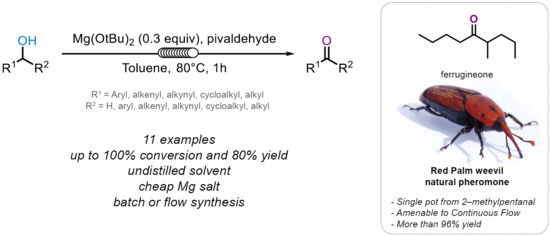
Mg-Catalyzed OPPenauer Oxidation—Application to the Flow Synthesis of a Natural Pheromone
Abstract
1. Introduction
2. Results and Discussions
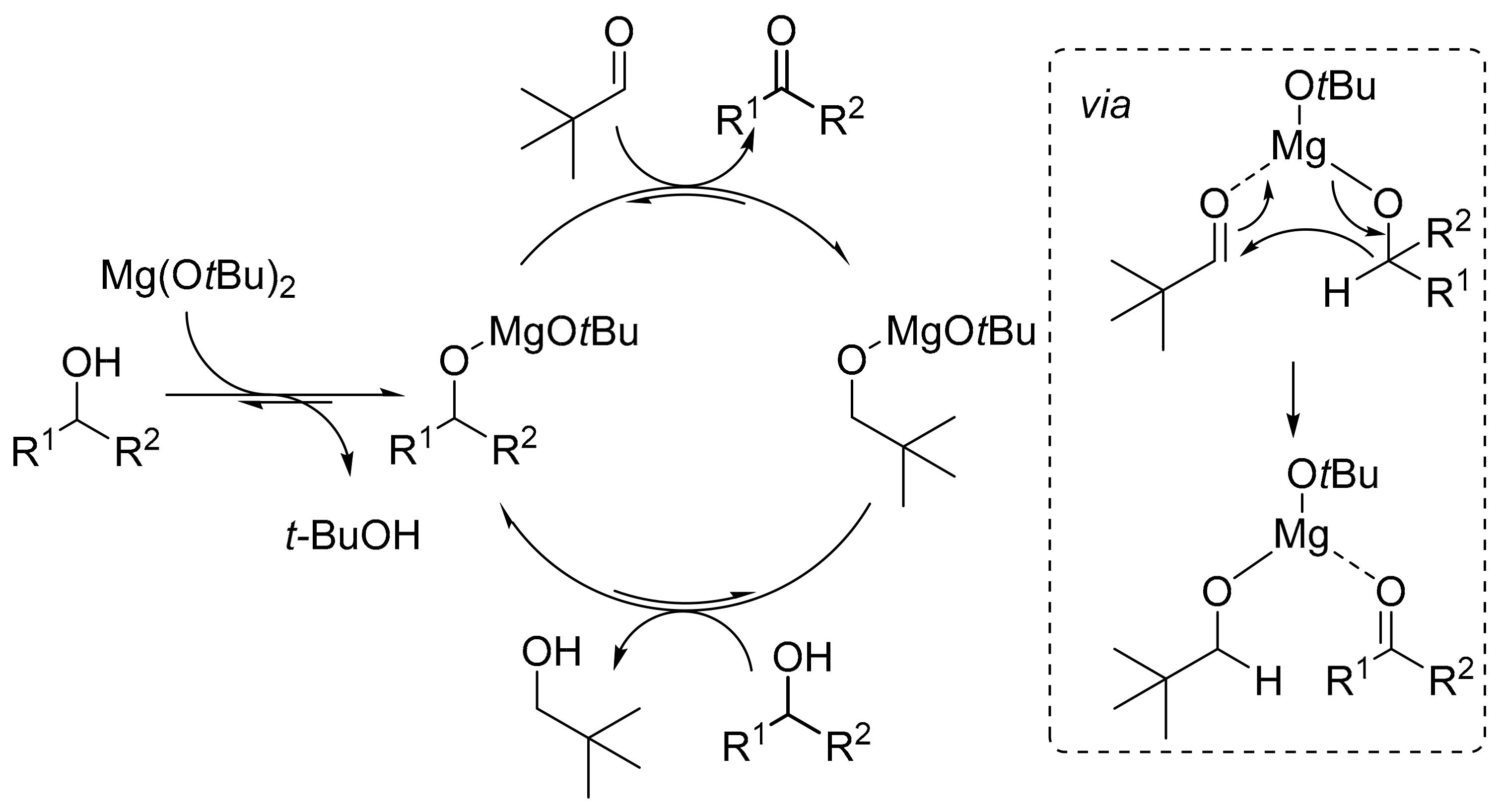
2.1. Preliminary Tests
2.2. Optimization of the Reaction Conditions
2.2.1. Aldehyde and Temperature Studies
2.2.2. Solvent Optimization
2.3. Batch Applications
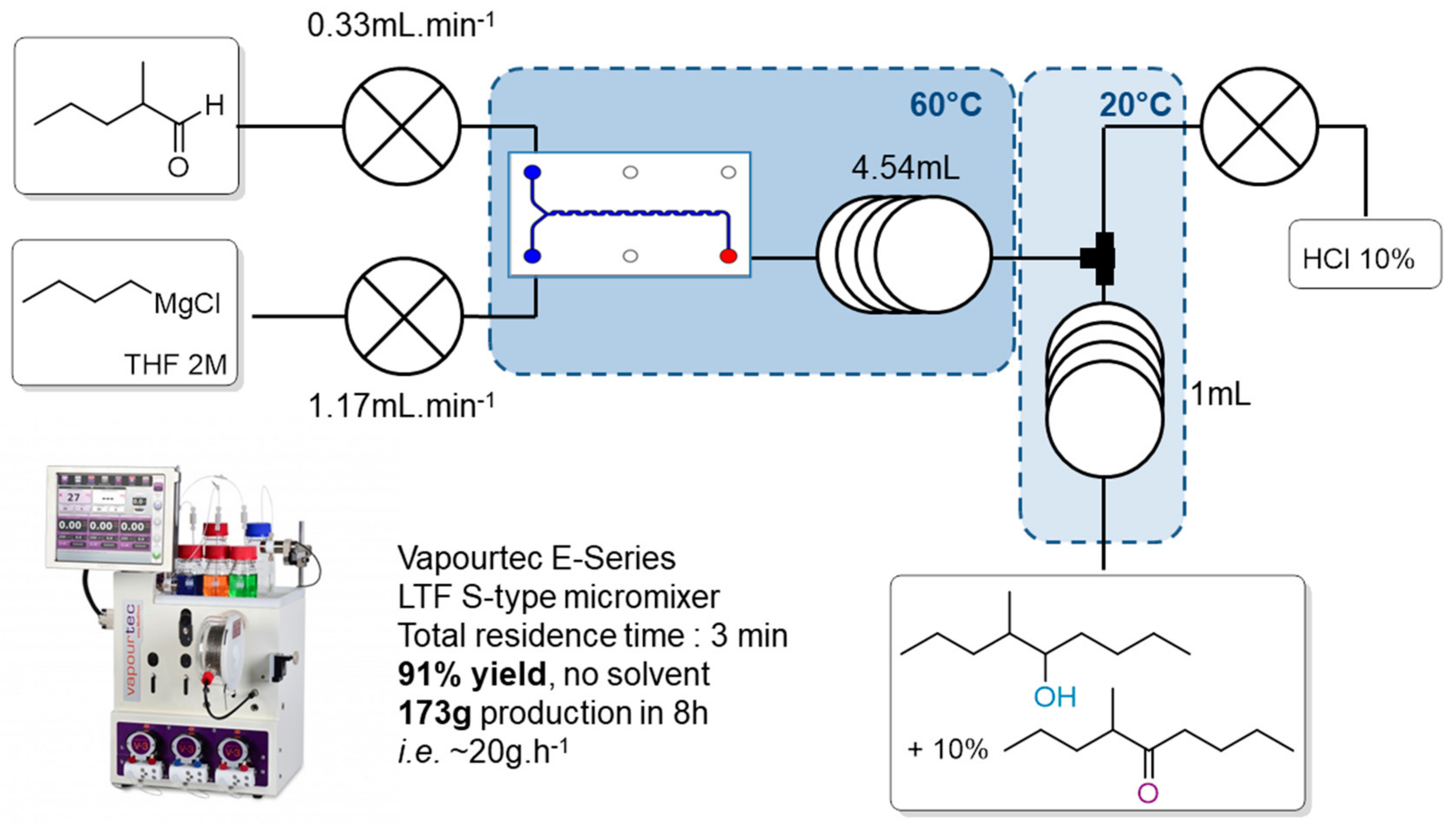
2.4. Flow Application
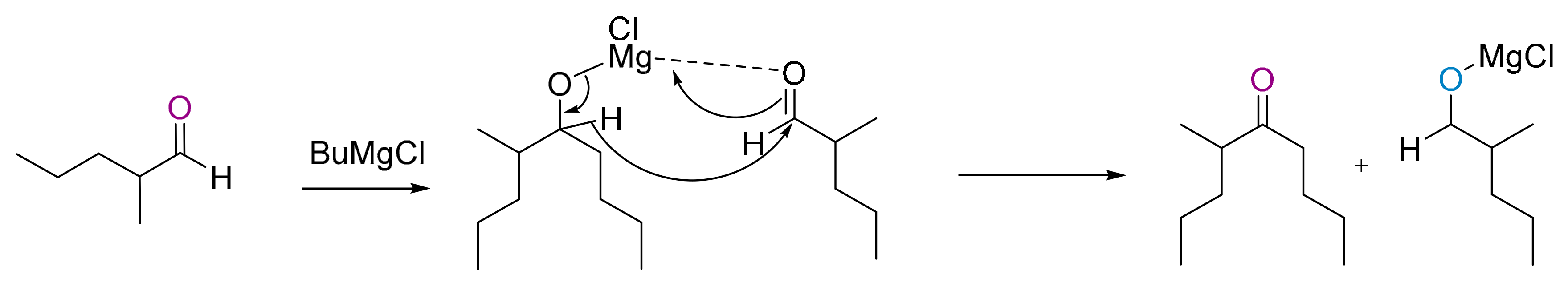
2.4.1. Using nBuMgCl in Catalytic Amounts
2.4.2. Proposed Synthesis of the 90/10 Mixture
2.4.3. Optimization of Flow Conditions
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oppenauer, R.V. Eine Methode der Dehydrierung von Sekundären Alkoholen zu Ketonen. I. Zur Herstellung von Sterinketonen und Sexualhormonen. Recl. Trav. Chim. Pays-Bas 1937, 56, 137–144. [Google Scholar] [CrossRef]
- Ponndorf, W.Z. Der reversible Austausch der Oxydationsstufen zwischen Aldehyden oder Ketonen einerseits und primären oder sekundären Alkoholen anderseits. Angew. Chem. 1926, 39, 138–143. [Google Scholar] [CrossRef]
- Verley, M. A Simple and Efficient Catalytic Method for the Reduction of Ketones. Bull. Soc. Chim. Fr. 1925, 37, 871–874. [Google Scholar] [CrossRef]
- Ramig, K.; Kuzemko, M.A.; Parrish, D.; Carpenter, B.K. A Novel Tandem Michael Addition/Meerwein−Ponndorf−Verley Reduction: Asymmetric Reduction of Acyclic α,β-Unsaturated Ketones Using A Chiral Mercapto Alcohol. Tetrahedron Lett. 1992, 33, 6279–6282. [Google Scholar] [CrossRef]
- Wanner, M.J.; Koomen, G.J.; Pandit, U.K. A Facile Stereoselective Synthesis of (±)-Sesbanine. Heterocycles 1981, 15, 377–379. [Google Scholar] [CrossRef]
- Bevinakatti, H.S.; Badiger, V.V.J. Synthesis and mass spectral studies of a coumarin analog of chloramphenicol. Heterocycl. Chem. 1980, 17, 1701–1703. [Google Scholar] [CrossRef]
