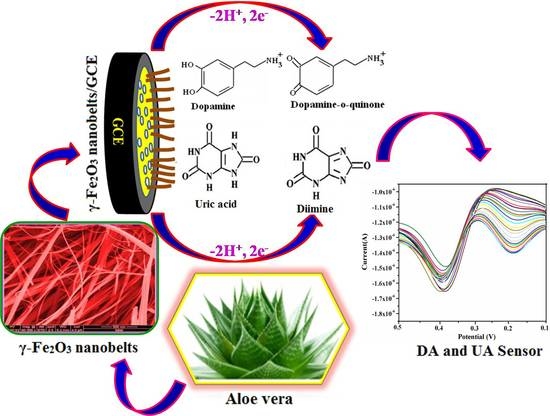
Sol-Gel Mediated Greener Synthesis of γ-Fe2O3 Nanostructures for the Selective and Sensitive Determination of Uric Acid and Dopamine
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Aloe vera Extract
2.3. Synthesis of γ-Fe2O3 Nanostructures
2.4. Characterizations
Fabrication of γ-Fe2O3 Nanostructures Modified GCE
3. Results and Discussion
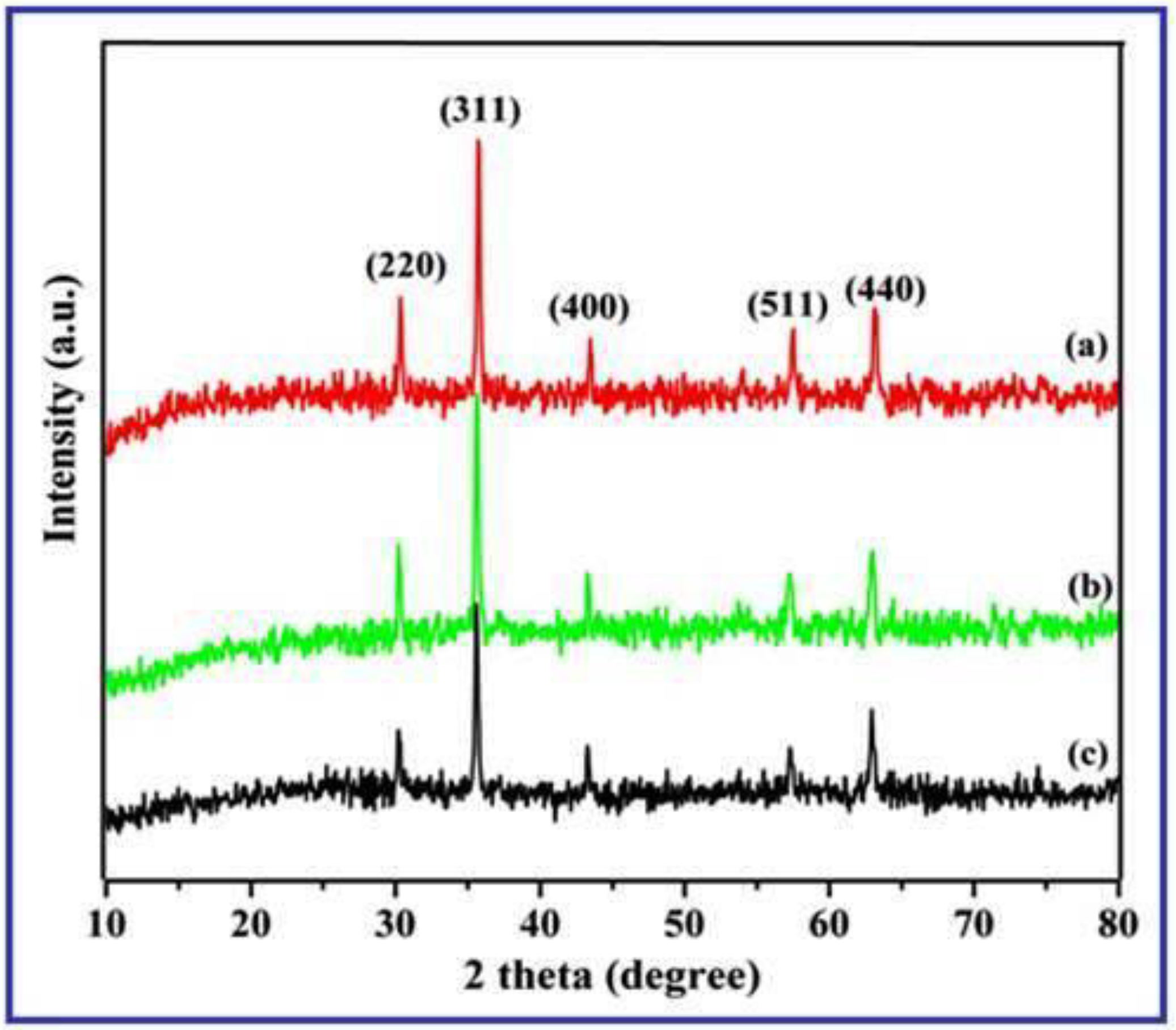
3.1. XRD Spectral Patterns of γ-Fe2O3 Nanostructures
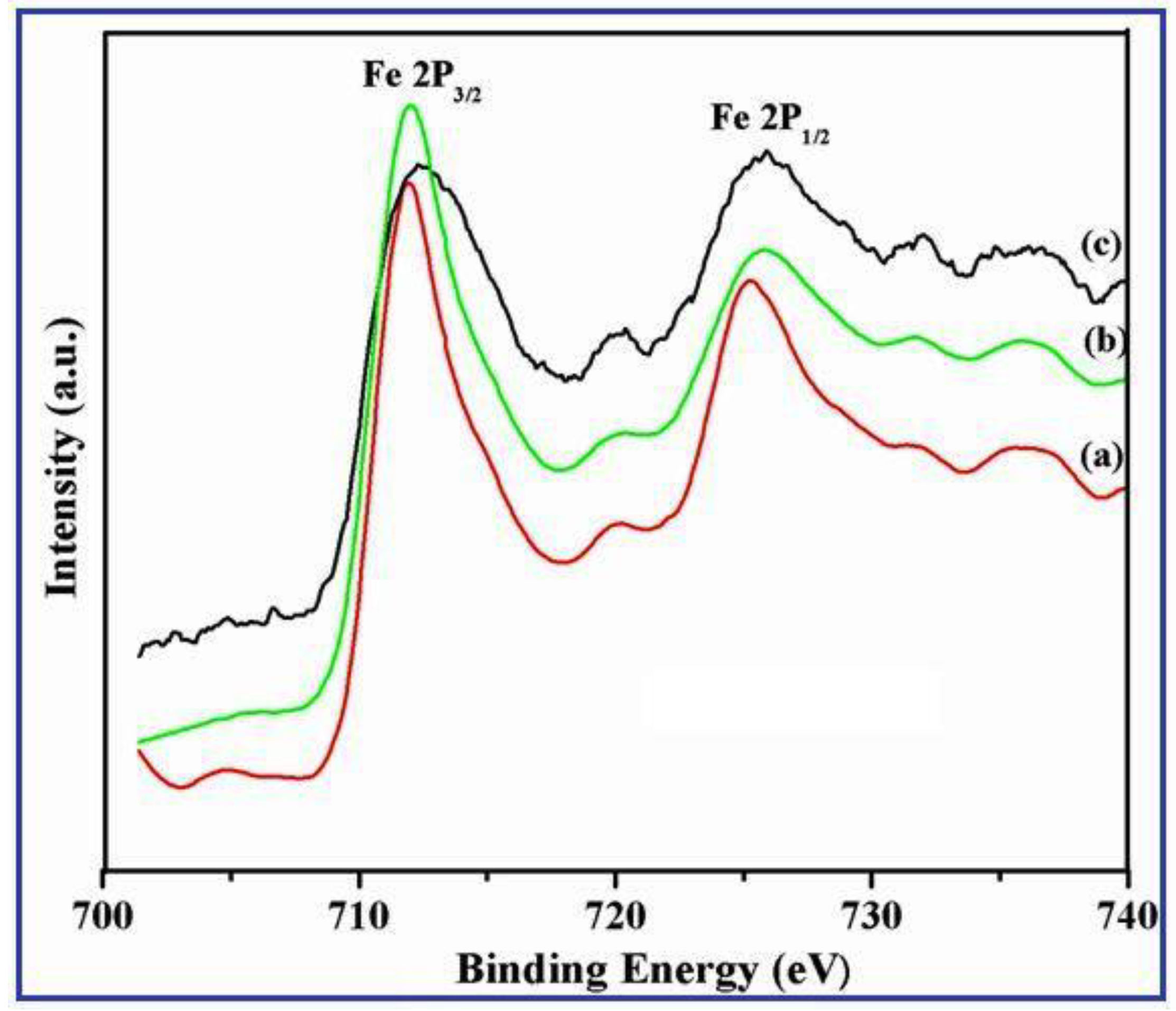
3.2. XPS Spectrum of γ-Fe2O3 Nanostructures
3.3. Field Emission-Scanning Electron Microscopic (FE-SEM) images of γ-Fe2O3 Nanostructures
3.4. Transmission Electron Microscopy (TEM) images of γ-Fe2O3 Nanostructures
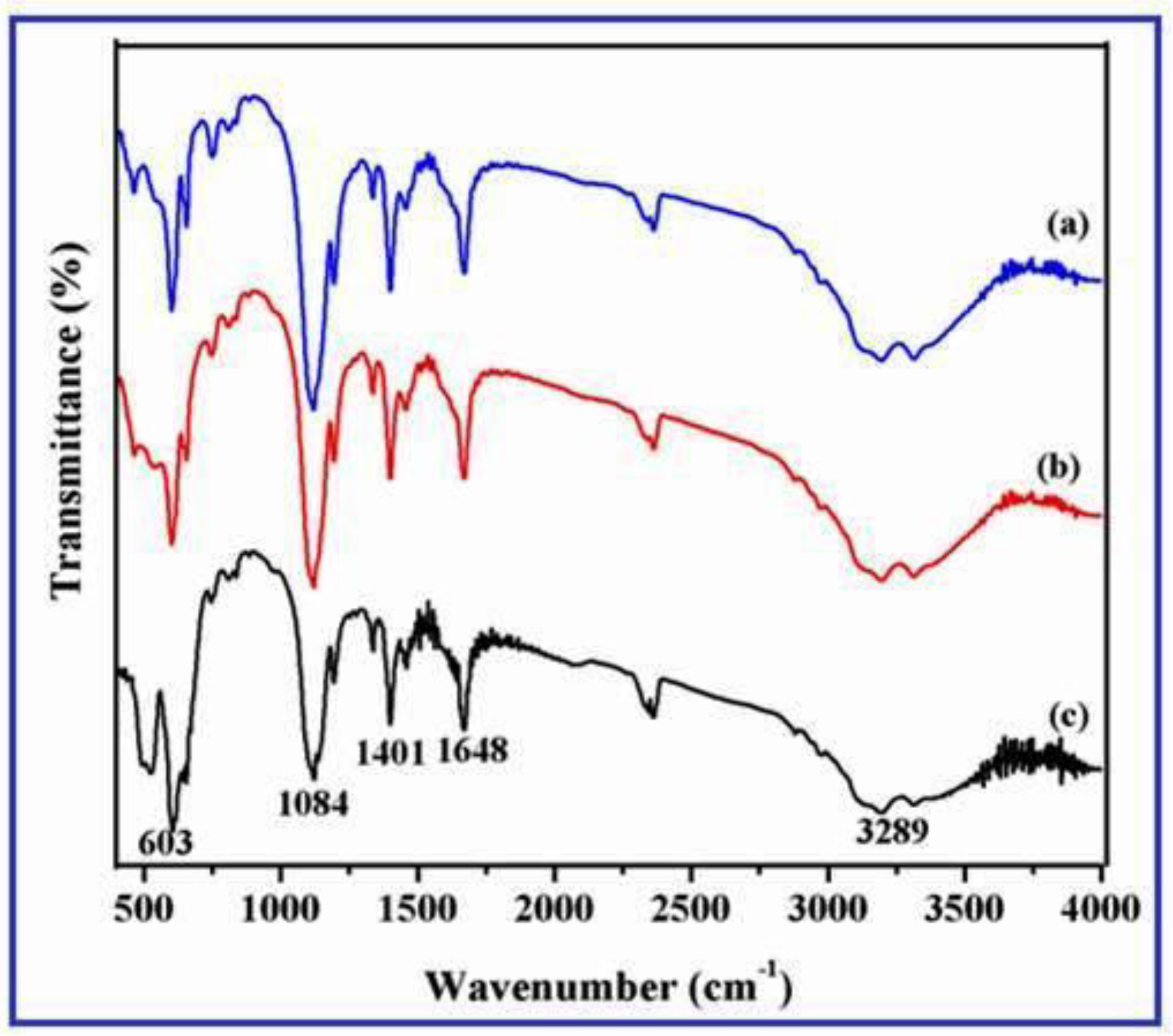
3.5. FT-IR Spectrum of γ-Fe2O3 Nanostructures
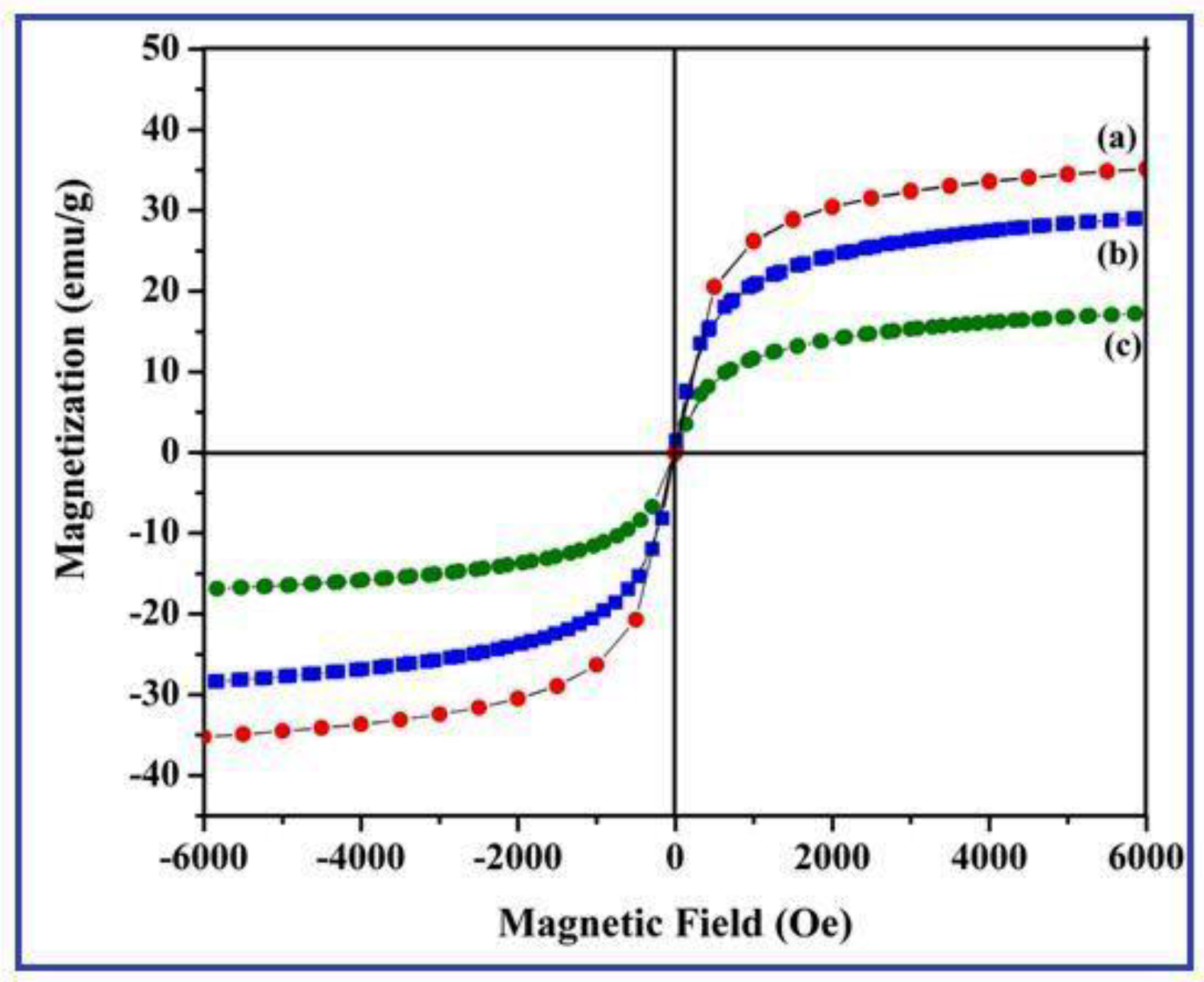
3.6. Magnetic Measurements of γ-Fe2O3 Nanostructures
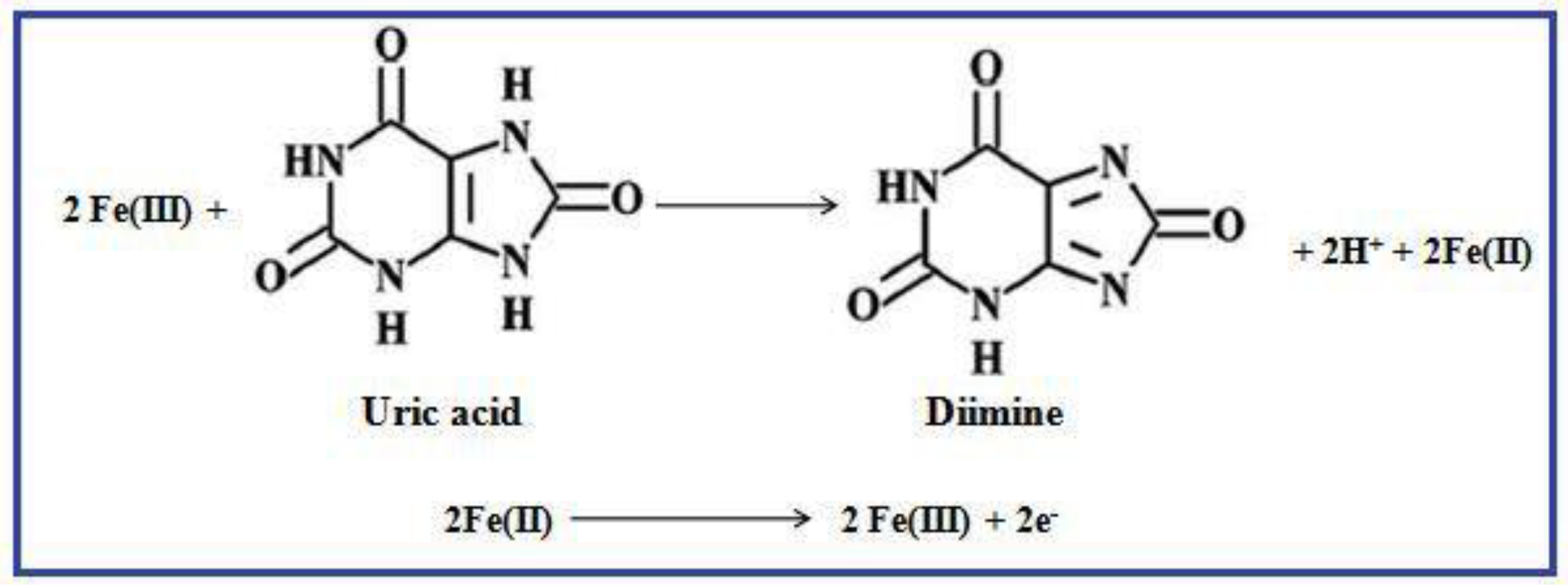
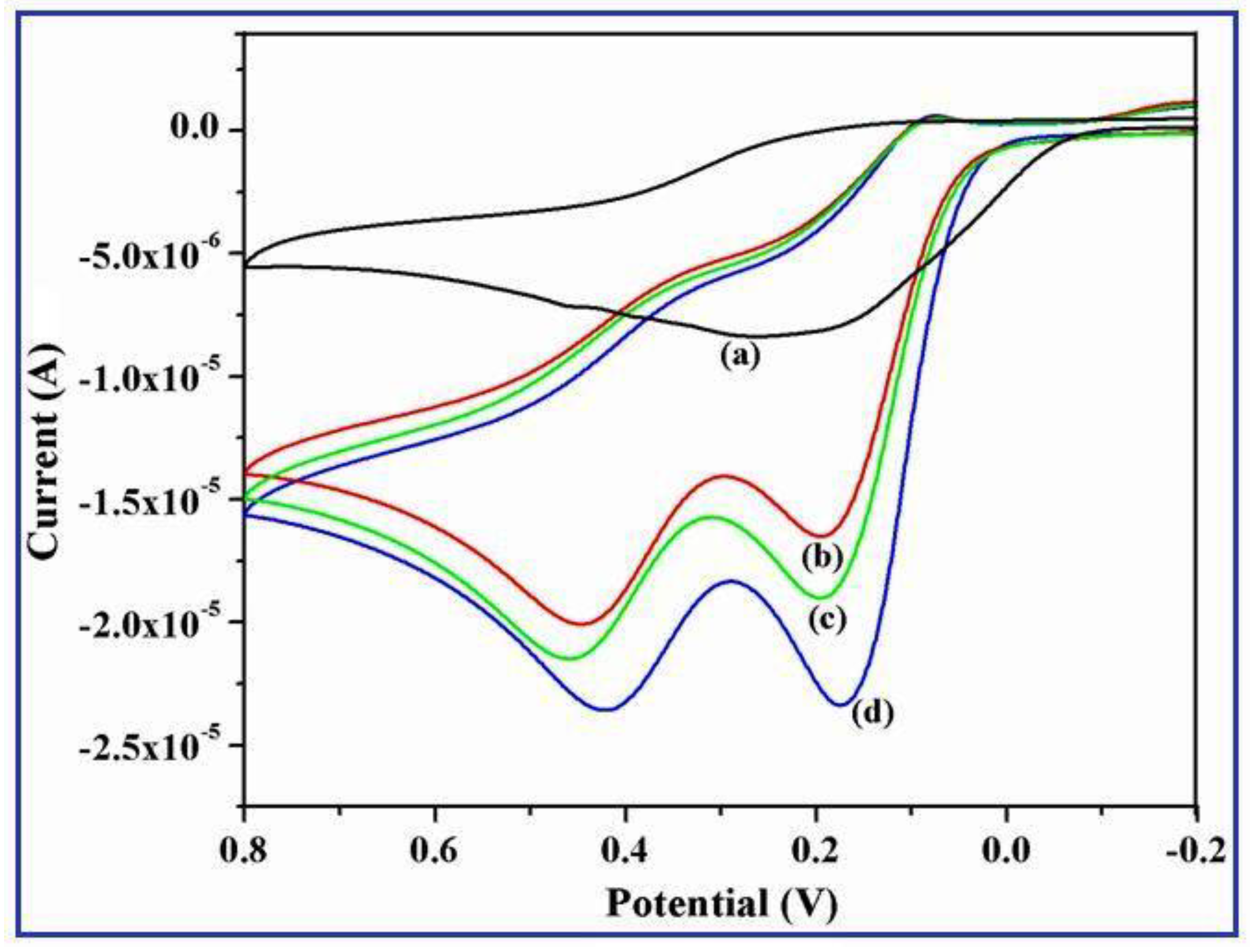
3.7. Electrocatalytic Activity of UA on γ-Fe2O3 Nanostructures Modified GCE
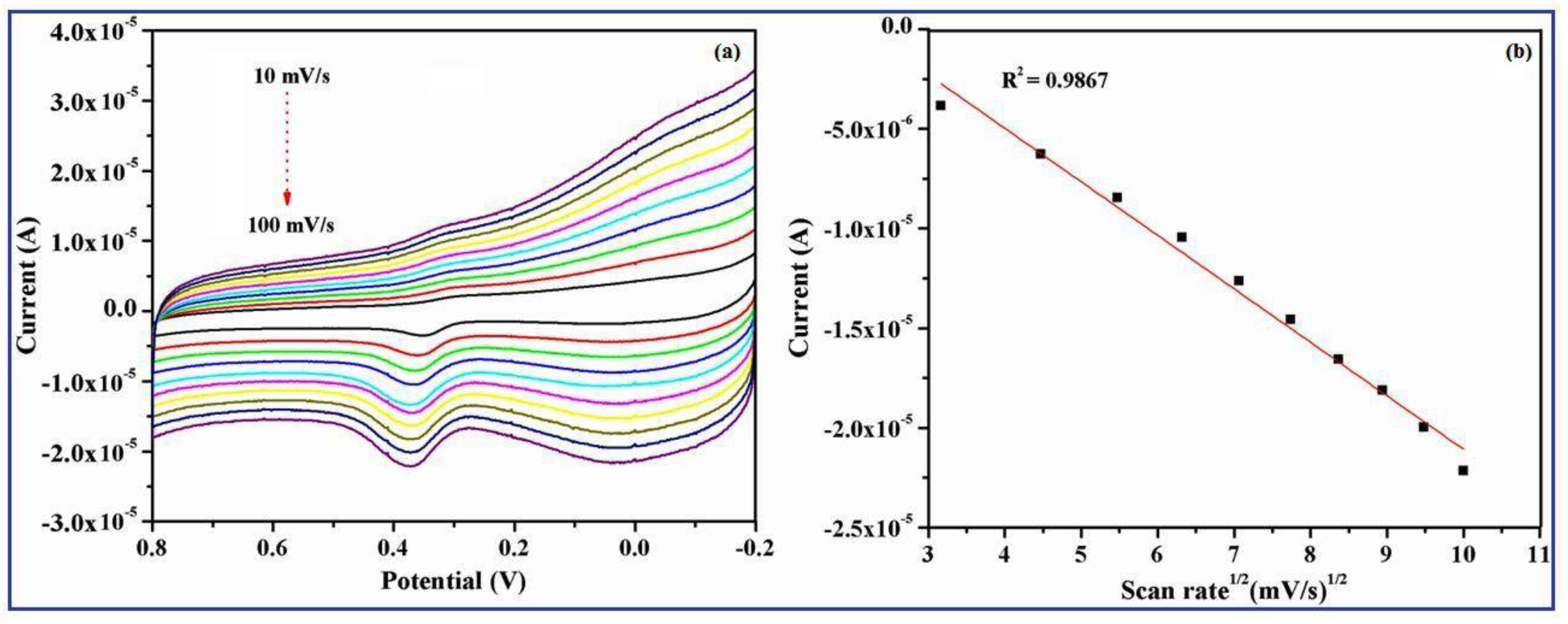
3.8. Effect of Scan Rates on the γ-Fe2O3 Nanobelts/GCE
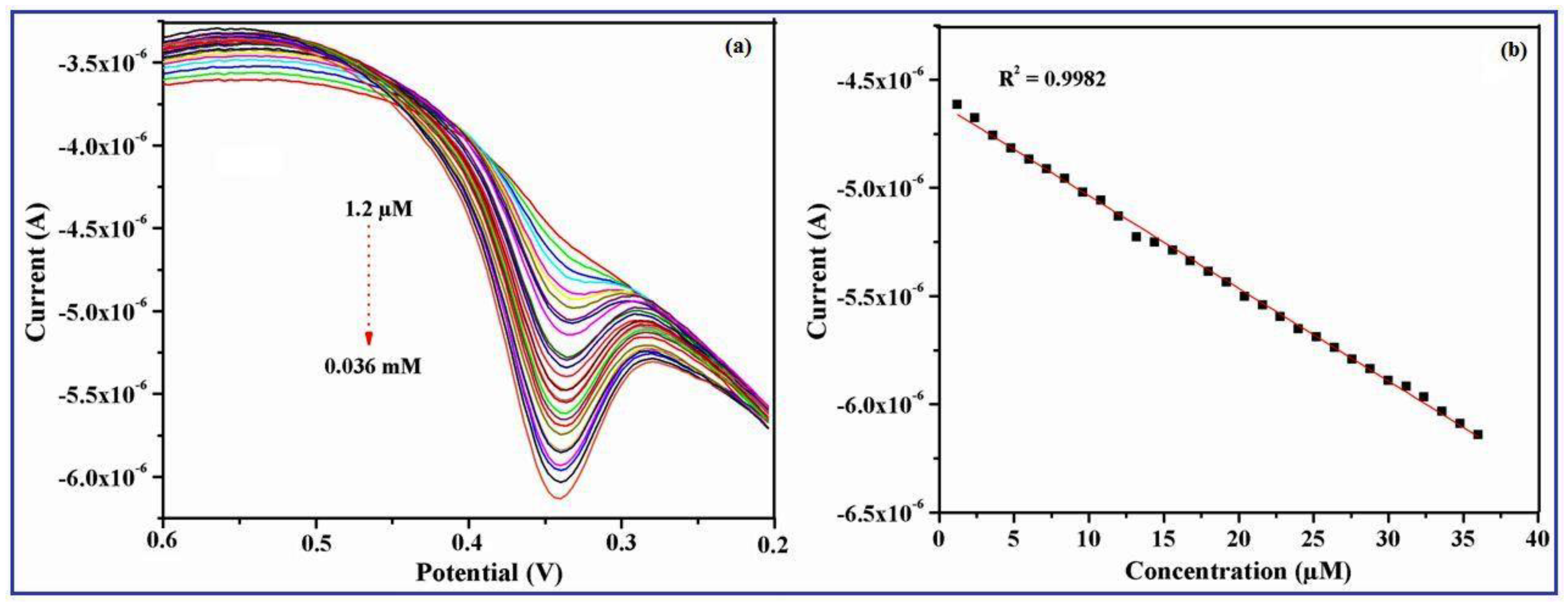
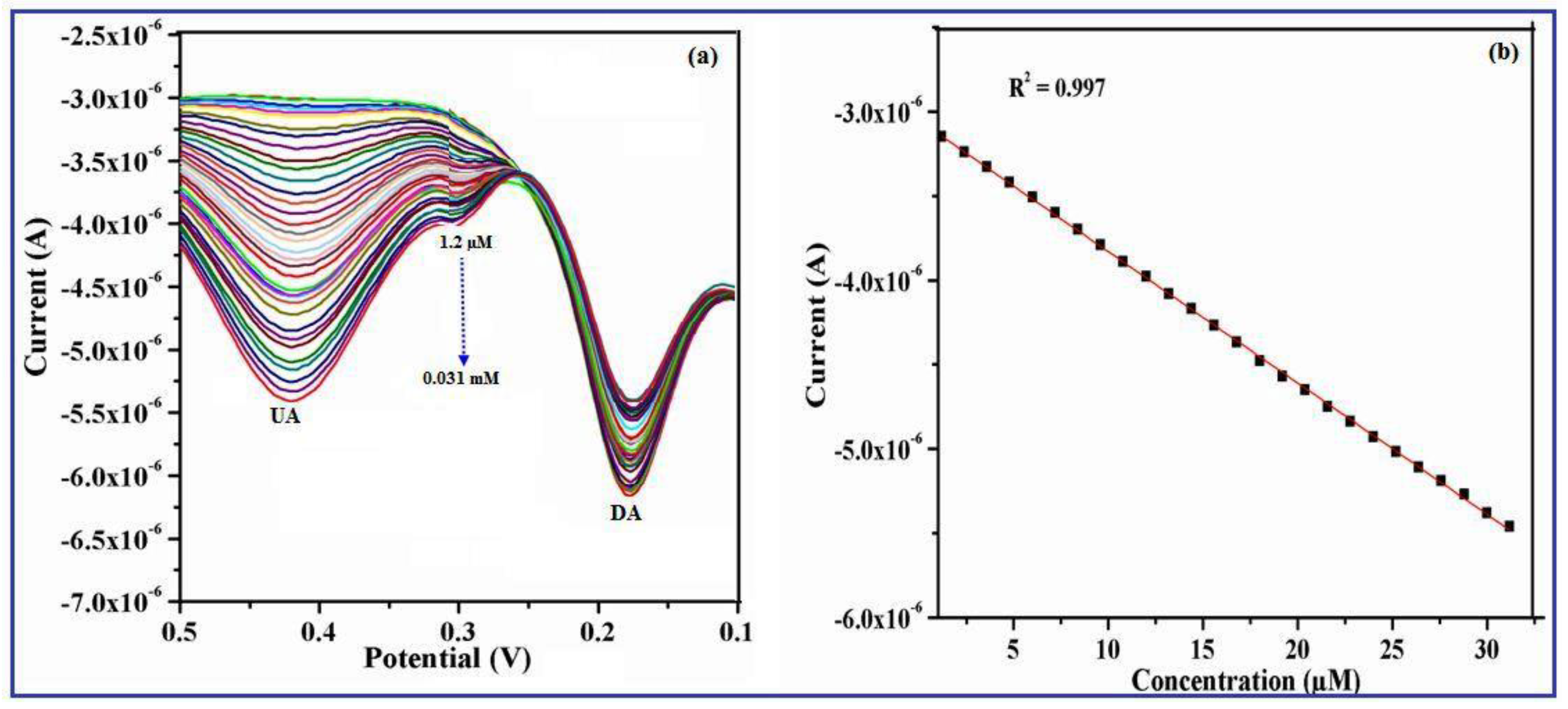
3.9. Effect of UA Concentration on the γ-Fe2O3 Nanobelts/GCE
3.10. Simultaneous Determination of UA and DA at the γ-Fe2O3 Nanostructures/GCE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuchibhatla, S.V.N.T.; Karakoti, A.S.; Bera, D.; Seal, S. One dimensional nanostructured materials. Prog. Mater. Sci. 2007, 52, 699–913. [Google Scholar] [CrossRef]
- Meng, L.R.; Chen, W.; Chen, C.; Zhou, H.; Peng, Q.; Li, Y. Uniform α-Fe2O3 Nanocrystal Moniliforme-Shape Straight-Chains. Cryst. Growth Des. 2010, 10, 479–482. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.A.; Petkowicz, D.I.; Smaniotto, A.; Pergher, S.B.C. Magnetic zeolites: A new adsorbent for removal of metallic contaminants from water. Water Res. 2004, 38, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Qu, H.H.; He, H.; Yu, Y.B. Removal of azo-dye Acid Red B (ARB) by adsorption and catalytic combustion using magnetic CuFe2O4 powder. Appl. Catal. B 2004, 48, 49–56. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Y.; Ji, H.; Wei, Y.G.; Wu, N.Z.; Zuo, B.J.; Wang, Q.L. Magnetic nanocomposite catalysts with high activity and selectivity for selective hydrogenation of ortho-chloronitrobenzene. J. Catal. 2005, 229, 114–118. [Google Scholar] [CrossRef]
- Shekhah, O.; Ranke, W.; Schule, A.; Kolios, G.; Schlogl, R. Styrene synthesis: High conversion over unpromoted iron oxide catalysts under practical working conditions. Angew. Chem. Int. Ed. 2003, 42, 5760–5763. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, J.; Liu, J.P.; Wang, Z.L.; Sun, S.H. Exchange-coupled nanocomposite magnets by nanoparticle self-assembly. Nature 2002, 420, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; Johannsen, M.; Wust, P.; Nadobny, J.; Schirra, H.; Schmidt, H.; Deger, S.; Loening, S.; et al. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2001, 225, 118–126. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.N.; Li, W.Y.; Gou, X.L. α-Fe2O3 Nanotubes in Gas Sensor and Lithium-Ion Battery Applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Q.; Gao, M.Y. Preparation of water-soluble magnetite nanocrystals from hydrated ferric salts in 2-pyrrolidone: Mechanism leading to Fe3O4. Angew. Chem. Int. Ed. 2005, 44, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G.X. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.J.; Hwang, Y.S.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Kratohvil, S.; Matijevic, E. Formation of monodispersed spindle-type hematite particles. J. Colloid Interface Sci. 1984, 102, 146–151. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Matijivic, E. Ferric hydrous oxide sols. IV. Preparation of uniform cubic hematite particles by hydrolysis of ferric chloride in alcohol—Water solutions. J. Colloid Interface Sci. 1981, 84, 274–277. [Google Scholar] [CrossRef]
