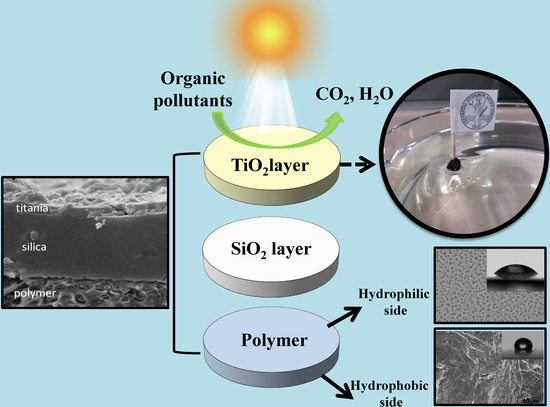
TiO2-SiO2-PMMA Terpolymer Floating Device for the Photocatalytic Remediation of Water and Gas Phase Pollutants
Abstract
:1. Introduction
2. Results and Discussion
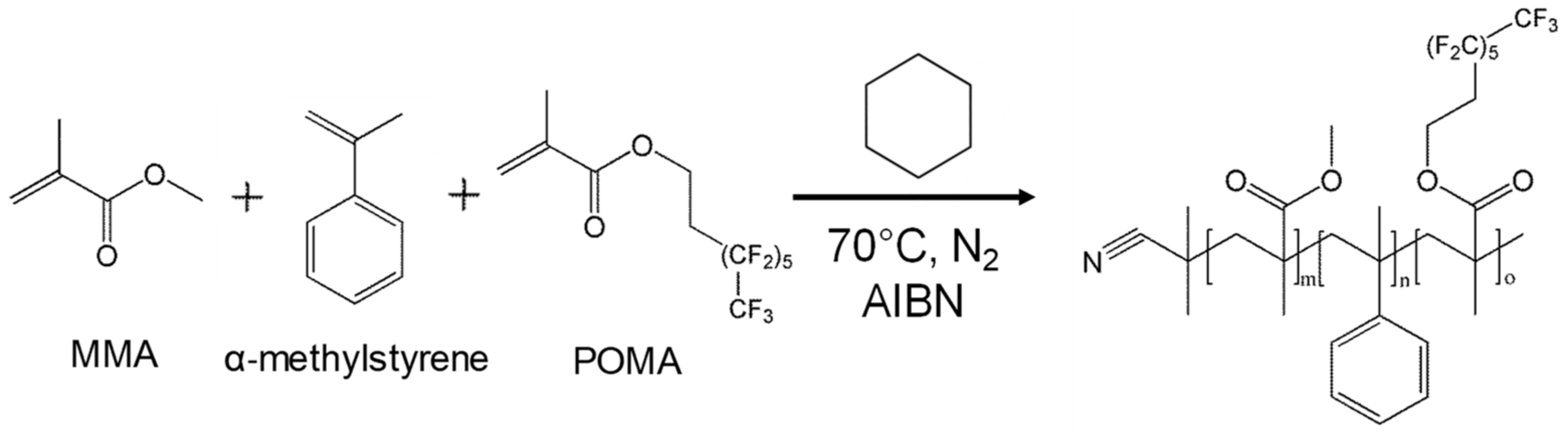
2.1. Synthesis and Characterization of MMA_α-Methylstyrene_POMA ter-Polymer
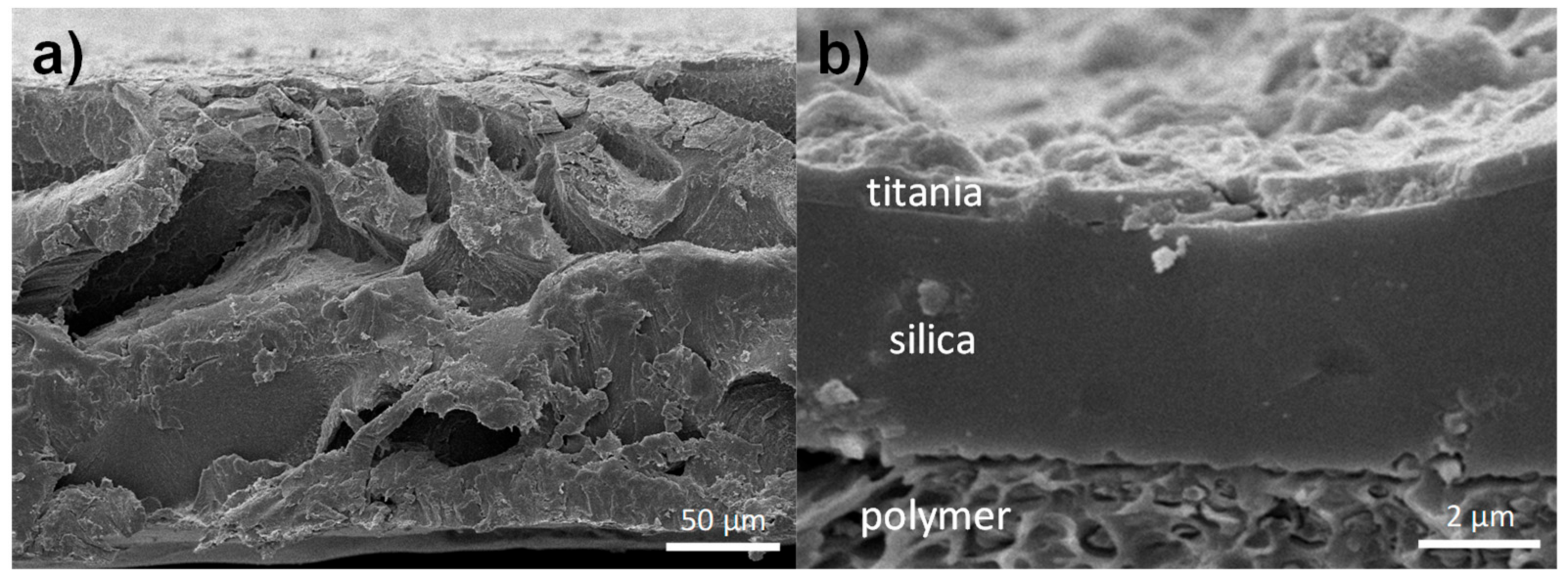
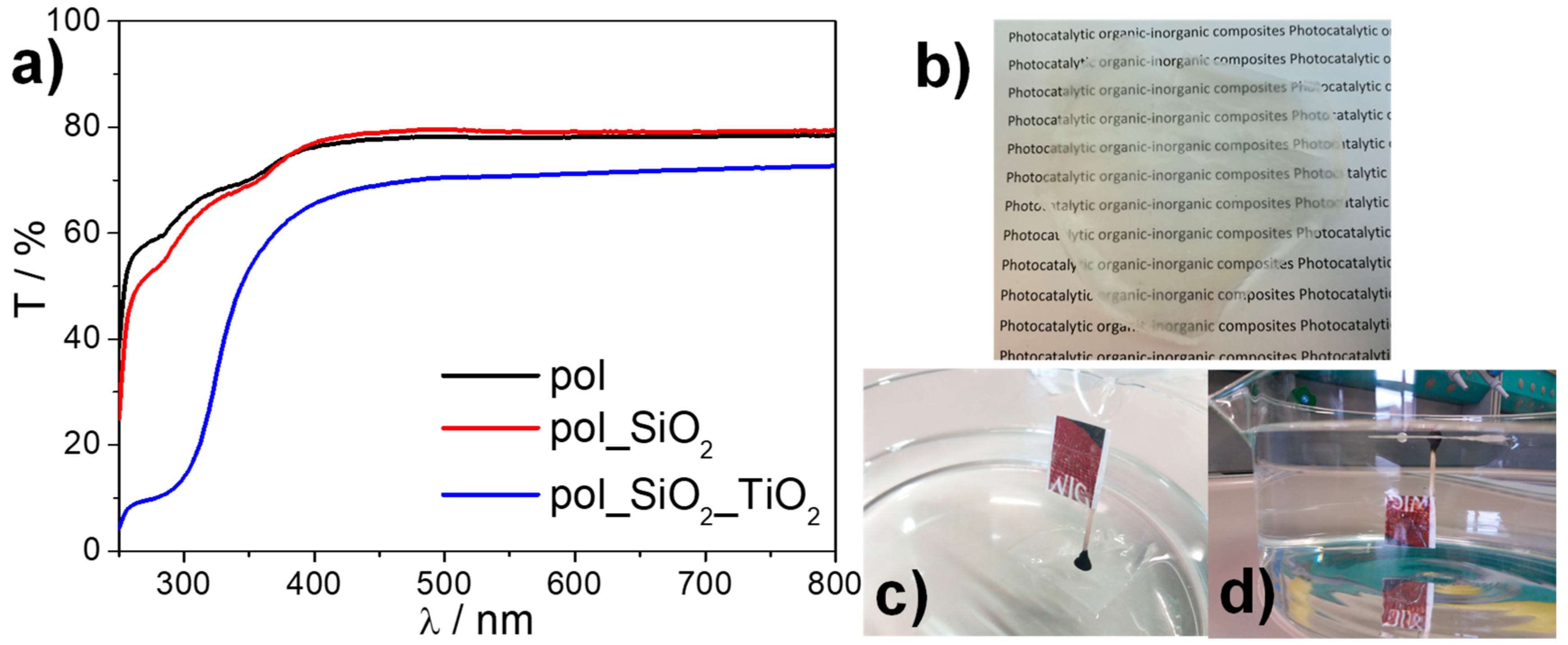
2.2. Device Preparation and Characterization
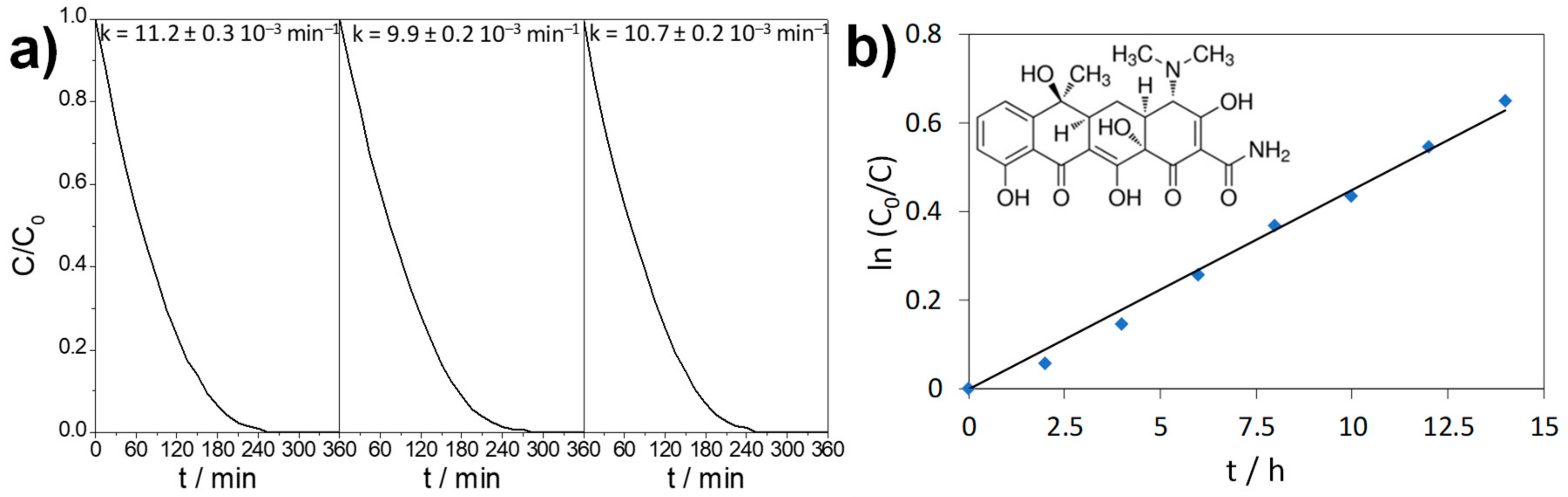
2.3. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation Procedures
3.2.1. Synthesis of POMA
3.2.2. Synthesis of MMA_α-Methylstyrene_POMA Ter-Polymer
3.2.3. Silica and Titania Sol Preparation
3.2.4. Device Preparation
3.3. Characterization Methods
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crippa, M.; Bianchi, A.; Cristofori, D.; D’Arienzo, M.; Merletti, F.; Morazzoni, F.; Scotti, R.; Simonutti, R. High dielectric constant rutile–polystyrene composite with enhanced percolative threshold. J. Mater. Chem. C 2013, 1, 484–492. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, J.; Shang, Y.; Wang, L.; He, X.; Li, J.; Zhao, P.; Wang, Y. Nanocomposite polymer membrane derived from nano TiO2 -PMMA and glass fiber nonwoven: High thermal endurance and cycle stability in lithium ion battery applications. J. Mater. Chem. A 2015, 3, 17697–17703. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zhang, Y.-M. Preparation and investigation of PVDF/PMMA/TiO2 composite film. J. Mater. Sci. 2009, 44, 2977–2984. [Google Scholar] [CrossRef]
- Motaung, T.E.; Luyt, A.S.; Bondioli, F.; Messori, M.; Saladino, M.L.; Spinella, A.; Nasillo, G.; Caponetti, E. PMMA–titania nanocomposites: Properties and thermal degradation behaviour. Polym. Degrad. Stab. 2012, 97, 1325–1333. [Google Scholar] [CrossRef]
- Pan, B.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.; Zheng, S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009, 151, 19–29. [Google Scholar] [CrossRef]