- Chinn, L.J.; Salamon, K.W. Synthesis of 9-deoxy-11-oxoprostaglandins. Selective reduction of an 11,15-dione. J. Org. Chem. 1979, 44, 168–172. [Google Scholar] [CrossRef]
- Jones, R.A.; Webb, T.C. The chemistry of terpenes—V. Tetrahedron 1972, 28, 2877–2879. [Google Scholar] [CrossRef]
- Rice, K.C.; Wilson, R.S. (-)-3-Isothujone, a small nonnitrogenous molecule with antinociceptive activity in mice. J. Med. Chem. 1976, 19, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- de Graauw, C.F.; Peters, J.A.; van Bekkum, H.; Huskens, J. Meerwein-Ponndorf-Verley Reductions and Oppenauer Oxidations: An Integrated Approach. J. Synthesis 1994, 1007–1017. [Google Scholar] [CrossRef]
- Graves, C.R.; Joseph Campbell, E.; Nguyen, S.T. Aluminum-based catalysts for the asymmetric Meerwein-Schmidt-Ponndorf- Verley-Oppenauer (MSPVO) reaction manifold. Tetrahedron Asymmetry 2005, 16, 3460–3468. [Google Scholar] [CrossRef]
- Graves, C.R.; Zeng, B.-S.; Nguyen, S.T. Efficient and Selective Al-Catalyzed Alcohol Oxidation via Oppenauer Chemistry. J. Am. Chem. Soc. 2006, 128, 12596–12597. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.; Otsuka, H.; Miura, T.; Ichikawa, H.; Maruoka, K. Practical Oppenauer (OPP) Oxidation of Alcohols with a Modified Aluminum Catalyst. Org. Lett. 2002, 4, 2669–2672. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.; Miura, T.; Itagaki, Y.; Ichikawa, H.; Maruoka, K. Catalytic Meerwein-Ponndorf-Verley (MPV) and Oppenauer (OPP) Reactions: Remarkable Acceleration of the Hydride Transfer by Powerful Bidentate Aluminum Alkoxides. Synthesis 2002, 279–291. [Google Scholar] [CrossRef]
- Gates, M.; Tschudi, G. The synthesis of Morphine. JACS 1956, 78, 1380. [Google Scholar] [CrossRef]