- Vayssieres, L.; Sathe, C.; Butorin, S.M.; Shuh, D.K.; Nordgren, J.; Guo, J.H. One-Dimensional Quantum-Confinement Effect in α-Fe2O3Ultrafine Nanorod Arrays. Adv. Mater. 2005, 17, 2320–2323. [Google Scholar] [CrossRef]
- Woo, K.; Lee, H.J.; Ahn, J.P.; Park, Y.S. Sol–Gel Mediated Synthesis of Fe2O3 Nanorods. Adv. Mater. 2003, 15, 1761–1764. [Google Scholar] [CrossRef]
- Wen, X.G.; Wang, S.H.; Ding, Y.; Wang, Z.L.; Yang, S.H. Optical Limiting of a Covalently Bonded Gold Nanoparticle/Polylysine Hybrid Material. J. Phys. Chem. B 2005, 109, 20854–20857. [Google Scholar] [CrossRef]
- Jia, C.J.; Sun, L.D.; Yan, Z.G.; You, L.P.; Luo, F.; Han, X.D.; Pang, Y.C.; Zhang, Z.; Yan, C.H. Single-crystalline iron oxide nanotubes. Angew. Chem. Int. Ed. 2005, 44, 4328–4333. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Chun, H.J.; Kim, D.S.; Kim, S.Y.; Park, J. Magnetic anisotropy of vertically aligned α-Fe2O3 nanowire array. Appl. Phys. Lett. 2006, 89, 223103–223105. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.P.; Chandhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, D.; Kong, Y.; Wang, X.; Pandoli, O.; Gao, G. Synergetic Antibacterial Effects of Silver Nanoparticles@Aloe Vera Prepared via a Green Method. Nano Biomed. Eng. 2010, 2, 252–257. [Google Scholar] [CrossRef]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: Optical properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 1140–1144. [Google Scholar] [CrossRef]
- Phumying, S.; Labuayai, S.; Thomas, C.; Amornkitbamrung, V.; Swatsitang, E.; Maensiri, S. Aloe vera plant-extract solution hyrothermal synthesis andmagnetic properties of magnetite (Fe3O4) nanoparticles. Appl. Phys. A 2013, 111, 1187–1193. [Google Scholar] [CrossRef]
