A Robust Two-Step Process for the Efficient Conversion of Acidic Soybean Oil for Biodiesel Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Soybean Acidic Oil Samples
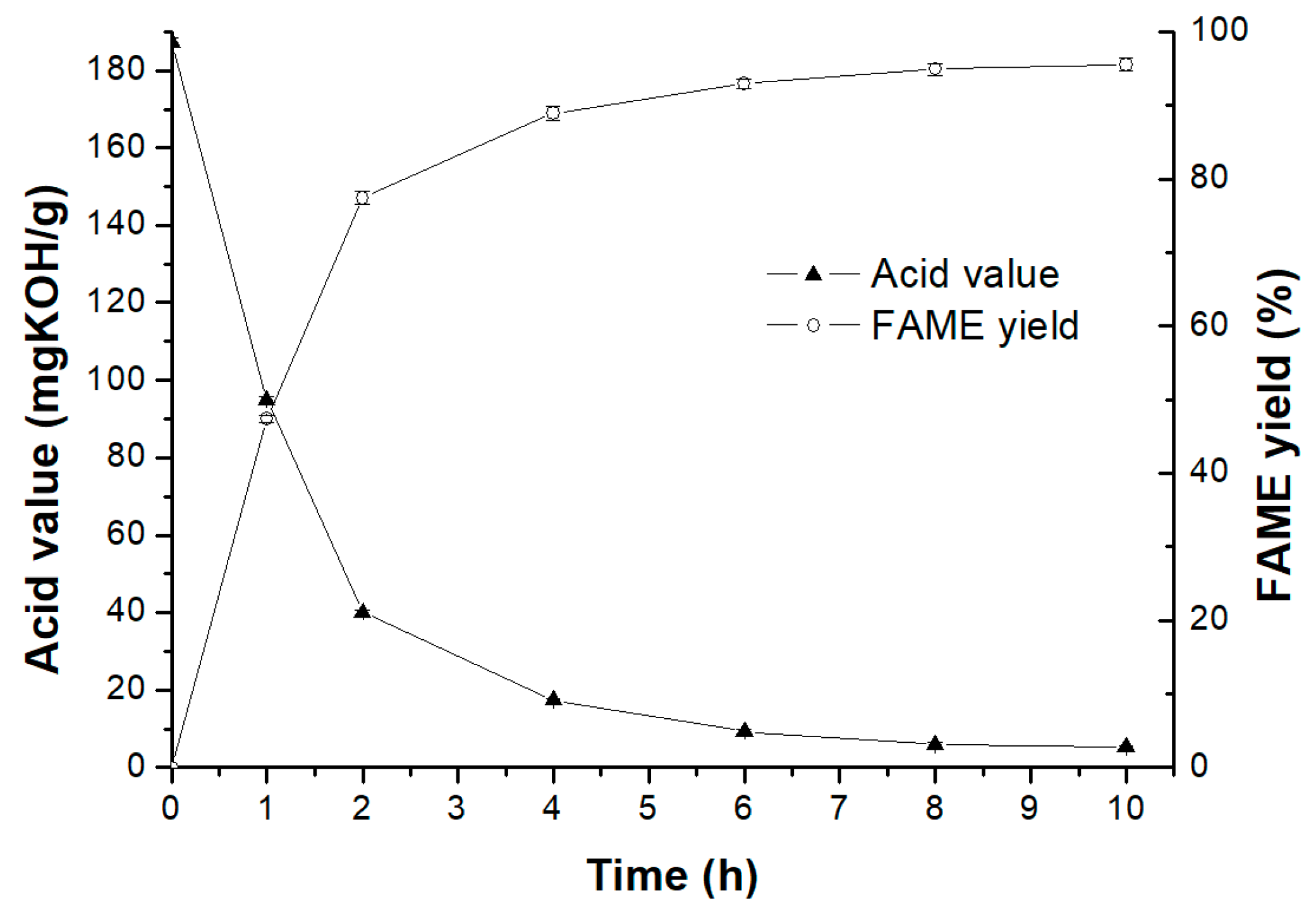
2.2. One-Step Conversion of Soybean Acidic Oils for Biodiesel Production
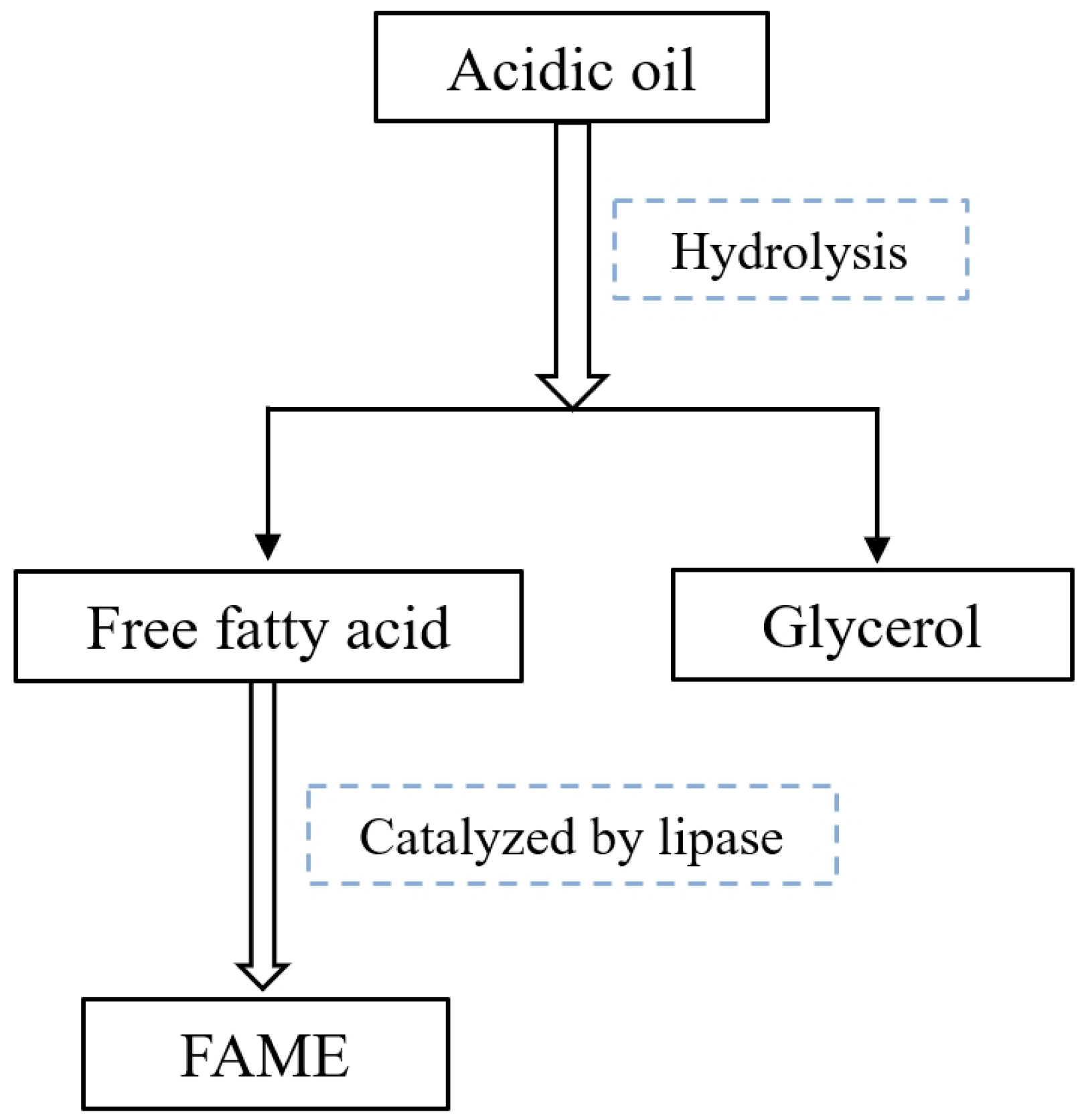
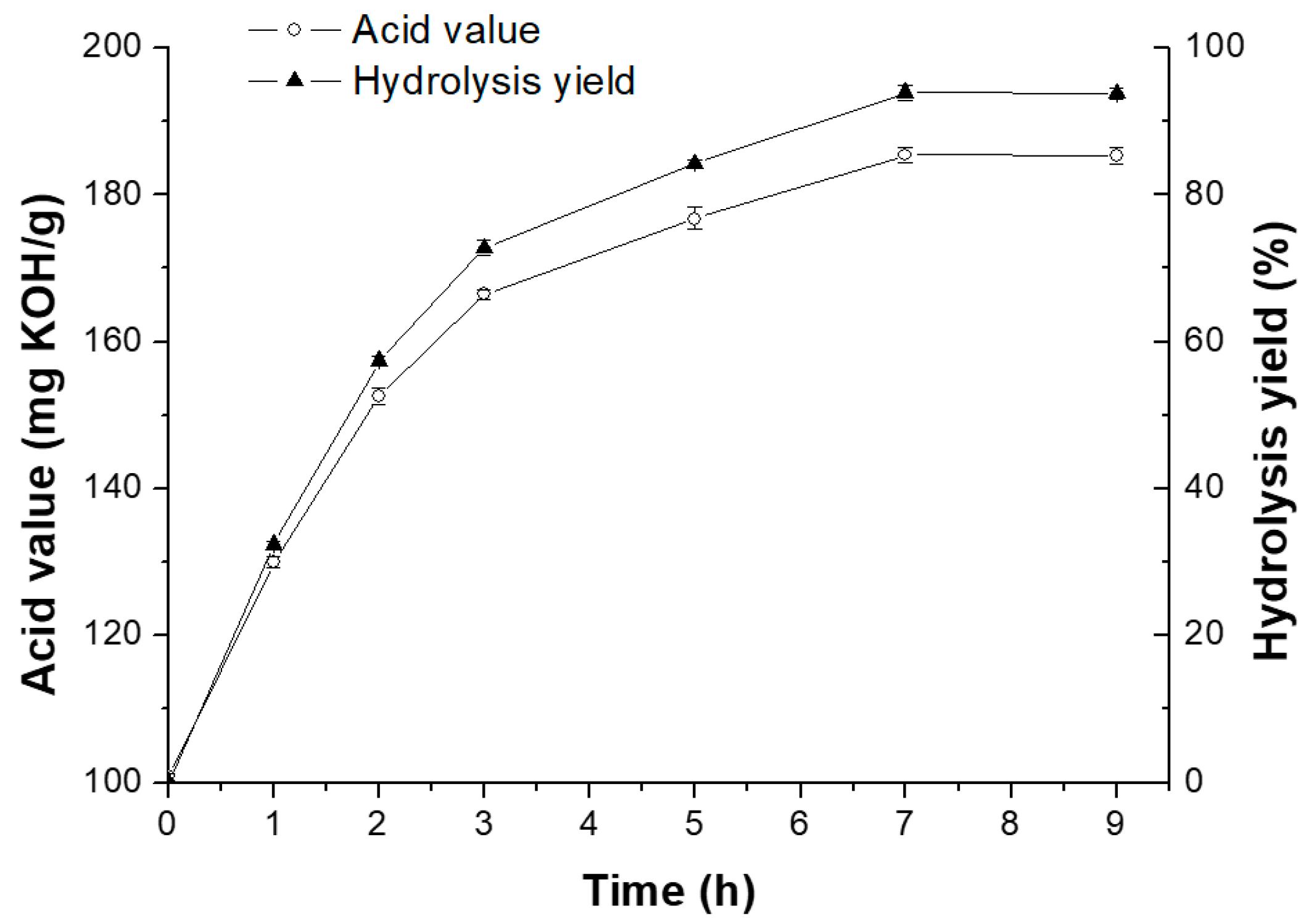
2.3. Sulfuric Acid-Catalyzed Hydrolysis of Acidic Soybean Oil
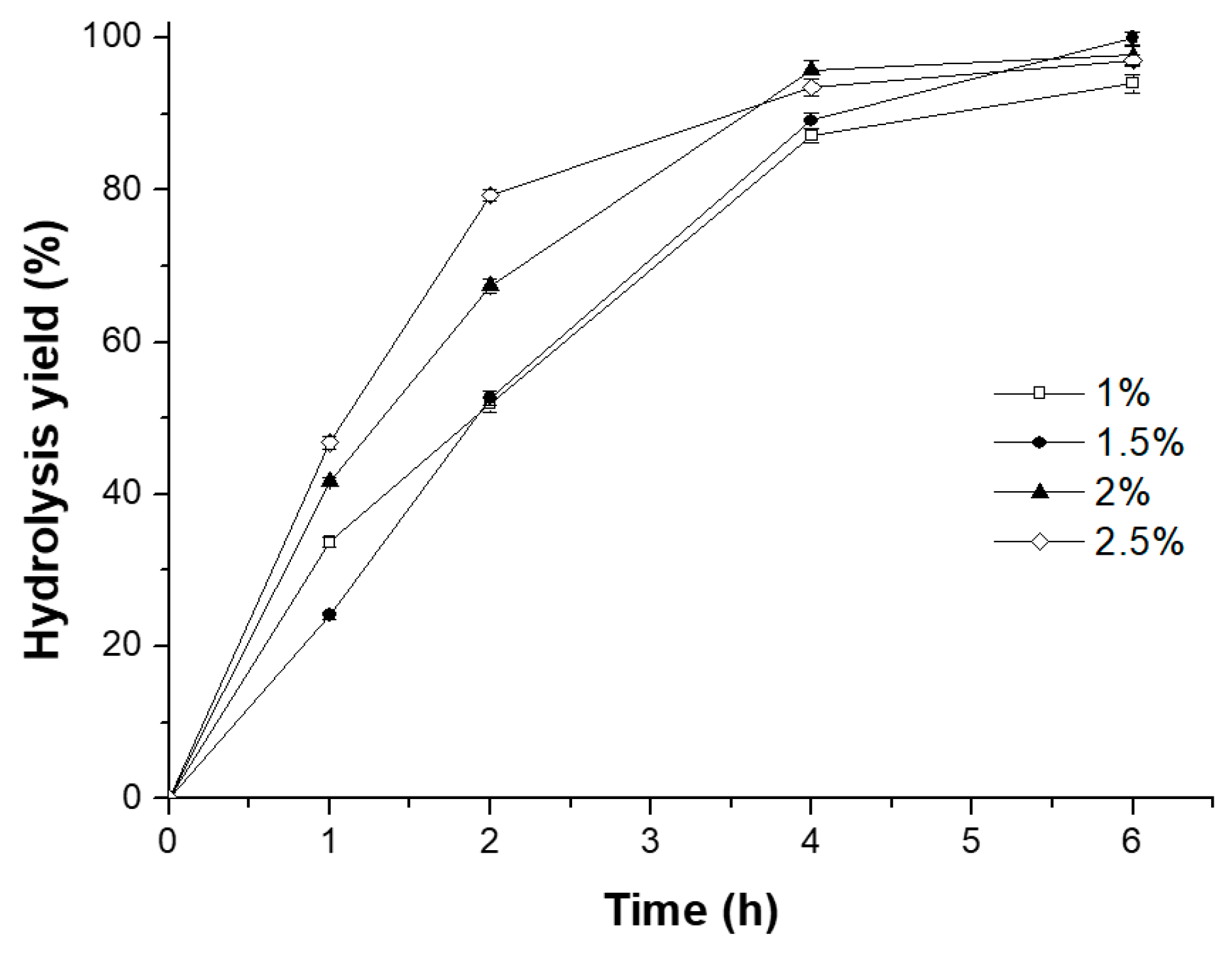
2.3.1. Effect of Catalyst Dosage
2.3.2. Effect of SDS (Sodium Dodecyl Sulfate) Dosage
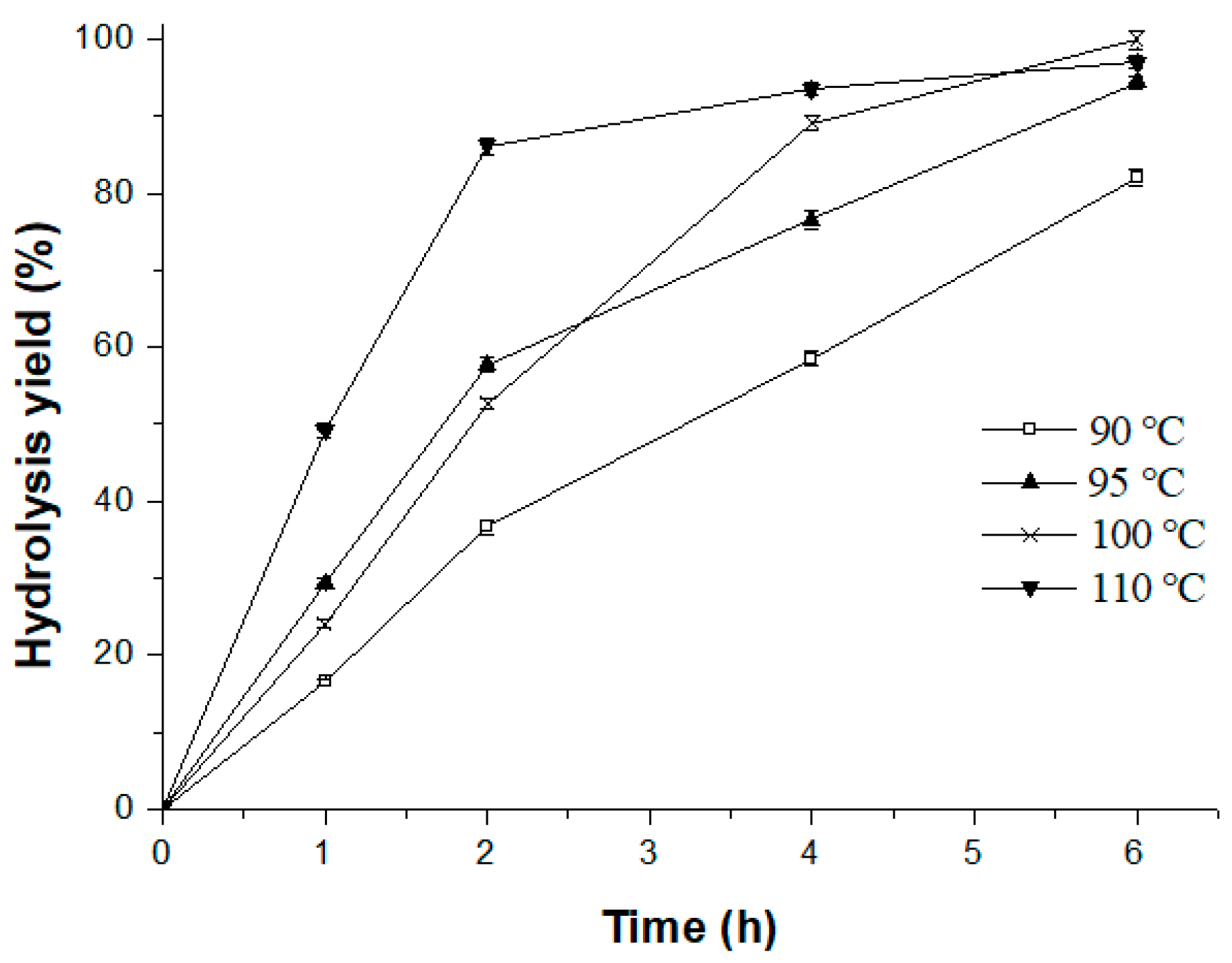
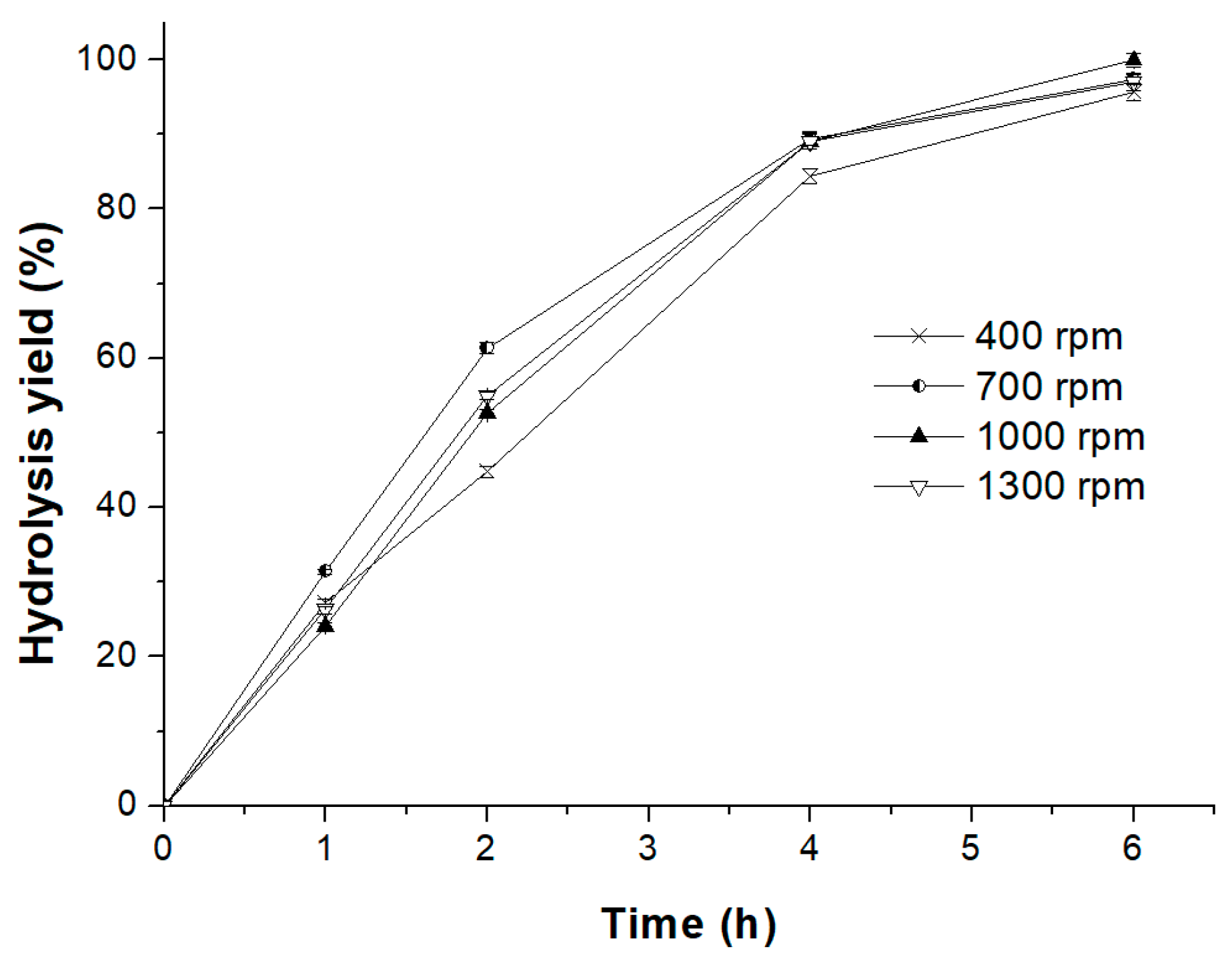
2.3.3. Effect of Reaction Temperature and Stirring Speed
2.3.4. Effect of Water to Oil Mass Ratio
2.4. Recycling of the Water Phase for the Next Batch of Hydrolysis
2.5. Novozym435-Mediated Esterification