- Woodward, R.B.; Wendler, N.E.; Brutschy, F.J. Quininone1. JACS 1945, 67, 1425. [Google Scholar] [CrossRef]
- Rapoport, H.; Naumann, R.; Bissell, E.R.; Bonner, R.M. The preparation of some dihydro ketones in the morphine series by Oppenauer oxidation. JOC 1950, 15, 1103. [Google Scholar] [CrossRef]
- Ballester, J.; Caminade, A.-M.; Majoral, J.-P.; Taillefer, M.; Ouali, A. Efficient and eco-compatible transition metal-free Oppenauer-type oxidation of alcohols. Catal. Commun. 2014, 47, 58–62. [Google Scholar] [CrossRef]
- Kirihara, M.; Okada, T.; Sugiyama, Y.; Akiyoshi, M.; Matsunaga, T.; Kimura, Y. Sodium Hypochlorite Pentahydrate Crystals (NaOCl·5H2O): A Convenient and Environmentally Benign Oxidant for Organic Synthesis. Org. Process Res. Dev. 2017, 21, 1925–1937. [Google Scholar] [CrossRef]
- Almeida, M.L.S.; Beller, M.; Wang, G.Z.; Bäckvall, J.E. Chromium Complexes with Oxido and Corrolato Ligands: Metal-Based Redox Processes versus Ligand Non-Innocence. Chem. A Eur. J. 2018, 2, 1533–1536. [Google Scholar] [CrossRef]
- Dani, P.; Karlen, T.; Gossage, R.A.; Gladiali, S.; van Koten, G. Hydrogen-Transfer Catalysis with Pincer-Aryl Ruthenium(II) Complexes. Angew. Chem. Int. Ed. 2000, 39, 743–745. [Google Scholar]
- Hanasaka, F.; Fujita, K.; Yamaguchi, R. Cp*Ir Complexes Bearing N-Heterocyclic Carbene Ligands as Effective Catalysts for Oppenauer-Type Oxidation of Alcohols. Organometallics 2004, 23, 1490–1492. [Google Scholar] [CrossRef]
- Ajjou, A.N.; Pinet, J.-L. Oppenauer-type oxidation of secondary alcohols catalyzed by homogeneous water-soluble complexes. Can. J. Chem. 2005, 83, 702–710. [Google Scholar] [CrossRef]
- Coleman, M.G.; Brown, A.N.; Bolton, B.A.; Guan, H. Iron-Catalyzed Oppenauer-Type Oxidation of Alcohols. Adv. Synth. Catal. 2010, 352, 967–970. [Google Scholar] [CrossRef]
