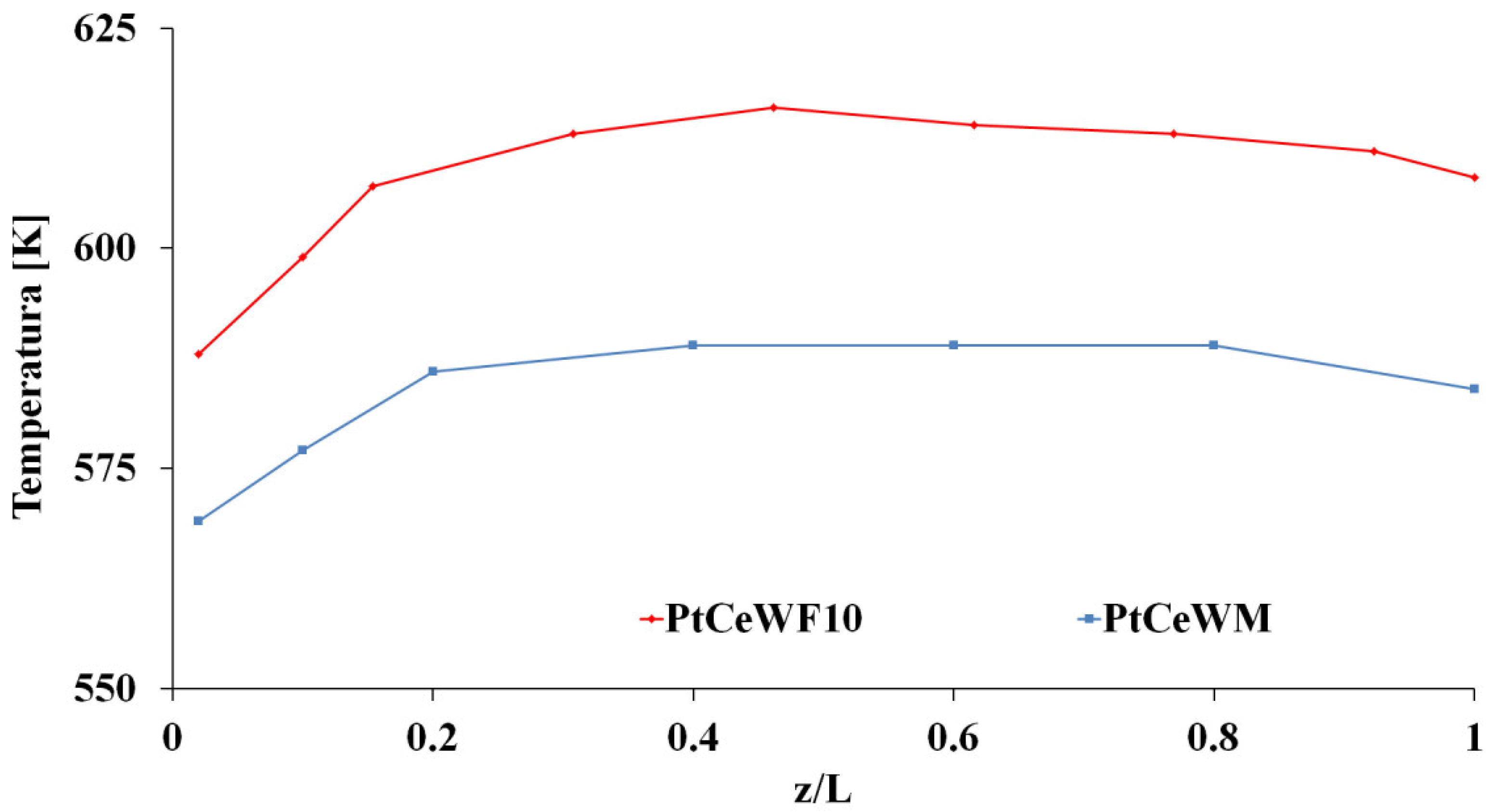
2.3. Transport Phenomena Aspects
3. Materials and Methods
3.1. Carriers Preparation
3.2. Washcoat Preparation
3.3. Dip-Coating Procedure
3.4. Catalysts Preparation
3.5. Catalysts Characterization
3.6. Catalytic Activity Tests
3.7. Experimental Apparatus
3.8. CFD Modeling
- Total volumetric flow rate: Q0 = 2000 Ncm3/min;
- Volumetric fraction of carbon monoxide: yCOIN = 0.21;
- Volumetric fraction of steam water: yH2OIN = 0.79;
- Temperature at the inlet of the reaction system: TIN = 533 K;
- Thermal conductivity of bulk aluminum: k = 171 W/m/K;
- Effective thermal conductivity of the solid carriers: calculated as volumetric average;
- Foam carrier void fraction: ε = 89%;
- Foam carrier permeability: K = 1 × 10−4 m2;
- Density of the noble metal-based catalyst: ρ = 600 kg/m3;
- Standard heat of reaction: ΔHR(298K) = −41.17 kJ/mol.
