Environmental-Friendly Synthesis of Alkyl Carbamates from Urea and Alcohols with Silica Gel Supported Catalysts
Abstract
:1. Introduction
2. Results and Discussion
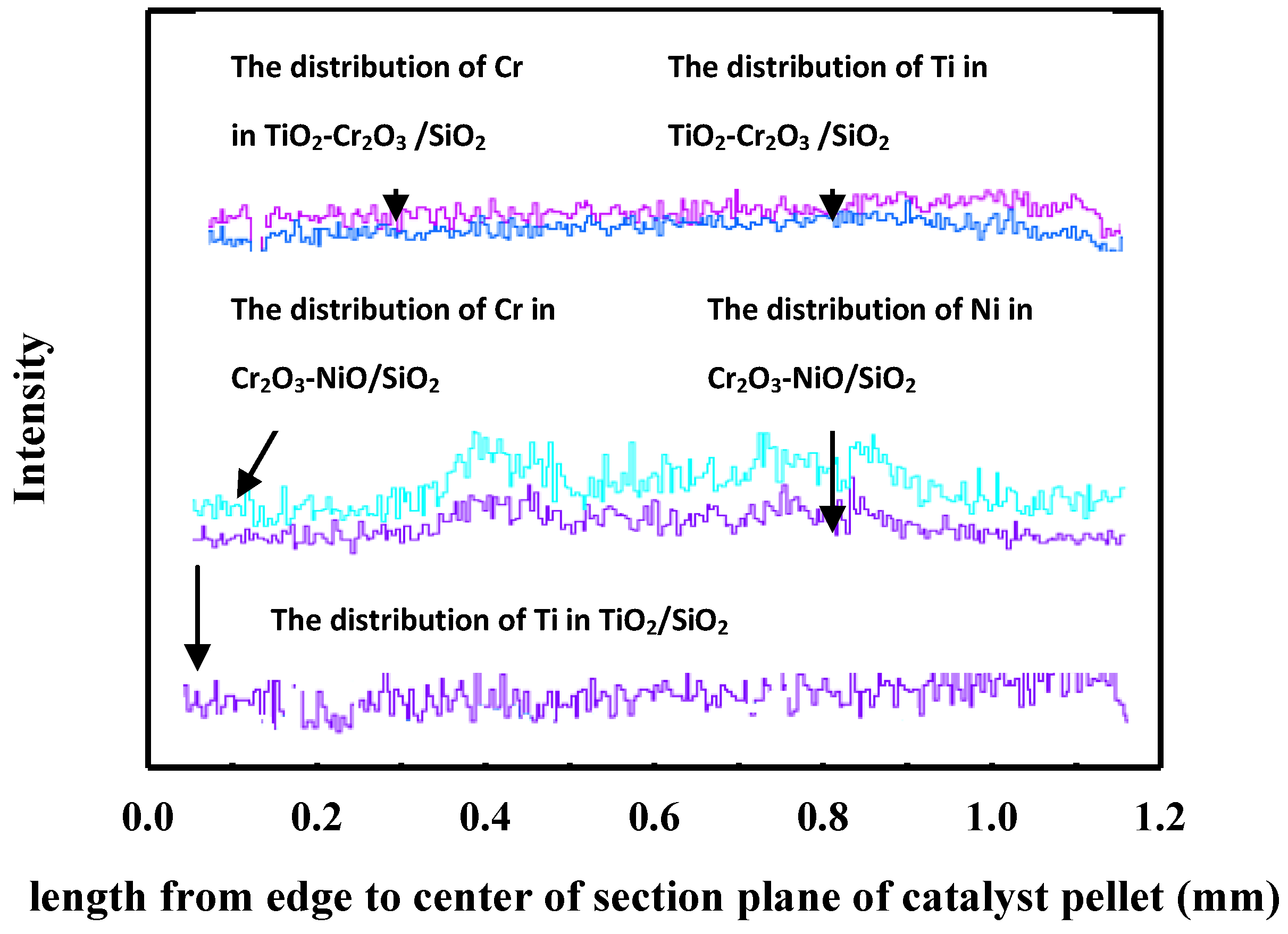
2.1. Characterization of Catalysts
2.2. Catalytic Activity Test
2.2.1. Effect of Active Metal Loading on Synthesis of Alkyl Carbamate
2.2.2. Performances of Different Catalysts for Synthesis of MC, EC, and BC
2.2.3. Effect of Calcination Temperature
2.2.4. Effect of Alcohol/Urea Molar Ratio
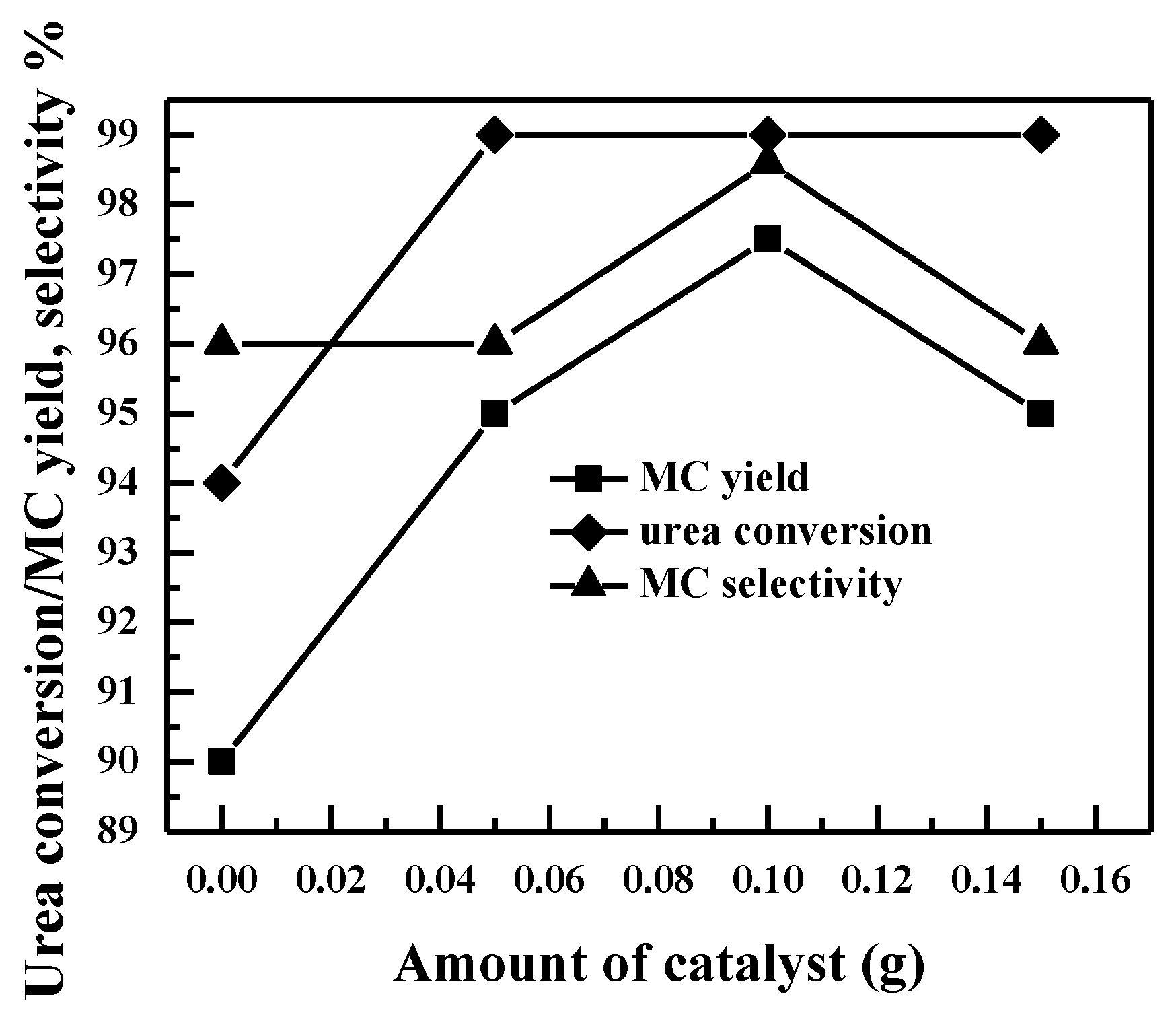
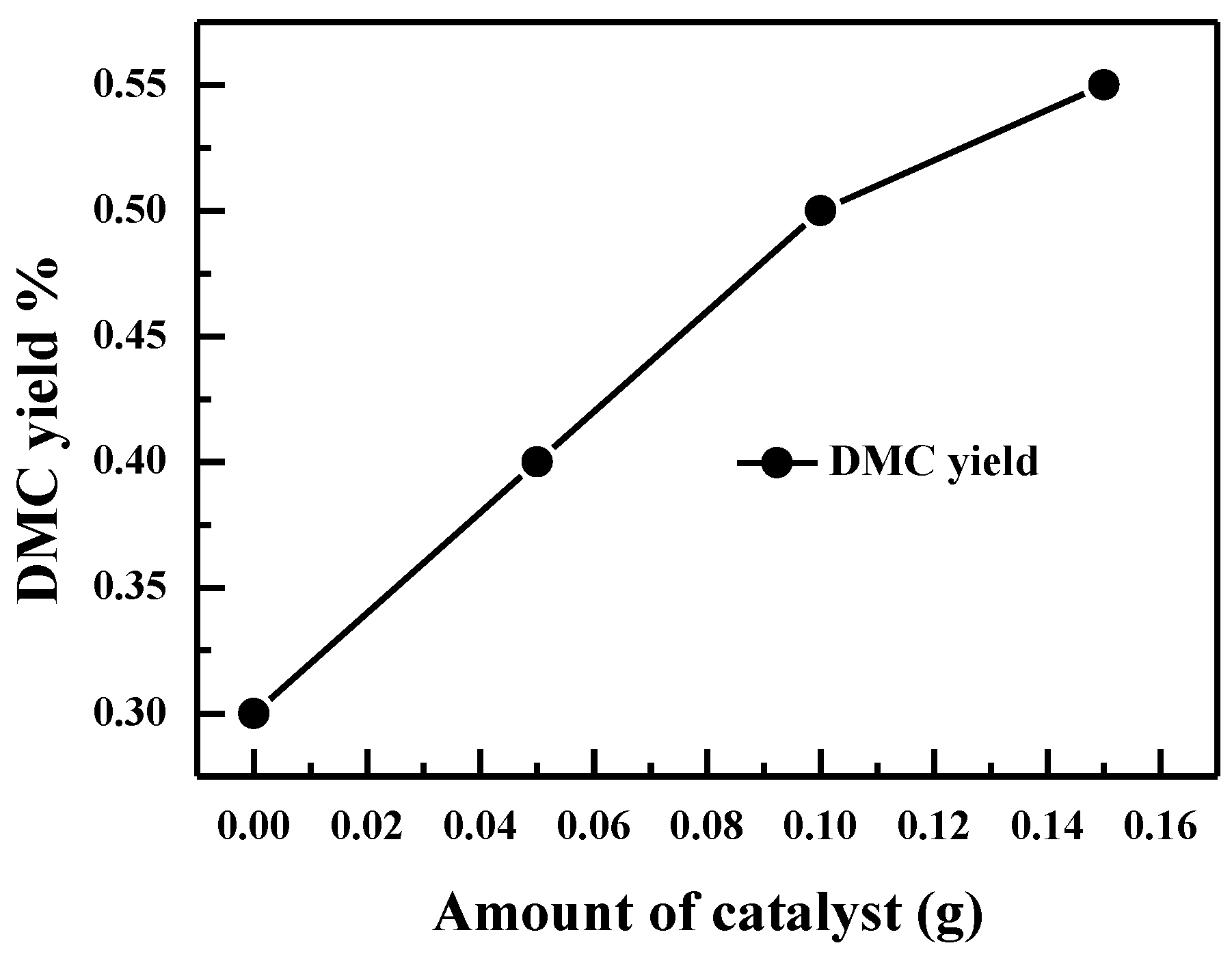
2.2.5. Effect of Catalyst Loadings
2.2.6. Effect of Reaction Temperature
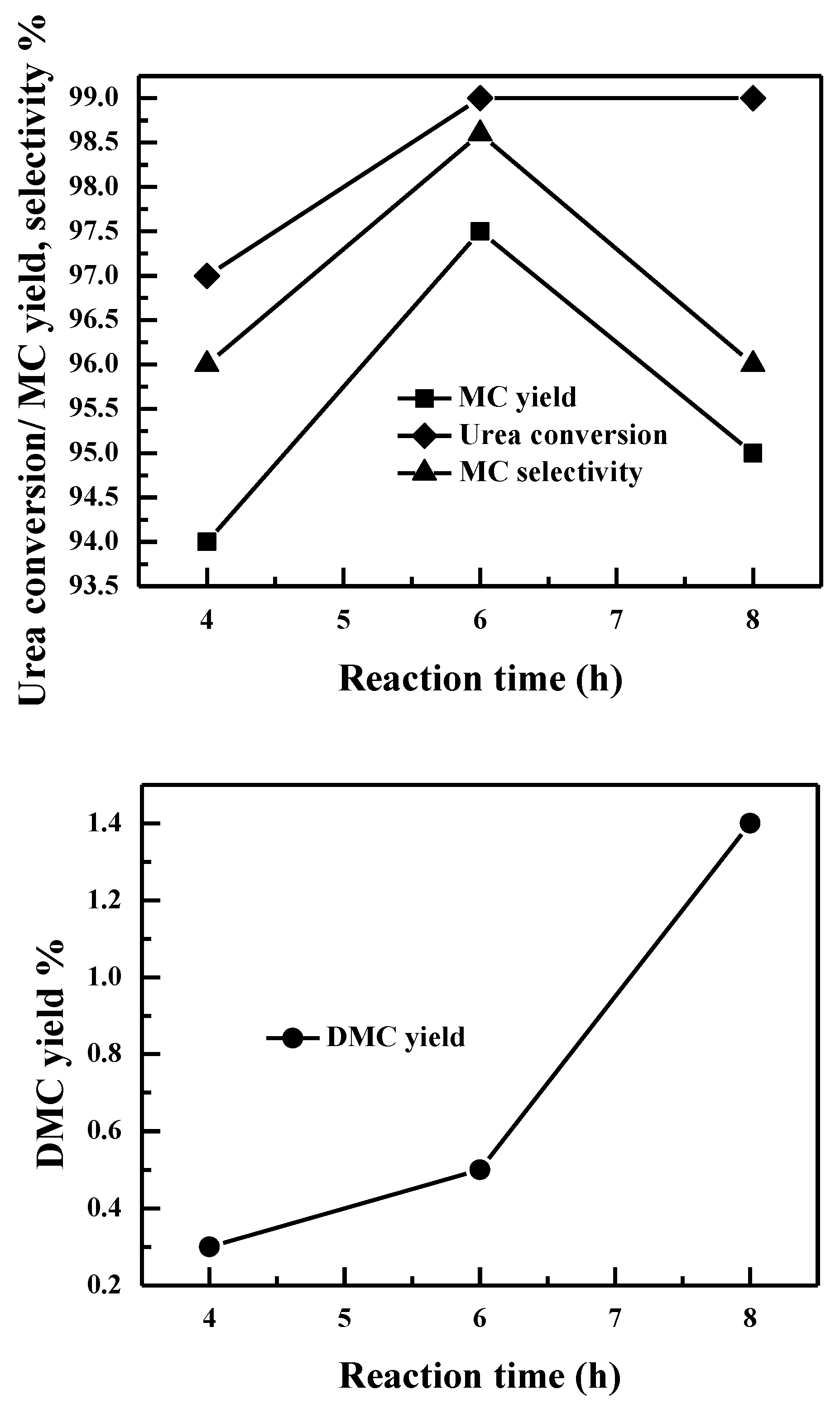
2.2.7. Effect of Reaction Time
2.2.8. Recyclability Test
2.2.9. Scaling Up in the 2 L Autoclave
3. Experimental
3.1. Materials
3.2. Preparation of Catalysts
3.3. Characterization of Catalysts
3.3.1. Elemental Analysis (EA)
3.3.2. BET Analysis
3.3.3. X-ray Photoelectron Spectroscopy (XPS) Measurement
3.3.4. X-ray Diffraction XRD Analysis
3.3.5. Temperature-Programmed Desorption (TPD) Study
3.3.6. EPMA Analysis
3.4. Reaction Conditions for Carbamates Synthesis
3.4.1. General Precedure for Synthesis of Alkyl Carbamate in a 90 mL Autoclave
3.4.2. A Demonstration for the Scaling up of the Synthesis of Carbamates
3.5. Recyclability of the Catalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adams, P.; Baron, F.A. Esters of Carbamic Acid. Chem. Rev. 1965, 65, 567–602. [Google Scholar] [CrossRef]