- Battilocchio, C.; Hawkins, J.M.; Ley, S.V. A Mild and Efficient Flow Procedure for the Transfer Hydrogenation of Ketones and Aldehydes using Hydrous Zirconia. Org. Lett. 2013, 15, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Bruneau-Voisine, A.; Wang, D.; Dorcet, V.; Roisnel, T.; Darcel, C.; Sortais, J.-B. Transfer Hydrogenation of Carbonyl Derivatives Catalyzed by an Inexpensive Phosphine-Free Manganese Precatalyst. Org. Lett. 2017, 19, 3656–3659. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, Y.; Ono, Y.; Sakai, N. Indium(III) Isopropoxide as a Hydrogen Transfer Catalyst for Conversion of Benzylic Alcohols into Aldehydes or Ketones via Oppenauer Oxidation. Synthesis 2016, 48, 4143–4148. [Google Scholar] [CrossRef]
- Yohei, O.; Masahito, K.; Kotaro, K.; Takeo, K.; Norio, S. Oxidative Coupling of Terminal Alkynes with Aldehydes Leading to Alkynyl Ketones by Using Indium(III) Bromide. Chem. A Eur. J. 2015, 21, 18598–18600. [Google Scholar] [CrossRef]
- Linghu, X.; Satterfield, A.D.; Johnson, J.S. Symbiotic Reagent Activation: Oppenauer Oxidation of Magnesium Alkoxides by Silylglyoxylates Triggers Second-Stage Aldolization. J. Am. Chem. Soc. 2006, 128, 9302–9303. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Karras, M. Magnesium-oppenauer oxidation of alcohols to aldehydes and ketones. Tetrahedron Lett. 1987, 28, 769–772. [Google Scholar] [CrossRef]
- Kloetzing, R.J.; Krasovskiy, A.; Knochel, P. The Mg-Oppenauer Oxidation as a Mild Method for the Synthesis of Aryl and Metallocenyl Ketones. Chem. A Eur. J. 2007, 13, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Knochel, P.; Millot, N.; Rodriguez, A.L.; Tucker, C.E. Preparation and Applications of Functionalized Organozinc Compounds. Org. React. 2004. [Google Scholar] [CrossRef]