- Laokul, P.; Amornkitbamrung, V.; Seraphin, S.; Maensiri, S. Characterization and magnetic properties of nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 powders prepared by the Aloe vera extract solution. Curr. Appl. Phys. 2011, 11, 101–108. [Google Scholar] [CrossRef]
- Klinkaewnarong, J.; Swatsitang, E.; Masingboon, C.; Seraphin, S.; Maensiri, S. Synthesis and characterization of nanocrystalline HAp powders prepared by using aloe vera plant extracted solution. Curr. Appl. Phys. 2010, 10, 521–525. [Google Scholar] [CrossRef]
- Juibari, N.M.; Eslami, A. Synthesis of nickel oxide nanorods by Aloe vera leaf extract. J. Therm. Anal. Calorim. 2018, 134, 1–11. [Google Scholar] [CrossRef]
- Shakkthivel, P.; Sasikala, S.; Ramalakshmi, M.; Kim, Y.Y.; Min, K. Nanospheres and nanoleaves of γ-Fe2O3 architecturing for magnetic and biomolecule sensing applications. Sens. Actuator. B Chem. 2016, 234, 386–394. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Peng, H.; Qi, R.; Luo, C. A glassy carbon electrode modified with MoS2 nanosheets and poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Microchim. Acta 2016, 183, 2517–2523. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-Assembled 3D flower like iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Sasikala, S.; Ramalakshmi, M.; Min, K.; Shakkthivel, P. Facile biosurfactant assisted biocompatible α-Fe2O3 nanorods and nanospheres synthesis, magneto physicochemical characteristics and their enhanced biomolecules sensing ability. RSC Adv. 2016, 6, 77133–77142. [Google Scholar] [CrossRef]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Colfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, J.; You, C.; Song, Z.; Yu, B.; Shen, Y. Synthesis and characterization of polyaniline/activated carbon composites and preparation of conductive films. Mater. Chem. Phys. 2009, 113, 46–53. [Google Scholar] [CrossRef]
- Apte, S.K.; Naik, S.D.; Sonawane, R.S.; Kale, B.B.; Baeg, J.O. Synthesis of Nanosize-Necked Structure α- and γ-Fe2O3 and its Photocatalytic Activity. J. Am. Ceram. Soc. 2007, 90, 412–414. [Google Scholar] [CrossRef]