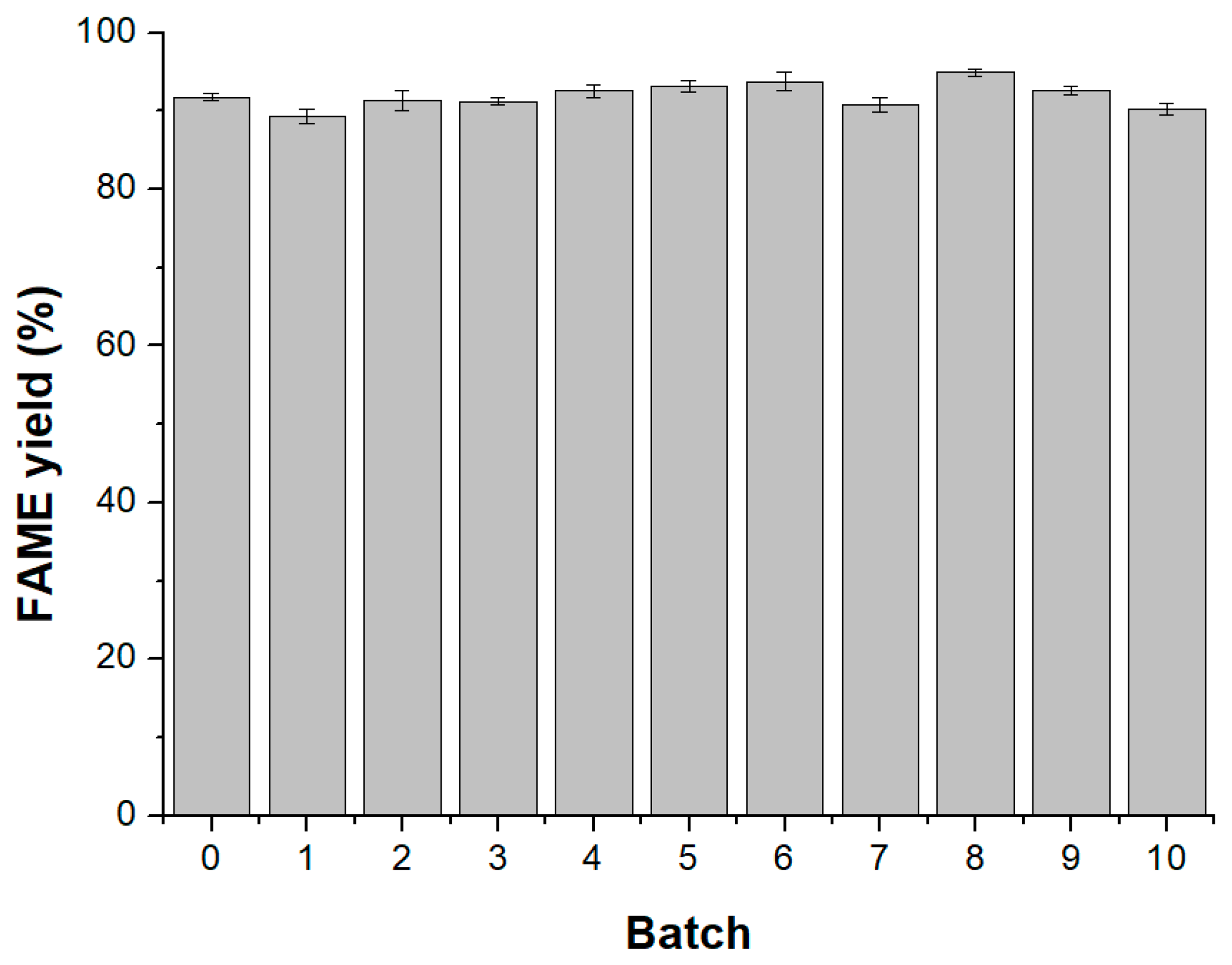
2.6. Study on the Catalytic Performance of Lipase during the Repeated Use
2.7. Investigation on the Applicability of the Proposed Two-Step Process
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Analysis of Free Fatty Acid (FFA) Composition and FAME Yield
3.3. Determination of Acid Value and Water Content
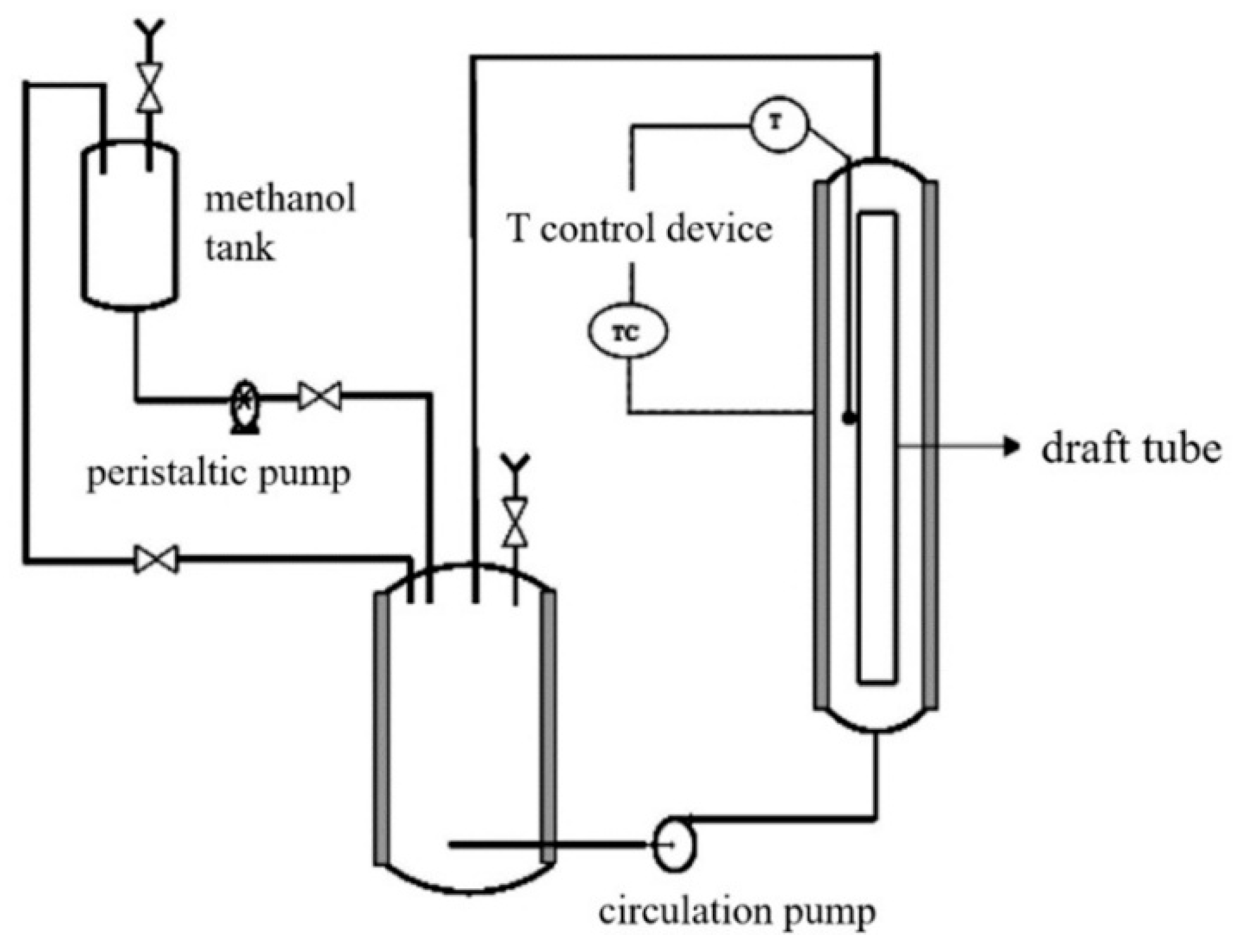
3.4. Sulfuric Acid-Catalyzed Hydrolysis of Soybean Acidic Oil
3.5. Recycling of the Water Phase for the Next Batch of Hydrolysis
3.6. Novozym435-Mediated Esterification
3.7. Recycling of Novozym435 for the Next Batch of Esterification
3.8. Analysis of Glycerol and Glycerides Composition of Oil by HPLC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, W.Y.; He, B.Q.; Li, J.X. Esterification of acidified oil with methanol by SPES/PES catalytic membrane. Bioresour. Technol. 