- Klatt, T.; Markiewicz, J.T.; Sämann, C.; Knochel, P. Strategies To Prepare and Use Functionalized Organometallic Reagents. J. Org. Chem. 2014, 79, 4253–4269. [Google Scholar] [CrossRef] [PubMed]
- Day, B.M.; Knowelden, W.; Coles, M.P. Synthetic and catalytic intermediates in a magnesium promoted Tishchenko reaction. Dalt. Trans. 2012, 41, 10930–10933. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, X.L.; Hügel, H.; Huang, D.; Du, Z.; Wang, K.; Hu, Y. Magnesium salt promoted tandem nucleophilic addition–Oppenauer oxidation of aldehydes with organozinc reagent. Org. Biomol. Chem. 2016, 14, 9720–9724. [Google Scholar] [CrossRef] [PubMed]
- Hallet, R.H.; Gries, G.; Gries, R.; Borden, J.H.; Czyzewska, E.; Oehlschlager, A.C.; Pierce, H.D.; Angerilli, N.P.D.; Rauf, A. Aggregation pheromones of two asian palm Weevils, Rhynchophorus ferrugineus and R. vulneratus. Naturwissenschaften 1993, 80, 328–331. [Google Scholar] [CrossRef]
- Perez, A.L.; Hallett, R.H.; Gries, R.; Gries, G.; Oehlschlager, B. Pheromone chirality of asian palm weevils, Rhynchophorus ferrugineus (Oliv.) andR. vulneratus (Panz.) (Coleoptera: Curculionidae). J. Chem. Ecology 1996, 22, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Guerret, O.; Dufour, S. Solid Composition for Controlled Delivery of Semiochemical Substances; EP3187046A1; Melchior Material and Life Science: Lacq, France, 2017. [Google Scholar]

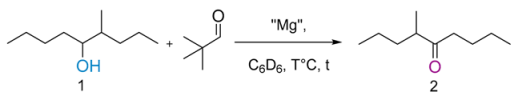
| Entry | “Mg” (equiv) | Temperature (°C) | Time (h) | 1H NMR (%) Conversion |
|---|---|---|---|---|
| entry 1 | MgCl2 (0.4) | 100 | 3 | 3 |
| entry 2 | Mg(OH)2 (0.4) | 100 | 3 | 2 |
| entry 3 | Mg(OEt)2 (0.4) | 100 | 3 | 31 |
| entry 4 | Mg(OEt)2 (0.4) | 100 | 6 | 33 |