- Gonçalves, L.C.; Seabra, A.B.; Pelegrino, M.T.; Araujo, D.R.; Bernardes, J.S.; Haddad, P.S. Superparamagnetic iron oxide nanoparticles dispersed in Pluronic F127 hydrogel: Potential uses in topical applications. RSC Adv. 2017, 7, 14496–14503. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Xiong, Z.; Hu, Y.; Jiang, J. Evolution of Useless Iron Rust into Uniform α-Fe2O3 Nanospheres: A Smart Way to Make Sustainable Anodes for Hybrid Ni–Fe Cell Devices. ACS Sustain. Chem. Eng. 2017, 5, 269–276. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, X.; Wu, D.; Chen, Q.; Huang, D.; Sun, C.; Xin, J.; Ni, K.; Gao, J. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem. Mater. 2015, 27, 3505–3515. [Google Scholar] [CrossRef]
- Suresh, R.; Prabu, R.; Vijayaraj, A.; Giribabu, K.; Stephen, A.; Narayanan, V. Fabrication of α-Fe2O3 Nanoparticles for the Electrochemical Detection of Uric Acid. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2012, 42, 303–307. [Google Scholar] [CrossRef]
- Sathish Kumar, P.; Prakash, P.; Balakumar, V. New Electrochemical Sensor Based on a Silver-Doped Iron Oxide Nanocomposite Coupled with Polyaniline and Its Sensing Application for Picomolar-Level Detection of Uric Acid in Human Blood and Urine Samples. J. Phys. Chem. B 2018, 122, 3037–3046. [Google Scholar] [CrossRef]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Li, G.; Liu, J. Determination of Uric Acid in Co-Presence of Dopamine and Ascorbic Acid Using Cuprous Oxide Nanoparticle-Functionalized Graphene Decorated Glassy Carbon Electrode. Catalysts 2018, 8, 407. [Google Scholar] [CrossRef]
- Sun, H.; Chao, J.; Zuo, X.; Su, S.; Liu, X.; Yuwen, L.; Fan, C.; Wang, L. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2014, 4, 27625–27629. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, M.; Liu, L.; Guo, R. A facile electrochemical uricase biosensor designed from gold/amino acid nanocomposites. Sens. Actuator B Chem. 2013, 176, 592–597. [Google Scholar] [CrossRef]