2011, 102, 5389–5393. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel production technologies: Review. AIMS Energy 2017, 5, 425–457. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G.; Sundaram, S.; Tišma, M.; Hessel, V. Cost analysis of enzymatic biodiesel production in small-scaled packed-bed reactors. Appl. Energy 2018, 210, 268–278. [Google Scholar] [CrossRef]
- Živković, S.B.; Veljković, M.V.; Banković-Ilić, I.B.; Krstić, I.M.; Konstantinović, S.S.; Ilić, S.B.; Avramović, J.M.; Stamenković, O.S.; Veljković, V.B. Technological, technical, economic, environmental, social, human health risk, toxicological and policy considerations of biodiesel production and use. Renew. Sustain. Energy Rev. 2017, 79, 222–247. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Qing, K.G. Biodiesel Production from Acidic Crude Palm Oil Using Perchloric Acid. Energy Procedia 2014, 61, 2745–2749. [Google Scholar] [CrossRef]
- Konwar, L.J.; Wärnå, J.; Mäki-Arvela, P.; Kumar, N.; Mikkola, J.P. Reaction kinetics with catalyst deactivation in simultaneous esterification and transesterification of acid oils to biodiesel (FAME) over a mesoporous sulphonated carbon catalyst. Fuel 2016, 166, 1–11. [Google Scholar] [CrossRef]
- Corro, G.; Telle, N.; Jimenez, T.; Tapia, A.; Banuelos, F.; Vazquez-Cuchillo, O. Biodiesel from waste frying oil. Two step process using acidified SiO2 for esterification step. Catal. Today 2011, 166, 116–122. [Google Scholar] [CrossRef]
- Lv, D.; Du, W.; Liu, D.H. Study on Soluble Lipase NS81006-Mediated Alcoholysis of Oils Containing FFA for Biodiesel Production. J. Chem. Eng. Chin. Univ. 2010, 24, 82–86. [Google Scholar]