- MOMENTUM BALANCE
- At z = 0 → U = U0 (average gas velocity) = Q0/S where S is the cross-sectional area;
- At z = L → p = 1 bar (atmospheric pressure).
- ENERGY BALANCE
- At z = 0 → T = TIN (temperature at the inlet of the reaction system);
- At z = L → −dT/dz = 0.
- MASS BALANCE
- At z = 0 → yCO = yCOIN, yH2O = yH2OIN;
- At z = L → −dCi/dz = 0 where Ci = molar concentration of the i-species.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bukur, D.B.; Todic, B.; Elbashir, N. Role of water-gas-shift reaction in Fischer-Tropsch synthesis on iron catalysts: a review. Catal. Today 2016, 275, 66–75. [Google Scholar] [CrossRef]
- Baschuk, J.J.; Li, X. Carbon monoxide poisoning of proton exchange membrane fuel cells. Int. J. Energy Res. 2001, 25, 695–713. [Google Scholar] [CrossRef]
- Zyryanova, M.M.; Snytnikov, P.V.; Amosov, Y.I.; Ven’yaminov, S.A.; Golosman, E.Z.; Sobyanin, V.A. Selective methanation of CO in the presence of CO2 in hydrogen-containing mixtures on nickel catalysts. Kinet. Catal. 2010, 51, 907–913. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, F.; Li, J.; Chen, K.; Yao, Y.; Li, P.; Zhu, M.; Shi, Y.; Wang, Q.; Guo, X. High CO Methanation Performance of Two-Dimensional Ni/MgAl Layered Double Oxide with Enhanced Oxygen Vacancies via Flash Nanoprecipitation. Catalysts 2018, 8, 363. [Google Scholar] [CrossRef]
- Wootsch, A.; Descorme, C.; Duprez, D. Preferential oxidation of carbon monoxide in the presence of hydrogen (PROX) over ceria–zirconia and alumina-supported Pt catalysts. J. Catal. 2004, 225, 259–266. [Google Scholar] [CrossRef]
- Fiorenza, R.; Spitaleri, L.; Gulino, A.; Scirè, S. Ru–Pd Bimetallic Catalysts Supported on CeO2-MnOX Oxides as Efficient Systems for H2 Purification through CO Preferential Oxidation. Catalysts 2018, 8, 203. [Google Scholar] [CrossRef]
- Fernandez, E.; Helmi, A.; Coenen, K.; Melendez, J.; Viviente, J.L.; Pacheco Tanaka, D.A.; Van Sint Annaland, M.; Gallucci, F. Development of thin Pd–Ag supported membranes for fluidized bed membrane reactors including WGS related gases. Int. J. Hydrogen Energy 2015, 40, 3506–3519. [Google Scholar] [CrossRef]
- Tocci, E.; Pullumbi, P. Molecular simulation of realistic membrane models of alkylated PEEK membranes. Mol. Simul. 2006, 32, 145–154. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Aghaeinejad-Meybodi, A.; Basile, A. Hydrogen production as a green fuel in silica membrane reactor: Experimental analysis and artificial neural network modeling. Fuel 2018, 222, 114–124. [Google Scholar] [CrossRef]
- Hallac, B.B.; Brown, J.C.; Stavitski, E.; Harrison, R.G.; Argyle, M.D. In Situ UV-Visible Assessment of Iron-Based High-Temperature Water-Gas Shift Catalysts Promoted with Lanthana: An Extent of Reduction Study. Catalysts 2018, 8, 63. [Google Scholar] [CrossRef]
- Kraussler, M.; Binder, M.; Fail, S.; Bosch, K.; Hackel, M.; Hofbauer, H. Performance of a water gas shift pilot plant processing product gas from an industrial scale biomass steam gasification plant. Biomass Bioenergy 2016, 89, 50–57. [Google Scholar] [CrossRef]