- Du, J.; Yue, R.; Ren, F.; Yao, Z.; Jiang, F.; Yang, P.; Du, Y. Novel graphene flowers modified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2014, 53, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.N.; Salleh, A.B.; Lim, H.N.; Tajudin, A.A. Electrochemical detection of uric acid via uricase-immobilized graphene oxide. Anal. Biochem. 2016, 509, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Jang, N.K.; Khang, G.; Hahn, Y.B. Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuator B Chem. 2015, 206, 146–151. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, W.; Wang, J.; Zhang, G.; Chai, S.; Zhang, L.; Liu, T. Selective determination of dopamine and uric acid using electrochemical sensor based on poly(alizarin yellow R) film-modified electrode. Anal. Methods 2014, 6, 3474–3481. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Hasebe, Y.; Zhang, Z.; Tao, D. Carbon Black-Carbon Nanotube Co-Doped Polyimide Sensors for Simultaneous Determination of Ascorbic Acid, Uric Acid, and Dopamine. Materials 2018, 11, 1691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, X.; Kang, Z.; Lin, P.; Fang, X.; Lei, Y.; Ma, S.; Zhang, Y. Highly sensitive uric acid biosensor based on individual zinc oxide micro/nanowires. Microchim. Acta 2013, 180, 759–766. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Zhu, Z.; Zhang, J.; Zhu, J. Novel Uric Acid Sensor Based on Enzyme Electrode Modified by ZnO Nanoparticles and Multiwall Carbon Nanotubes. Anal. Lett. 2009, 42, 775–789. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Wu, D.; Wei, Q. Electrochemical Determination of Dopamine in the Presence of Uric Acid Using Palladium-Loaded Mesoporous Fe3O4 Nanoparticles. Measurement 2015, 60, 1–5. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, A.H. One-pot synthesis of Au Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Yang, Y.; Li, S.; Yu, Q.; Xu, X.; Hu, X. Graphene–Au nanoparticles nanocomposite film for selective electrochemical determination of dopamine. Anal. Methods 2012, 4, 1725–1728. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, M.; Cai, B.; Wang, W.; Ye, Z.; Huang, J. 3D graphene network@WO3 nanowire composites: A multifunctional colorimetric and electrochemical biosensing platform. Chem. Commun. 2014, 50, 11135–11138. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Bong, S.; Kang, Y.J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.S.J.; Ramaprabhu, S. Dopamine biosensor with metal oxide nanoparticles decorated multi-walled carbon nanotubes. Nanosci. Methods 2012, 1, 102–114. [Google Scholar] [CrossRef]
- Abdelwahab, A.A.; Shim, Y. Simultaneous determination of ascorbic acid, dopamine, uric acid and folic acid based on activated graphene/MWCNT nanocomposite loaded Au nanoclusters. Sens. Actuator B Chem. 2015, 221, 659–665. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Kaur, B.; Pandiyan, T.; Satpati, B.; Srivastava, R. Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloids Surf. B 2013, 111, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, J.; Wang, H.; Zou, C.E.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuator B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]




| Electrode Material | Technique | LOD | Linear Range | Electrolyte (pH) | Ref. |
|---|---|---|---|---|---|
| AuNPs@MoS2/GCE | DPV | 10 μM | 10–7000 μM | pH 7.0 | [46,47] |
| GNP superstructure/uricase electrode | CA | 50 µM | 0.02–2.5 mM | pH 7.0 | [47] |
| Graphene flowers/CFE | DPV | 3.98 μM | 3.98–371.4 μM | pH 7.0 | [48] |
| GOU/GCE | CV | 3.45 μM | 0.02–0.154 mM | pH 6.5 | [49] |
| Nafion/Uricase/ZnO NSs/Ag/Si electrode | CA | 0.05 mM | 0.05–0.5 mM | pH 7.0 | [50] |
| PAYR/CPE electrode | DPV | 9.5 µM | 27.8–304.4 µM | pH 7.0 | [51] |
| CB-CNT/PI/GCE | CA | 8.8 µM | 10–1000 µM | pH 7.0 | [52] |
| Nafion/uricase/ZnO/Au Electrode | CA | 0.1 mM | 0.1–0.59 mM | pH 7.4 | [53] |