- Wu, T.T.; Huang, J.; Arrington, N.D.; Dill, G.M. Synthesis and herbicidal activity of .alpha.-heterocyclic carbinol carbamates. J. Agric. Food Chem. 1987, 35, 817–823. [Google Scholar] [CrossRef]
- Guo, X.; Shang, J.; Ma, X.; Li, J.; Zhang, H.; Cui, X.; Shi, F.; Deng, Y. Synthesis of dialkyl hexamethylene-1,6-dicarbamate from 1,6-hexamethylenediamine and alkyl carbamate over FeCl3 as catalyst. Catal. Commun. 2009, 10, 1248–1251. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Y.; You, F.; Peng, T.; He, Z.; Li, K. Exploring to direct the reaction pathway for hydrogenation of levulinic acid into γ-valerolactone for future Clean-Energy Vehicles over a magnetic Cu-Ni catalyst. Int. J. Hydrog. Energy 2017, 42, 25185–25194. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Z. Lag quasi-synchronization for memristive neural networks with switching jumps mismatch. Neural Comput. Appl. 2017, 28, 4011–4022. [Google Scholar] [CrossRef]
- Lin, S.J.; Hsu, M.F. Incorporated risk metrics and hybrid AI techniques for risk management. Neural Comput. Appl. 2017, 28, 3477–3489. [Google Scholar] [CrossRef]
- Asteris, P.G.; Plevris, V. Anisotropic masonry failure criterion using artificial neural networks. Neural Comput. Appl. 2017, 28, 2207–2229. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, H.; Ma, Y.; Zhang, R. High selective production of 5-hydroxymethylfurfural from fructose by sulfonic acid functionalized SBA-15 catalyst. Compos. Part B Eng. 2019, 156, 88–94. [Google Scholar] [CrossRef]
- Singh, P. High-order fuzzy-neuro-entropy integration-based expert system for time series forecasting. Neural Comput. Appl. 2017, 28, 3851–3868. [Google Scholar] [CrossRef]