- Wang, M.; Yang, G.; Jin, P.; Tang, H.; Wang, H.; Chen, Y. Highly hydrophilic poly(vinylidene fluoride)/meso-titania hybrid mesoporous membrane for photocatalytic membrane reactor in water. Sci. Rep. 2016, 6, 19148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Nano-TiO2 Reinforced PEEK/PEI Blends as Biomaterials for Load-Bearing Implant Applications. ACS Appl. Mater. Interfaces 2015, 7, 5561–5573. [Google Scholar] [CrossRef] [PubMed]
- Salabat, A.; Mirhoseini, F. Applications of a new type of poly(methyl methacrylate)/TiO2 nanocomposite as an antibacterial agent and a reducing photocatalyst. Photochem. Photobiol. Sci. 2015, 14, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Ravirajan, P.; Haque, S.A.; Durrant, J.R.; Bradley, D.D.C.; Nelson, J. The Effect of Polymer Optoelectronic Properties on the Performance of Multilayer Hybrid Polymer/TiO2 Solar Cells. Adv. Funct. Mater. 2005, 15, 609–618. [Google Scholar] [CrossRef]
- Lee, H.J.; Leventis, H.C.; Haque, S.A.; Torres, T.; Grätzel, M.; Nazeeruddin, M.K. Panchromatic response composed of hybrid visible-light absorbing polymers and near-IR absorbing dyes for nanocrystalline TiO2-based solid-state solar cells. J. Power Sources 2011, 196, 596–599. [Google Scholar] [CrossRef]
- Wen, R.; Guo, J.; Zhao, C.; Liu, Y. Nanocomposite Capacitors with Significantly Enhanced Energy Density and Breakdown Strength Utilizing a Small Loading of Monolayer Titania. Adv. Mater. Interfaces 2018, 5, 1701088. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; He, X.; Fang, M.; Gao, J.; Li, J.; Deng, L.; Chen, H.; Tian, G.; Wang, J.; et al. In situ prepared nano-crystalline TiO2–poly(methyl methacrylate) hybrid enhanced composite polymer electrolyte for Li-ion batteries. J. Mater. Chem. A 2013, 1, 5955–5961. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Liu, Y.-Y.; Lin, R.-H.; Yen, F.