- Gog, A.; Roman, M.; Tosa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification—Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Lee, E.Y. Lipase-catalyzed in-situ biosynthesis of gycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.L.; Du, W.; Liu, D.H. Kinetics of Liquid Lipase NS81006-Ctalyzed Alcoholysis of Oil for Biodiesel Production. Chin. J. Catal. 2012, 33, 1857–1861. [Google Scholar]
- Lv, L.L.; Dai, L.M.; Du, W.; Liu, D.H. Effect of water on lipase NS81006-catalyzed alcoholysis for biodiesel production. Process Biochem. 2017, 58, 239–244. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Hashim, M.A.; Hayyan, M.; AlNashef, I.M.; Al-Zahrani, S.M.; Al-Saadi, M.A. Ethanesulfonic acid-based esterification of industrial acidic crude palm oil for biodiesel production. Bioresour. Technol. 2011, 102, 9564–9570. [Google Scholar] [CrossRef] [PubMed]
- Takisawa, K.; Kanemoto, K.; Miyazaki, T.; Kitamura, Y. Hydrolysis for direct esterification of lipids from wet microalgae. Bioresour. Technol. 2013, 144, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Takisawa, K.; Kanemoto, K.; Kartikawati, M.; Kitamura, Y. Simultaneous hydrolysis-esterification of wet microalgal lipid using acid. Bioresour. Technol. 2013, 149, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, X.; Du, W.; Liu, D. Preparation of Epoxidized Soybean Oil by Catalysis of Small Amount of Sulfuric Acid. Chin. J. Process Eng. 2010, 10, 714–719. [Google Scholar]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. Surfactant-assisted direct biodiesel production from wet Nannochloropsis occulata by in situ transesterification/reactive extraction. Biofuel Res. J. 2016, 9, 366–371. [Google Scholar] [CrossRef]