- Ghasemi, E. Particle swarm optimization approach for forecasting backbreak induced by bench blasting. Neural Comput. Appl. 2017, 28, 1855–1862. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, N.; Cao, C.; Hardeberg, J.Y. Design of four-band multispectral imaging system with one single-sensor. Future Gener. Comput. Syst. 2018, 86, 670–679. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, J.; He, Z.; Hu, Z.; Hui, D. Energy absorption of bio-inspired multi-cell CFRP and aluminum square tubes. Compos. Part B Eng. 2017, 121, 134–144. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, X.; Ma, J.; Wang, J.; Shi, Y.; Hui, D. Lateral crushing and bending responses of CFRP square tube filled with aluminum honeycomb. Compos. Part B Eng. 2017, 118, 104–115. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Fu, J.; Li, Q.; Hui, D. Dynamic crash responses of bio-inspired aluminum honeycomb sandwich structures with CFRP panels. Compos. Part B Eng. 2017, 121, 122–133. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Zhu, X.L.; Zhang, R.H. Energy Recovery Strategy Numerical Simulation for Dual Axle Drive Pure Electric Vehicle Based on Motor Loss Model and Big Data Calculation. Complexity 2018, 2018, 4071743. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Wang, L.; Song, Z.; Li, H.; Wang, T.; Li, H.; Eli, W. Catalytic Conversion of Glucose into 5-Hydroxymethylfurfural by Hf(OTf)4 Lewis Acid in Water. Catalysts 2016, 6, 1. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, J.; Gao, Z.; Zhang, L.; Li, C.; Wang, T. Dicyclopentadiene Hydroformylation to Value-Added Fine Chemicals over Magnetically Separable Fe3O4-Supported Co-Rh Bimetallic Catalysts: Effects of Cobalt Loading. Catalysts 2017, 7, 103. [Google Scholar] [CrossRef]
- Lin, C.; Wu, C.; Guo, P.; Chang, S. Innovative encapsulated oxygen-releasing beads for bioremediation of BTEX at high concentration in groundwater. J. Environ. Manag. 2017, 204, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, U.P.; Iwaasa, A.D.; Theodori, G.L.; Ansley, R.J.; Moya, E.G. State of knowledge about energy development impacts on North American rangelands: An integrative approach. J. Environ. Manag. 2016, 180, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Wang, J.W.; Dong, W.S.; Kang, M.Q.; Wang, X.K.; Peng, S.Y. A phosgene-free process for the synthesis of methyl N-phenyl carbamate by the reaction of aniline with methyl carbamate. J. Mol. Catal. A Chem. 2004, 212, 99–105. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Liu, S.; Fei, Y.; Deng, Y. Catalytic degradation of polyurea: Synthesis of N-substituted carbamates with CuO–ZnO as the catalyst. Green Chem. 2016, 18, 6091–6098. [Google Scholar] [CrossRef]
- Shang, J.; Liu, S.; Ma, X.; Lu, L.; Deng, Y. A new route of CO2 catalytic activation: Syntheses of N-substituted carbamates from dialkyl carbonates and polyureas. Green Chem. 2012, 14, 2899–2906. [Google Scholar] [CrossRef]
- Beinfest, S.; Halpern, J. Preparation of Lower Alkyl Carbamates. U.S. Patent 3,013,064, 12 December 1961. [Google Scholar]