| Cationic polydiallyldimethylammonium chloride/Uricase/ZnO | DPV | 2000 µM | 0.005–1.0 mM | pH 6.9 | [54] |
| γ-Fe2O3 nanobelts/GCE | DPV | 1.2 µM | 1.2 μM–0.036 mM | pH 7.4 | This work |
| Electrode Material | Technique | LOD | Linear Range | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Au-Cu2O/rGO | DPV | 3.9 µM | 10–90 µM | pH 7.0 | [56] |
| Au–graphene | DPV | 1.86 mM | 5–1000 mM | pH 6.0 | [57] |
| 3D-GN@WO3 nanowire | CA | 238 µM | 10–150 mM | pH 6.0 | [58] |
| Graphene | DPV | 2.64 mM | 4–100 mM | pH 7.0 | [59] |
| ZnO/MWNTs/GCE | CV | 3 µM | 3–200 µM | pH 7.0 | [60] |
| AGR-MWCNT/GCE | DPV | 1.4 µM | 2–120 µM | pH 7.0 | [61] |
| GR–CS/GCE | DPV | 5 µM | 15–175 µM | pH 7.0 | [62] |
| Ag/RGO | LSV | 5.4 µM | 10–800 µM | pH 6.0 | [63] |
| Au/RGO/GCE | DPV | 1.40 μM | 6.8–41 μM | pH 7.0 | [64] |
| γ-Fe2O3 nanobelts/GCE | DPV | 0.6 μM | 0.6 μM–0.015 mM | pH 7.4 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundar, S.; Venkatachalam, G.; Kwon, S.J. Sol-Gel Mediated Greener Synthesis of γ-Fe2O3 Nanostructures for the Selective and Sensitive Determination of Uric Acid and Dopamine. Catalysts 2018, 8, 512. https://doi.org/10.3390/catal8110512
Sundar S, Venkatachalam G, Kwon SJ. Sol-Gel Mediated Greener Synthesis of γ-Fe2O3 Nanostructures for the Selective and Sensitive Determination of Uric Acid and Dopamine. Catalysts. 2018; 8(11):512. https://doi.org/10.3390/catal8110512
Chicago/Turabian StyleSundar, Sasikala, Ganesh Venkatachalam, and Seong Jung Kwon. 2018. "Sol-Gel Mediated Greener Synthesis of γ-Fe2O3 Nanostructures for the Selective and Sensitive Determination of Uric Acid and Dopamine" Catalysts 8, no. 11: 512. https://doi.org/10.3390/catal8110512
APA StyleSundar, S., Venkatachalam, G., & Kwon, S. J. (2018). Sol-Gel Mediated Greener Synthesis of γ-Fe2O3 Nanostructures for the Selective and Sensitive Determination of Uric Acid and Dopamine. Catalysts, 8(11), 512. https://doi.org/10.3390/catal8110512