-S. Photocatalytic degradation of p-phenylenediamine with TiO2-coated magnetic PMMA microspheres in an aqueous solution. J. Hazard. Mater. 2009, 163, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chen, L.-L.; Shang, N.-C. Photocatalytic degradation of dimethyl phthalate in an aqueous solution with Pt-doped TiO2-coated magnetic PMMA microspheres. J. Hazard. Mater. 2009, 172, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, W.; Wu, J.-M. Titania nanowires functionalized polyester fabrics with enhanced photocatalytic and antibacterial performances. J. Hazard. Mater. 2018, 343, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shao, C.; Yang, S.; Li, X.; Guo, X.; Wang, X.; Li, X.; Liu, Y. Heterojunction of g-C3N4/BiOI Immobilized on Flexible Electrospun Polyacrylonitrile Nanofibers: Facile Preparation and Enhanced Visible Photocatalytic Activity for Floating Photocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 2316–2323. [Google Scholar] [CrossRef]
- Krýsa, J.; Waldner, G.; Měšt’ánková, H.; Jirkovský, J.; Grabner, G. Photocatalytic degradation of model organic pollutants on an immobilized particulate TiO2 layer. Appl. Catal. B Environ. 2006, 64, 290–301. [Google Scholar] [CrossRef]
- Tu, W.; Lin, Y.-P.; Bai, R. Enhanced performance in phenol removal from aqueous solutions by a buoyant composite photocatalyst prepared with a two-layered configuration on polypropylene substrate. J. Environ. Chem. Eng. 2016, 4, 230–239. [Google Scholar] [CrossRef]
- Ni, L.; Li, Y.; Zhang, C.; Li, L.; Zhang, W.; Wang, D. Novel floating photocatalysts based on polyurethane composite foams modified with silver/titanium dioxide/graphene ternary nanoparticles for the visible-light-mediated remediation of diesel-polluted surface water. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Han, H.; Bai, R. Highly effective buoyant photocatalyst prepared with a novel layered-TiO2 configuration on polypropylene fabric and the degradation performance for methyl orange dye under UV–Vis and Vis lights. Sep. Purif. Technol. 