- Slate, J.D.; Culbreth, J. Carbamic Ester Process and Fertilizer Values Therein. U.S. Patent 3,554,730, 12 January 1971. [Google Scholar]
- Sun, J.J.; Yang, B.L.; Lin, H.Y. A semicontinuous process for the synthesis of methyl carbamate from urea and methanol. Chem. Eng. Technol. 2004, 27, 435–439. [Google Scholar] [CrossRef]
- Zhang, R.H.; He, Z.C.; Wang, H.W.; You, F.; Li, K.N. Study on self-tuning tyre friction control for developing main-servo loop integrated chassis control system. IEEE Access 2017, 5, 6649–6660. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, J. Process for Preparing Aminoformate. C.N. Patent 1,365,969, 28 August 2002. [Google Scholar]
- Sun, Y.H.; Wei, W.; Zhao, N.; Wang, M.H. Method of Synthesizing Methyl Carbamate. C.N. Patent 1,475,481, 18 February 2004. [Google Scholar]


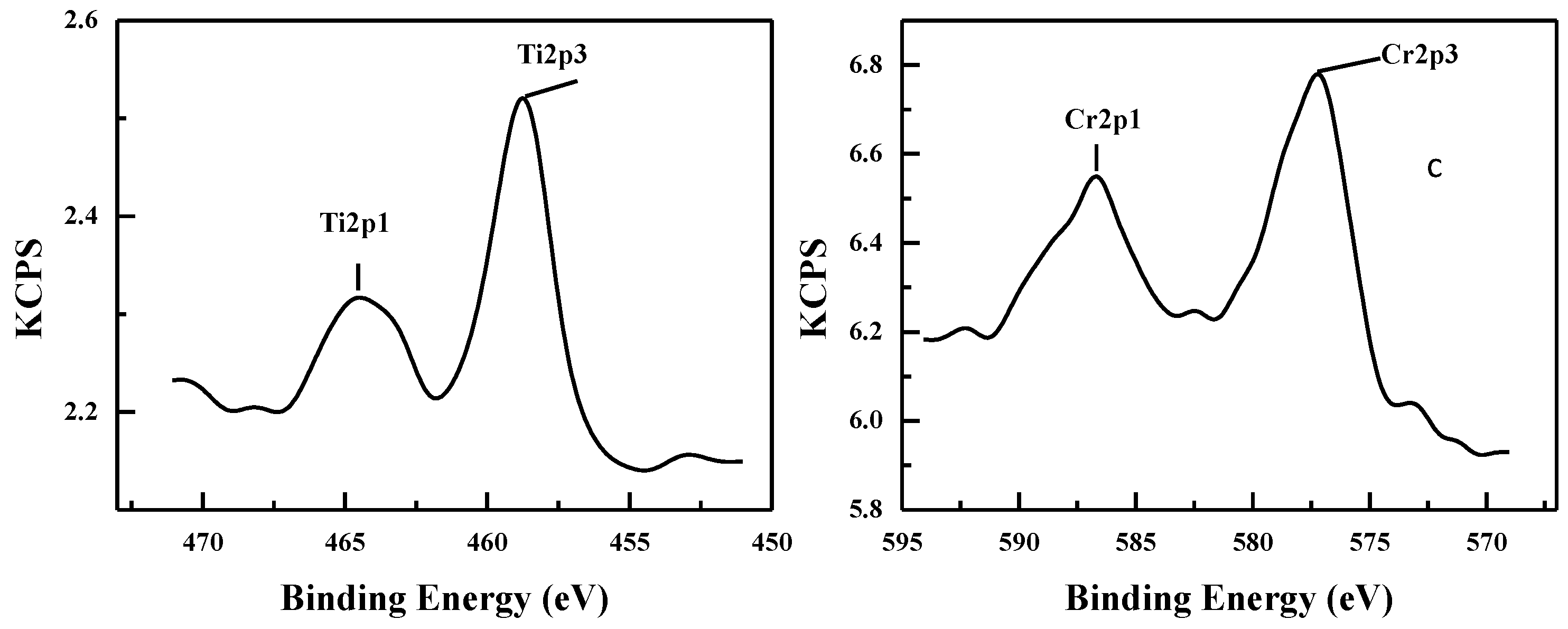
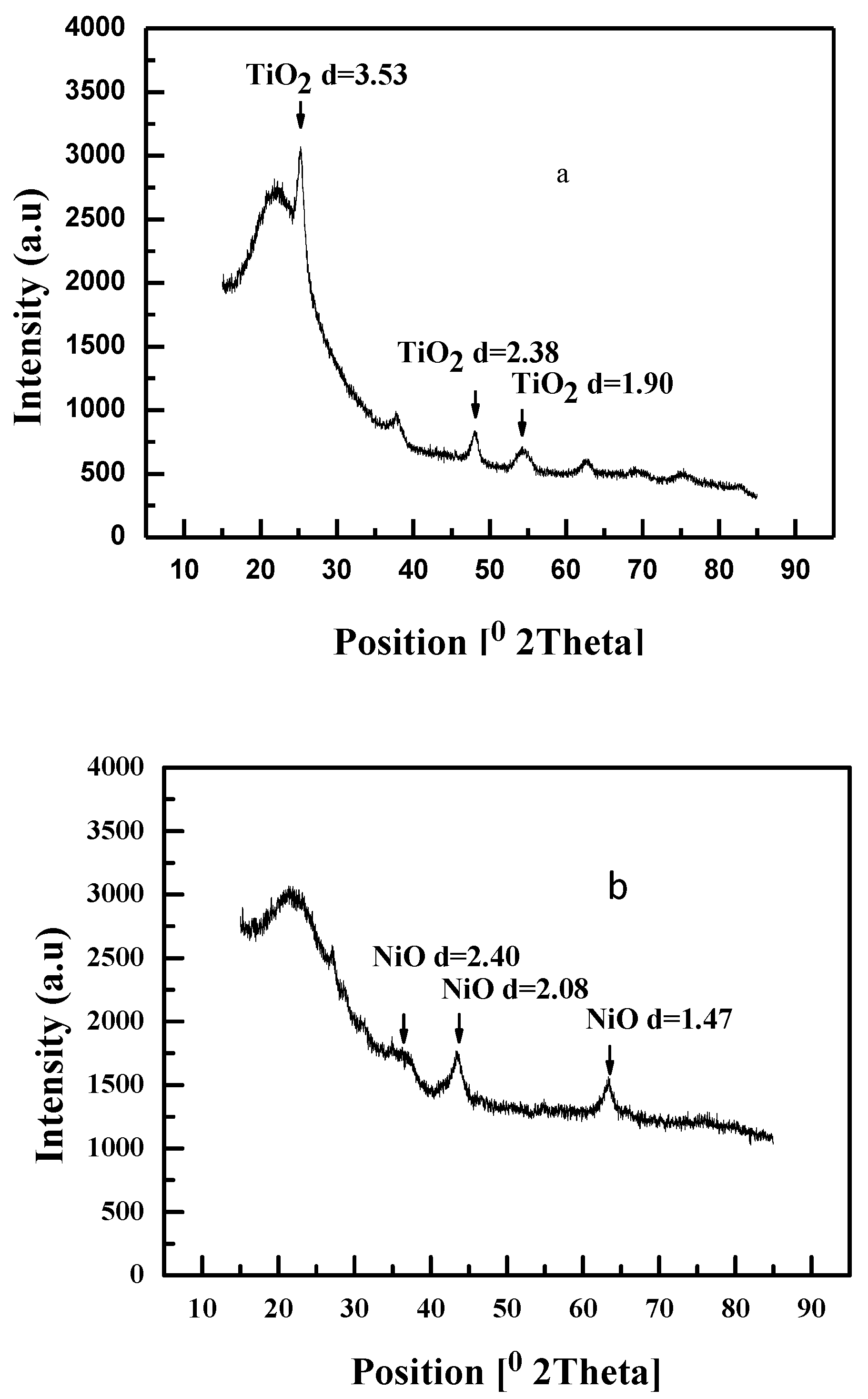

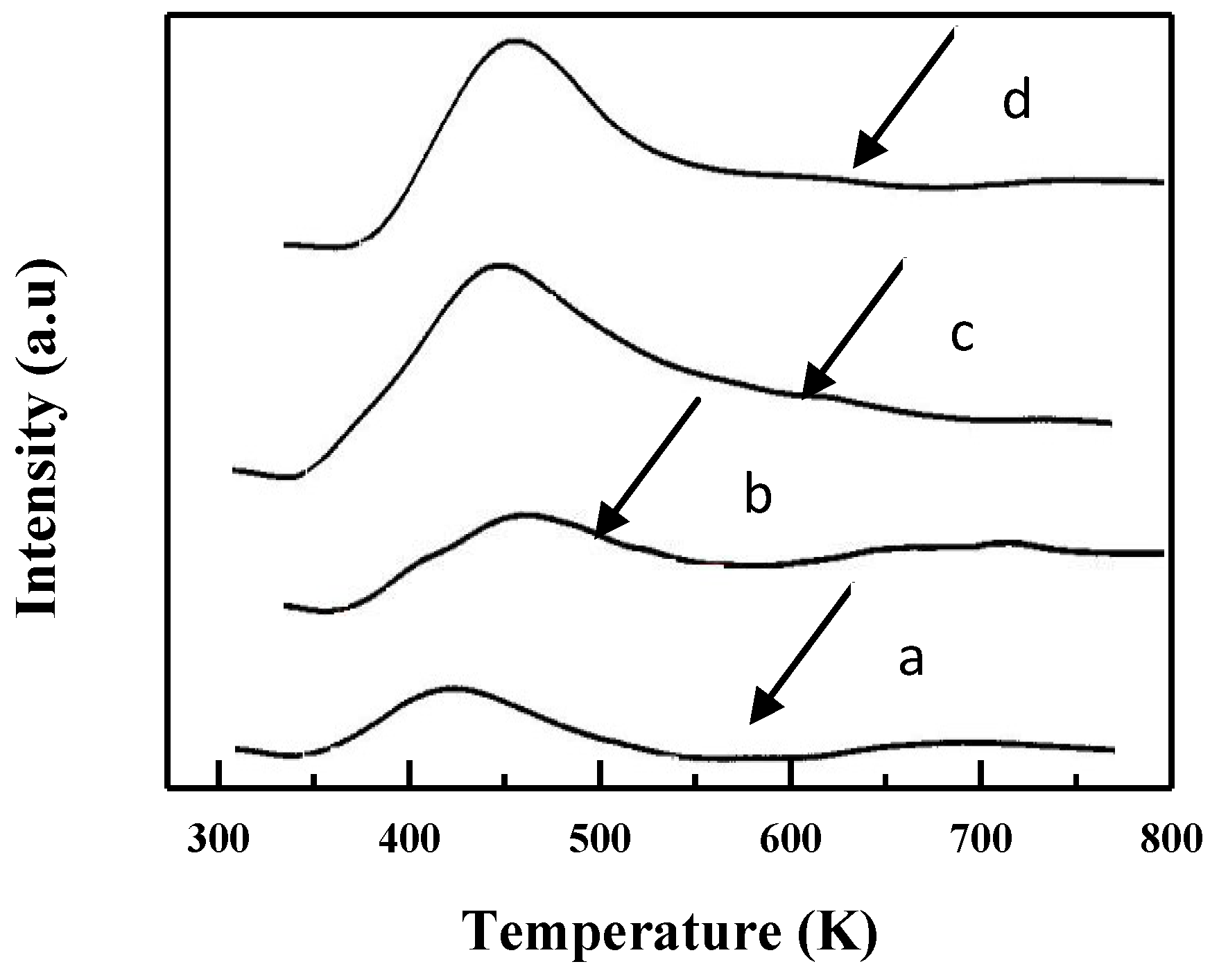


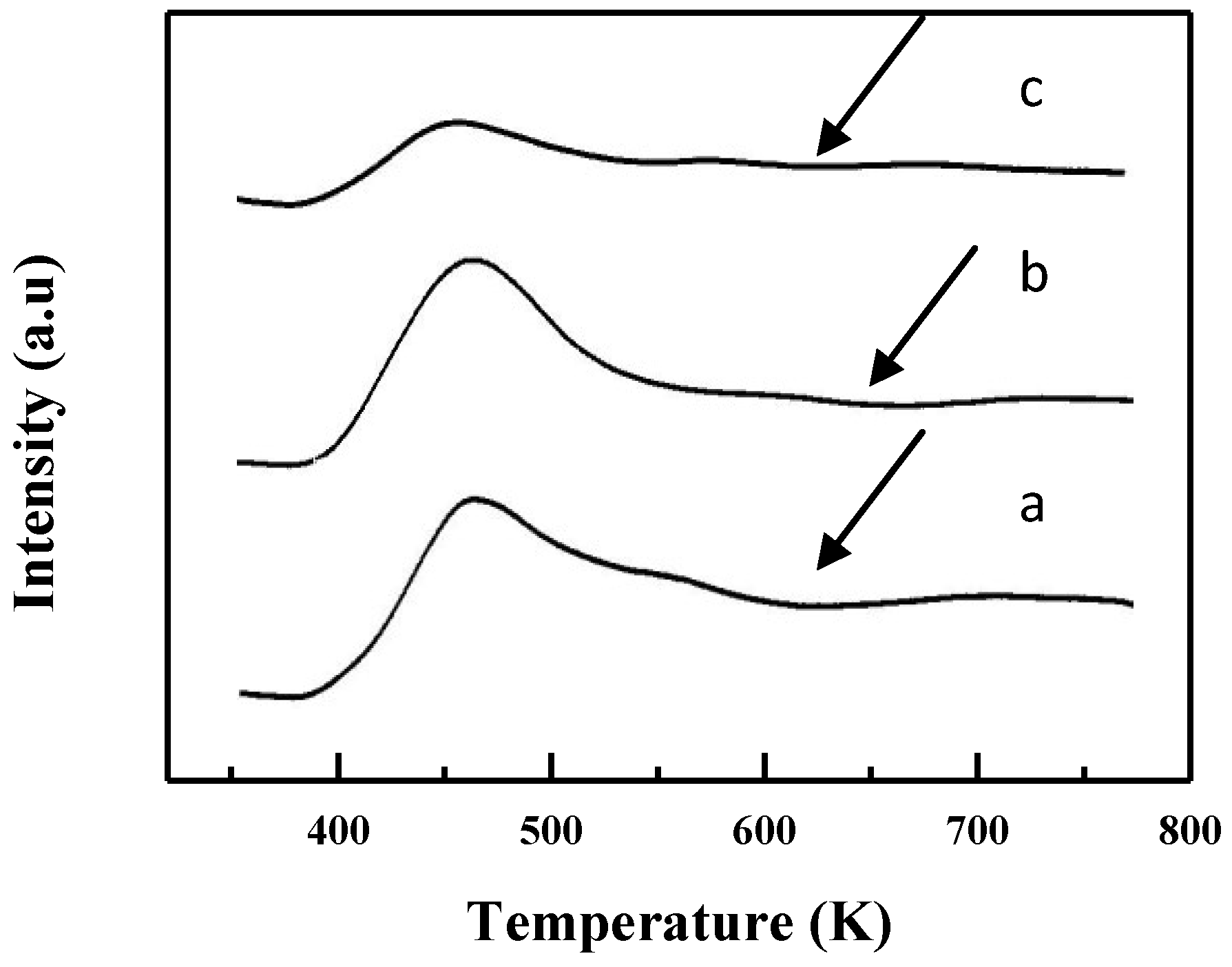



| Catalyst | M/Si atom% Bulk | Surface Area/m2/g | Pore volume /cm3/g | B. E. /eV | M/Si atm% Surface |
|---|---|---|---|---|---|
| SiO2 | 601 | 0.74 | |||
| 2.2 wt% TiO2/SiO2-500 | |||||
| 2.9 wt% TiO2/SiO2-500 | Ti/Si 3.8 | 554 | 0.69 | Ti 2p3/2 459.1 | Ti/Si 3.0 |
| 3.6 wt% TiO2/SiO2-500 | |||||
| 3.5 wt% Cr2O3-8.0 wt% NiO/SiO2-500 | |||||
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | Cr/Si 10 Ni/Si 7.9 | 370 | 0.47 | Cr 2p3/2 577.6 Ni 2p3/2 856.6 | Cr /Si 12.6 Ni/Si 7.8 |
| 9.0 wt% Cr2O3-3.6 wt% NiO/SiO2-500 | |||||
| 1.7 wt% TiO2-4.9 wt% Cr2O3/SiO2-500 | |||||