| entry 5 | MgO (0.4) | 100 | 3 | 2 |
| entry 6 | Mg(OtBu)2 (0.3) | 110 | 1 | 70 |
| entry 7 | Mg(OtBu)2 (0.3) | 60 | 1 | 21 |
| entry 8 | Mg(OtBu)2 (0.3) | 60 | 3 | 32 |
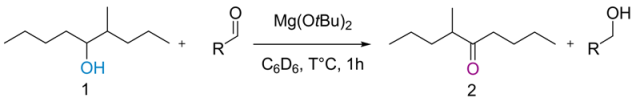
| Entry | Aldehyde | Temperature (°C) | 1H NMR (%) Conversions 1 |
|---|---|---|---|
| entry 1 | Pivaldehyde | 110 | 70 |
| entry 2 | 2-methylpentanal | 60 | 21 |
| entry 3 | isobutyraldehyde | 100 | 25 |
| entry 4 | Cyclohexane carboxaldehyde | 100 | 54 |
| entry 5 | Bromaldehyde | 100 | 84 |
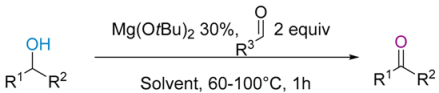
| Entry | Alcohol | Aldehyde | Solvent | 1H NMR (%) Conversions 1 |
|---|---|---|---|---|
| entry 1 | Ferrugineol | Pivaldehyde | C6D6 a | 70 |
| entry 2 | Ferrugineol | Pivaldehyde | Toluene b | 54 |
| entry 3 | Ferrugineol | Pivaldehyde | DMF b | 16 |
| entry 4 | Ferrugineol | Pivaldehyde | THF b | 3 |
| entry 5 | Ferrugineol | Pivaldehyde | MeTHF b | 16 |
| entry 6 | Ferrugineol | Pivaldehyde | DME b | traces |
| entry 7 | Cyclohexanol | Pivaldehyde | C6D6 b | 62 |
| entry 8 | Cyclohexanol | Pivaldehyde | Toluene c | 71 |
| entry 9 | Cyclohexanol | Pivaldehyde | Dry Toluene c | 46 |
| entry 10 | Cyclohexanol | Pivaldehyde | DCE c | 55 |
| entry 11 | Cyclohexanol | Pivaldehyde | MTBE c | 57 |
| entry 12 | Cyclohexanol | Bromaldehyde | C6D6 d | 60 |
| entry 13 | Cyclohexanol | Bromaldehyde | Dry C6D6 d | 31 |
| entry 14 | Cyclohexanol | Bromaldehyde | Dry C6D6 + H2O d | traces |
| entry 15 | Cyclohexanol | Bromaldehyde | Toluene c | 45 |
| entry 16 | Cyclohexanol | Bromaldehyde | DCE c | 36 |
| entry 17 | Cyclohexanol | Bromaldehyde | MTBE c | 38 |
| entry 18 | Cyclohexanol | Bromaldehyde | Dry THF c | 3 |
| entry 19 | Cyclohexanol | Bromaldehyde | Dry DCM d | 39 |
| entry 10 | Cyclohexanol | Bromaldehyde | Dry Toluene d | 49 |