2010, 73, 142–150. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Yang, M.; Di, Z.; Lee, J.-K. Facile control of surface wettability in TiO2/poly(methyl methacrylate) composite films. J. Colloid Interface Sci. 2012, 368, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Soliveri, G.; Sabatini, V.; Farina, H.; Ortenzi, M.A.; Meroni, D.; Colombo, A. Double side self-cleaning polymeric materials: The hydrophobic and photoactive approach. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 285–291. [Google Scholar] [CrossRef]
- Ma, J.Z.; Hu, J.; Zhang, Z.J. Polyacrylate/silica nanocomposite materials prepared by sol-gel process. Eur. Polym. J. 2007, 43, 4169–4177. [Google Scholar] [CrossRef]
- McLaren, A.C.; McLaren, S.G.; Hickmon, M.K. Sucrose, xylitol, and erythritol increase PMMA permeability for depot antibiotics. Clin. Orthop. Relat. Res. 2007, 461, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, V.; Farina, H.; Montarsolo, A.; Ardizzone, S.; Ortenzi, M.A. Novel Synthetic Approach to Tune the Surface Properties of Polymeric Films: Ionic Exchange Reaction between Sulfonated Polyarylethersulfones and Ionic Liquids. Polym. Plast. Technol. Eng. 2017, 56, 296–309. [Google Scholar] [CrossRef]
- Sabatini, V.; Cattò, C.; Cappelletti, G.; Cappitelli, F.; Antenucci, S.; Farina, H.; Ortenzi, M.A.; Camazzola, S.; Di Silvestro, G. Protective features, durability and biodegration study of acrylic and methacrylic fluorinated polymer coatings for marble protection. Prog. Org. Coat. 2018, 114, 47–57. [Google Scholar] [CrossRef]
- Cappelletti, G.; Ardizzone, S.; Meroni, D.; Soliveri, G.; Ceotto, M.; Biaggi, C.; Benaglia, M.; Raimondi, L. Wettability of bare and fluorinated silanes: A combined approach based on surface free energy evaluations and dipole moment calculations. J. Colloid Interface Sci. 2013, 389, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kim, J.-B.; Bruening, M.L.; Baker, G.L. Functionalization of Surfaces by Water-Accelerated Atom-Transfer Radical Polymerization of Hydroxyethyl Methacrylate and Subsequent Derivatization. Macromolecules 2002, 35, 1175–1179. [Google Scholar] [CrossRef]