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | Ti/Si 2.0 Cr/Si 7.9 | 434 | 0.49 | Ti 2p3/2 458.7 Cr 2p3/2 577.2 | Ti/Si 2.4 Cr/Si 8.6 |
| 1.0 wt% TiO2-8.5 wt% Cr2O3/SiO2-500 |
| Entry | Catalyst | Con./% | Y. Carbamate% | Y. Dialkyl Carbonate/% |
|---|---|---|---|---|
| 1 | 94 | MC 89 | DMC 0.3 | |
| 2 | SiO2 | 94 | MC 90 | DMC 0.4 |
| 3 | 2.2 wt% TiO2/SiO2-500 | >99 | MC 94 | DMC 0.4 |
| 4 | 2.9 wt% TiO2/SiO2-500 | >99 | MC 97.5 | DMC 0.6 |
| 5 | 3.6 wt% TiO2/SiO2-500 | >99 | MC 96 | DMC 0.7 |
| 6 | 85 | EC 80 | DEC 0.2 | |
| 7 | SiO2 | 85 | EC 80 | DEC 0.2 |
| 8 | 3.5 wt% Cr2O3-8.5 wt% NiO/SiO2-500 | >99 | EC 94 | DEC 0.3 |
| 9 | 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | >99 | EC 97 | DEC 0.5 |
| 10 | 9.0 wt% Cr2O3-3.6 wt% NiO/SiO2-500 | >99 | EC 96 | DEC 0.55 |
| 11 | 92 | BC 88 | DBC 0.4 | |
| 12 | SiO2 | 92 | BC 88 | DBC 0.4 |
| 13 | 1.0 wt% TiO2-8.5 wt% Cr2O3/SiO2-500 | >99 | BC 95 | DBC 0.2 |
| 14 | 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | >99 | BC 96 | DBC 0.5 |
| 15 | 1.7 wt% TiO2-4.9 wt% Cr2O3/SiO2-500 | >99 | BC 94 | DBC 0.65 |
| Entry | Catalyst | Time | GC Yield of Alkyl Carbamate |
|---|---|---|---|
| 1 | 2.9 wt% TiO2/SiO2-400 | 6 h | MC 95% |
| 2 | 2.9 wt% TiO2/SiO2-500 | 6 h | MC 97.5% |
| 3 | 2.9 wt% TiO2/SiO2-600 | 6 h | MC 94% |
| 4 | 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-400 | 4 h | EC 95% |
| 5 | 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 4 h | EC 97% |
| 6 | 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-600 | 4 h | EC 96% |
| 7 | 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-400 | 4 h | BC 95% |