- Yang, W.; Du, W.; Liu, D.H. Production of Ricinoleic Acid from Castor Oil by Free Lipase-mediated Hydrolysis. Curr. Biotechnol. 2014, 4, 373–378. [Google Scholar]
- Chen, J.Z.; Wang, S.M.; Zhou, B.Y.; Dai, L.M.; Liu, D.H.; Du, W. A robust process for lipase-mediated biodiesel production from microalgae lipid. RSC Adv. 2016, 6, 48515–48522. [Google Scholar] [CrossRef]
- Ma, G.; Dai, L.; Liu, D.H.; Du, W. Lipase-Mediated Selective Methanolysis of Fish Oil for Biodiesel Production and Polyunsaturated Fatty Acid Enrichment. Energy Fuels 2018, 32, 7630–7635. [Google Scholar] [CrossRef]




| FFA | Acidic Soybean Oil | Refined Soybean Oil |
|---|---|---|
| wt% | wt% | |
| C16:0 | 14.5 | 11.8 |
| C18:0 | 2.65 | 4.29 |
| C18:1 | 22.3 | 24.9 |
| C18:2 | 54.3 | 52.0 |
| C18:3 | 6.06 | 6.99 |
| Other FFA | 0.19 | nd |
| Item | Value |
|---|---|
| Acid value (mgKOH/g) | 0.44 |
| FAME content (%) | 97 |
| Water content (%) | 0.04 |
| Monoglycerides content (%) | 2.76 |
| Diglycerides content (%) | 0 |
| Glycerol content (%) | 0 |
| Phosphorus content (ppm) | 4.90 |
| Sulphur content (ppm) | 9.60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Dai, L.; Liu, D.; Du, W. A Robust Two-Step Process for the Efficient Conversion of Acidic Soybean Oil for Biodiesel Production. Catalysts 2018, 8, 527. https://doi.org/10.3390/catal8110527
Ma G, Dai L, Liu D, Du W. A Robust Two-Step Process for the Efficient Conversion of Acidic Soybean Oil for Biodiesel Production. Catalysts. 2018; 8(11):527. https://doi.org/10.3390/catal8110527
Chicago/Turabian StyleMa, Gaojian, Lingmei Dai, Dehua Liu, and Wei Du. 2018. "A Robust Two-Step Process for the Efficient Conversion of Acidic Soybean Oil for Biodiesel Production" Catalysts 8, no. 11: 527. https://doi.org/10.3390/catal8110527