- Uchida, H.; Isogai, N.; Oba, M.; Hasegawa, T. The Zinc Oxide-Copper Catalyst for Carbon Monoxide-Shift Conversion. I. The Dependency of the Catalytic Activity on the Chemical Composition of the Catalyst. Bull. Chem. Soc. Jpn. 1967, 40, 1981–1986. [Google Scholar] [CrossRef]
- Reay, D.; Ramshaw, C.; Harvey, A. Process Intensification, Engineering for Efficiency: Sustainability and Flexibility, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Palma, V.; Pisano, D.; Martino, M.; Ricca, A.; Ciambelli, P. High Thermal Conductivity Structured Carriers for Catalytic Processes Intensification. Chem. Eng. Trans. 2015, 43, 2047–2052. [Google Scholar] [CrossRef]
- Palma, V.; Martino, M. Pt-Re Based Catalysts for the Realization of a Single Water Gas Shift Process. Chem. Eng. Trans. 2017, 57, 1657–1662. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, L.; Zhou, L.; Ma, L.; He, Y.; Zhang, X.; Gao, J. Structured interlocked-microcapsules: A novel scaffold for enzyme immobilization. Catal. Commun. 2017, 88, 35–38. [Google Scholar] [CrossRef]
- Tomašić, V.; Gomzi, Z. Development of the Structured Catalysts for the Exhaust Gas Treatment. Chem. Biochem. Eng. Q. 2001, 15, 109–115. [Google Scholar]
- Tronconi, E.; Groppi, G.; Visconti, C.G. Structured catalysts for non-adiabatic applications. Curr. Opin. Chem. Eng. 2014, 5, 55–67. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Camacho, Y.S.M.; Bensaid, S.; Lorentzou, S.; Russo, N.; Fino, D. Structured catalytic reactor for soot abatement in a reducing atmosphere. Fuel Process. Technol. 2017, 167, 462–473. [Google Scholar] [CrossRef]
- Brautsch, A.; Griffin, T.; Schlegel, A. Heat transfer characterization of support structures for catalytic combustion. Int. J. Heat Mass Transfer 2002, 45, 3223–3231. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Mass-Transfer Characterization of Metallic Foams as Supports for Structured Catalysts. Ind. Eng. Chem. Res. 2005, 44, 4993–5002. [Google Scholar] [CrossRef]
- Wu, D.; Kong, S.; Zhang, H.; Li, Y. Mechanical stability of monolithic catalysts: Factors affecting washcoat adhesion and cohesion during preparation. Aiche J. 2014, 60, 2765–2773. [Google Scholar] [CrossRef]
- Palma, V.; Pisano, D.; Martino, M. CFD modeling of the influence of carrier thermal conductivity for structured catalysts in the WGS reaction. Chem. Eng. Sci. 2018, 178, 1–11. [Google Scholar] [CrossRef]
- Palma, V.; Pisano, D.; Martino, M.; Ciambelli, P. Structured catalysts with high thermoconductive properties for the intensification of Water Gas Shift process. Chem. Eng. J. 2016, 304, 544–551. [Google Scholar] [CrossRef]
- Baronskaya, N.A.; Khasin, A.A.; Smirnov, E.I.; Yur’eva, T.M. Variants of the organization of a controlled-temperature-profile catalyst bed in a tubular reactor for the single-step water gas shift reaction. Theor. Found. Chem. Eng. 2009, 43, 366–373. [Google Scholar] [CrossRef]
- Van Dijk, H.A.J.; Boon, J.; Nyqvist, R.N.; Van Den Brink, R.W. Development of a single stage heat integrated water-gas shift reactor for fuel processing. Chem. Eng. J. 2010, 159, 182–189. [Google Scholar] [CrossRef]