| 8 | 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 4 h | BC 96% |
| 9 | 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-600 | 4 h | BC 95% |
| Catalyst | Alcohol/Urea | Urea Con./% | Y. Carbamate/% | Y. Dialkyl Carbonate/% |
|---|---|---|---|---|
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 10.4 | >99 | EC 91 | DEC 0.8 |
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 15.7 | >99 | EC 97 | DEC 0.5 |
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 21 | >99 | EC 93 | DEC 0.3 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 9.7 | >99 | BC 92 | DBC 2.5 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 13 | >99 | BC 96 | DBC 0.5 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 16.2 | >99 | BC 94 | DBC 0.3 |
| Entry | Catalyst/% | Alcohol | Con./% | Y. Carbamate/% | Y. Dialkyl Carbonate/% |
|---|---|---|---|---|---|
| 1 a | 0 | Ethanol | 85 | EC 80 | DEC 0.2 |
| 2 a | 5 | Ethanol | >99 | EC 90 | DEC 0.4 |
| 3 a | 10 | Ethanol | >99 | EC 97 | DEC 0.5 |
| 4 a | 15 | Ethanol | >99 | EC 94 | DEC 0.55 |
| 5 b | 0 | Butanol | 92 | BC 88 | DBC 0.4 |
| 6 b | 5 | Butanol | >99 | BC 92 | DBC 2.5 |
| 7 b | 10 | Butanol | >99 | BC 96 | DBC 0.6 |
| 8 b | 15 | Butanol | >99 | BC 90 | DBC 2.5 |
| Catalyst | T/°C | Con./% | Y. Carbamate/% | Y. Dialkyl Carbonate/% |
|---|---|---|---|---|
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 150 | 72 | EC 70 | DEC 0 |
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 160 | 80 | EC 78 | DEC 0.3 |