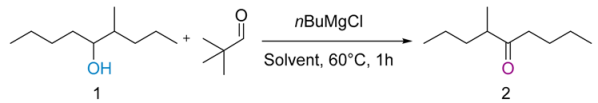
| Entry | nBuMgCl (equiv) | Solvent | 1H NMR (%) Conversions 1 |
|---|---|---|---|
| entry 1 | 0.1 to 0.3 | neat | NR |
| entry 2 | 0.4 | neat | 18 |
| entry 3 | 0.5 | neat | traces |
| entry 4 | 0.4 | THF | NR |
| entry 5 | 0.4 | C6D6 | 21 |
| entry 6 | 0.4 | CDCl3 | traces |
| entry 7 | 0.4 | DME | traces |
| entry 8 | 0.4 | DMF | traces |
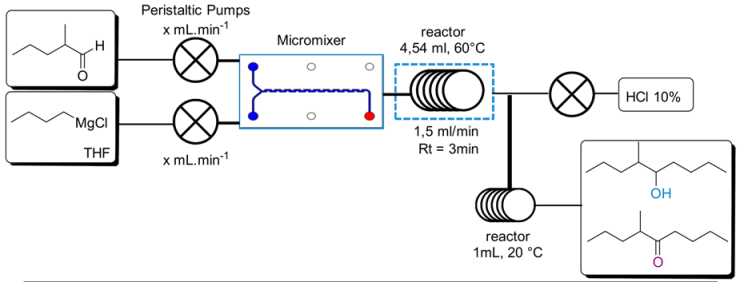
| Entry | Aldehyde Flow (mL/min) | nBuMgCl Flow (mL/min) | nBuMgCl Conc. | nBuMgCl | % (2) (GC/MS) | % (1) (GC/MS) | Yield (%) |
|---|---|---|---|---|---|---|---|
| entry 1 | 0.281 | 1.22 | 2.04 | 1.10 | 0.7 | 99.3 | 60 |
| entry 2 | 0.297 | 1.2 | 2.04 | 1.03 | 1.8 | 98.2 | 79 |
| entry 3 | 0.329 | 1.17 | 2.04 | 0.90 | 14.0 | 86.0 | 91 |
| entry 4 | 0.4 | 1.11 | 2.04 | 0.70 | 21.0 | 78.4 | 97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liautard, V.; Birepinte, M.; Bettoli, C.; Pucheault, M. Mg-Catalyzed OPPenauer Oxidation—Application to the Flow Synthesis of a Natural Pheromone. Catalysts 2018, 8, 529. https://doi.org/10.3390/catal8110529
Liautard V, Birepinte M, Bettoli C, Pucheault M. Mg-Catalyzed OPPenauer Oxidation—Application to the Flow Synthesis of a Natural Pheromone. Catalysts. 2018; 8(11):529. https://doi.org/10.3390/catal8110529
Chicago/Turabian StyleLiautard, Virginie, Mélodie Birepinte, Camille Bettoli, and Mathieu Pucheault. 2018. "Mg-Catalyzed OPPenauer Oxidation—Application to the Flow Synthesis of a Natural Pheromone" Catalysts 8, no. 11: 529. https://doi.org/10.3390/catal8110529
APA StyleLiautard, V., Birepinte, M., Bettoli, C., & Pucheault, M. (2018). Mg-Catalyzed OPPenauer Oxidation—Application to the Flow Synthesis of a Natural Pheromone. Catalysts, 8(11), 529. https://doi.org/10.3390/catal8110529