- Eigenberger, G. Catalytic fixed-bed reactors. Handb. Heterogen. Catal. 2008, 10, 2075–2106. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-Based Catalysts for the High-Temperature Water-Gas Shift (HTWGS) Reaction: A Review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Kam, R.; Scott, J.; Amal, R.; Selomulya, C. Pyrophoricity and stability of copper and platinum based water-gas shift catalysts during oxidative shut-down/start-up operation. Chem. Eng. Sci. 2010, 65, 6461–6467. [Google Scholar] [CrossRef]
- Song, L.; Lu, Z.; Zhang, Y.; Su, Q.; Li, L. Hydrogen-Etched TiO2-x as Efficient Support of Gold Catalysts for Water-Gas Shift Reaction. Catalysts 2018, 8, 26. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Dosa, M.; Novara, C.; Giorgis, F.; Russo, N.; Fino, D. Nanostructured Ceria-Based Materials: Effect of the Hydrothermal Synthesis Conditions on the Structural Properties and Catalytic Activity. Catalysts 2017, 7, 174. [Google Scholar] [CrossRef]
- Santos, J.L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Operando DRIFTS-MS Study of WGS and rWGS Reaction on Biochar-Based Pt Catalysts: The Promotional Effect of Na. J. Carbon Res. 2018, 4, 47. [Google Scholar] [CrossRef]
- Jeong, D.-W.; Potdar, H.S.; Shim, J.-O.; Jang, W.-J.; Roh, H.-S. H2 production from a single stage water gas shift reaction over Pt/CeO2, Pt/ZrO2, and Pt/Ce(1Lx)Zr(x)O2 catalysts. Int. J. Hydrogen Energy 2013, 38, 4502–4507. [Google Scholar] [CrossRef]
- Palma, V.; Pisano, D.; Martino, M. Structured noble metal-based catalysts for the WGS process intensification. Int. J. Hydrogen Energy 2018, 43, 11745–11754. [Google Scholar] [CrossRef]
- Palma, V.; Pisano, D.; Martino, M. The influence of the textural properties of aluminum foams as catalyst carriers for water gas shift process. Int. J. Hydrogen Energy 2017, 42, 23517–23525. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Cataly. Rev. Sci. Eng. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Valentini, M.; Groppi, G.; Cristiani, C.; Levi, M.; Tronconi, E.; Forzatti, P. The deposition of γ-Al2O3 layers on ceramic and metallic supports for the preparation of structured catalysts. Catal. Today 2001, 69, 307–314. [Google Scholar] [CrossRef]
- Barbero, B.P.; Costa-Almeida, L.; Sanz, O.; Morales, M.R.; Cadus, L.E.; Montes, M. Washcoating of metallic monoliths with a MnCu catalyst for catalytic combustion of volatile organic compounds. Chem. Eng. J. 2008, 139, 430–435. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Kocaefe, Y.; Cheng, J.; Liu, W. The Effect of Novel Synthetic Methods and Parameters Control on Morphology of Nano-alumina Particles. Nanosc. Res. Lett. 2016, 11, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Adamowska, M.; Da Costa, P. Structured Pd/γ-Al2O3 Prepared by Washcoated Deposition on a Ceramic Honeycomb for Compressed Natural Gas Applications. J. Nanopart. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.-K.; Kim, N.; Park, J.-G.; Paik, U. Crystalline structure of ceria particles controlled by the oxygen partial pressure and STI CMP performances. Ultramicroscopy 2008, 108, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Borodziński, A.; Bonarowska, M. Relation between Crystallite Size and Dispersion on Supported Metal Catalysts. Langmuir 1997, 13, 5613–5620. [Google Scholar] [CrossRef]