- Chiantore, O.; Trossarelli, L.; Lazzari, M. Photooxidative degradation of acrylic and methacrylic polymers. Polymer 2000, 41, 1657–1668. [Google Scholar] [CrossRef]
- McNeill, I.C.; Sadeghi, S.M.T. Thermal stability and degradation mechanisms of poly(acrylic acid) and its salts: Part 1—Poly(acrylic acid). Polym. Degrad. Stab. 1990, 29, 233–246. [Google Scholar] [CrossRef]
- Zuev, V.V.; Bertini, F.; Audisio, G. Investigation on the thermal degradation of acrylic polymers with fluorinated side-chains. Polym. Degrad. Stab. 2006, 91, 512–516. [Google Scholar] [CrossRef]
- Carradò, A.; Sokolova, O.; Donnio, B.; Palkowski, H. Influence of corona treatment on adhesion and mechanical properties in metal/polymer/metal systems. J. Appl. Polym. Sci. 2011, 120, 3709–3715. [Google Scholar] [CrossRef]
- Soliveri, G.; Pifferi, V.; Panzarasa, G.; Ardizzone, S.; Cappelletti, G.; Meroni, D.; Sparnacci, K.; Falciola, L. Self-cleaning properties in engineered sensors for dopamine electroanalytical detection. Analyst 2015, 140, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Arabatzis, I.; Antonaraki, S.; Stergiopoulos, T.; Hiskia, A.; Papaconstantinou, E.; Bernard, M.; Falaras, P. Preparation, characterization and photocatalytic activity of nanocrystalline thin film TiO2 catalysts towards 3,5-dichlorophenol degradation. J. Photochem. Photobiol. A Chem. 2002, 149, 237–245. [Google Scholar] [CrossRef]
- Pifferi, V.; Rimoldi, L.; Meroni, D.; Segrado, F.; Soliveri, G.; Ardizzone, S.; Falciola, L. Electrochemical characterization of insulating silica-modified electrodes: Transport properties and physicochemical features. Electrochem. Commun. 2017, 81, 102–105. [Google Scholar] [CrossRef]