- Damyanova, S.; Bueno, J.M.C. Effect of CeO2 loading on the surface and catalytic behaviors of CeO2-Al2O3-supported Pt catalysts. Appl. Catal. A Gen. 2003, 253, 135–150. [Google Scholar] [CrossRef]
- Dutta, G.; Waghmare, U.V.; Baidya, T.; Hegde, M.S. Hydrogen spillover on CeO2/Pt: enhanced storage of active hydrogen. Chem. Mater. 2007, 19, 6430–6436. [Google Scholar] [CrossRef]
- Tiana, J.; Lin, J.; Xua, M.; Wan, S.; Lin, J.; Wang, Y. Hexagonal boron nitride catalyst in a fixed-bed reactor for exothermic propane oxidation dehydrogenation. Chem. Eng. Sci. 2018, 186, 142–151. [Google Scholar] [CrossRef]
- Salzano, E.; Cammarota, F.; Di Benedetto, A.; Di Sarli, V. Explosion behavior of hydrogenemethane/air mixtures. J. Loss Prev. Process Ind. 2012, 25, 443–447. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A. The effect of powder characteristics on washcoat quality. Part I: Alumina washcoats. J. Eur. Ceram. Soc. 2000, 20, 815–824. [Google Scholar] [CrossRef]
- Yasaki, S.; Yoshino, Y.; Ihara, K.; Ohkubo, K. Method of Manufacturing an Exhaust Gas Purifying Catalyst. U.S. Patent 5,208,206, 4 May 1993. [Google Scholar]







| Sample | H2 Uptake (mmol/g) | Chemical Composition (wt %) | SSAB.E.T. (m2/g) |
|---|---|---|---|
| Washcoat powder (W) | - | γ-Al2O3 (100) | 186 |
| Ceria/washcoat (CeW) | - | γ-Al2O3 (73)—CeO2 (27) | 138 |
| Pt/ceria/washcoat (PtCeW) | - | γ-Al2O3 (73) CeO2 (26)—Pt (1) | 132 |
| Pt/ceria/washcoat/foam 10_10/12 (PtCeWF10) | 1.01 | - | - |
| Pt/ceria/washcoat/monolith (PtCeWM) | 1.06 | - | - |
| Species | Sample | Peak | Crystallite Size (nm) |
|---|---|---|---|
| Ceria | PtCeW | 111 | 7.5 |
| γ-alumina | - | 440 | 4.8 |
| Platinum | - | - | Not determined |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, V.; Pisano, D.; Martino, M. Comparative Study Between Aluminum Monolith and Foam as Carriers for The Intensification of The CO Water Gas Shift Process. Catalysts 2018, 8, 489. https://doi.org/10.3390/catal8110489
Palma V, Pisano D, Martino M. Comparative Study Between Aluminum Monolith and Foam as Carriers for The Intensification of The CO Water Gas Shift Process. Catalysts. 2018; 8(11):489. https://doi.org/10.3390/catal8110489
Chicago/Turabian StylePalma, Vincenzo, Domenico Pisano, and Marco Martino. 2018. "Comparative Study Between Aluminum Monolith and Foam as Carriers for The Intensification of The CO Water Gas Shift Process" Catalysts 8, no. 11: 489. https://doi.org/10.3390/catal8110489
APA StylePalma, V., Pisano, D., & Martino, M. (2018). Comparative Study Between Aluminum Monolith and Foam as Carriers for The Intensification of The CO Water Gas Shift Process. Catalysts, 8(11), 489. https://doi.org/10.3390/catal8110489