- Antonello, A.; Soliveri, G.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Photocatalytic remediation of indoor pollution by transparent TiO2 films. Catal. Today 2014, 230, 35–40. [Google Scholar] [CrossRef]
- Piera, E.; Ayllón, J.A.; Doménech, X.; Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76, 259–270. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; U.S. Food and Drug Administration: Washington, DC, USA, 2016. [Google Scholar]
- Rimoldi, L.; Pargoletti, E.; Meroni, D.; Falletta, E.; Cerrato, G.; Turco, F.; Cappelletti, G. Concurrent role of metal (Sn, Zn) and N species in enhancing the photocatalytic activity of TiO2 under solar light. Catal. Today 2018, 313, 40–46. [Google Scholar] [CrossRef]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Pérez-Moya, M.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Calamari, D.; Zuccato, E.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Strategic Survey of Therapeutic Drugs in the Rivers Po and Lambro in Northern Italy. Environ. Sci. Technol. 2003, 37, 1241–1248. [Google Scholar] [CrossRef]
- Czekalski, N.; Berthold, T.; Caucci, S.; Egli, A.; Bürgmann, H. Increased Levels of Multiresistant Bacteria and Resistance Genes after Wastewater Treatment and Their Dissemination into Lake Geneva, Switzerland. Front. Microbiol. 2012, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Addamo, M.; Augugliaro, V.; Di Paola, A.; García-López, E.; Loddo, V.; Marcì, G.; Palmisano, L. Removal of drugs in aqueous systems by photoassisted degradation. J. Appl. Electrochem. 2005, 35, 765–774. [Google Scholar] [CrossRef]
- Magalhães, F.; Lago, R.M. Floating photocatalysts based on TiO2 grafted on expanded polystyrene beads for the solar degradation of dyes. Sol. Energy 2009, 83, 1521–1526. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Maino, G.; Meroni, D.; Pifferi, V.; Falciola, L.; Soliveri, G.; Cappelletti, G.; Ardizzone, S. Electrochemically assisted deposition of transparent, mechanically robust TiO2 films for advanced applications. J. Nanopart. Res. 2013, 15, 2087. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Green and low cost tetracycline degradation processes by nanometric and immobilized TiO2 systems. Catal. Today 2017, 281, 38–44. [Google Scholar] [CrossRef]
- Reyes, C.; Fernández, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]

| Tg (°C) | D | wCA Air-Side (°) | wCA Mould-Side (°) | Elastic Modulus (GPa) | Tensile Strength (Mpa) | Elongation at Break (%) | ||
|---|---|---|---|---|---|---|---|---|
| before UV test | 123.9 | 28100 | 2.1 | 67 ± 2 | 114 ± 3 | 3.1 | 70 | 2.3 |
| after UV test | 124.0 | 28000 | 2.1 | 66 ± 3 | 116 ± 2 | 2.9 | 68 | 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatini, V.; Rimoldi, L.; Tripaldi, L.; Meroni, D.; Farina, H.; Ortenzi, M.A.; Ardizzone, S. TiO2-SiO2-PMMA Terpolymer Floating Device for the Photocatalytic Remediation of Water and Gas Phase Pollutants. Catalysts 2018, 8, 568. https://doi.org/10.3390/catal8110568
Sabatini V, Rimoldi L, Tripaldi L, Meroni D, Farina H, Ortenzi MA, Ardizzone S. TiO2-SiO2-PMMA Terpolymer Floating Device for the Photocatalytic Remediation of Water and Gas Phase Pollutants. Catalysts. 2018; 8(11):568. https://doi.org/10.3390/catal8110568
Chicago/Turabian StyleSabatini, Valentina, Luca Rimoldi, Laura Tripaldi, Daniela Meroni, Hermes Farina, Marco Aldo Ortenzi, and Silvia Ardizzone. 2018. "TiO2-SiO2-PMMA Terpolymer Floating Device for the Photocatalytic Remediation of Water and Gas Phase Pollutants" Catalysts 8, no. 11: 568. https://doi.org/10.3390/catal8110568
APA StyleSabatini, V., Rimoldi, L., Tripaldi, L., Meroni, D., Farina, H., Ortenzi, M. A., & Ardizzone, S. (2018). TiO2-SiO2-PMMA Terpolymer Floating Device for the Photocatalytic Remediation of Water and Gas Phase Pollutants. Catalysts, 8(11), 568. https://doi.org/10.3390/catal8110568