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 170 | >99 | EC 97 | DEC 0.5 |
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 180 | >99 | EC 94 | DEC 2 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 150 | 80 | BC 78 | DBC 0 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 160 | 92 | BC 90 | DBC 0.4 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 170 | >99 | BC 96 | DBC 0.6 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 180 | >99 | BC 92 | DBC 2.5 |
| Catalyst | T/h | Con./% | Y. Carbamate/% | Y. Dialkyl Carbonate/% |
|---|---|---|---|---|
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 3 | 96 | EC 93 | DEC 0.2 |
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 4 | >99 | EC 97 | DEC 0.5 |
| 7.1 wt% Cr2O3-6.3 wt% NiO/SiO2-500 | 5 | >99 | EC 94 | DEC 1.3 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 3 | 97 | BC 94 | DBC 0.4 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 4 | >99 | BC 96 | DBC 0.6 |
| 1.4 wt% TiO2-6.1 wt% Cr2O3/SiO2-500 | 5 | >99 | BC 95 | DBC 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, L.; Yang, X.; Zhang, R. Environmental-Friendly Synthesis of Alkyl Carbamates from Urea and Alcohols with Silica Gel Supported Catalysts. Catalysts 2018, 8, 579. https://doi.org/10.3390/catal8120579
Ma Y, Wang L, Yang X, Zhang R. Environmental-Friendly Synthesis of Alkyl Carbamates from Urea and Alcohols with Silica Gel Supported Catalysts. Catalysts. 2018; 8(12):579. https://doi.org/10.3390/catal8120579
Chicago/Turabian StyleMa, Yubo, Lei Wang, Xiaodong Yang, and Ronghui Zhang. 2018. "Environmental-Friendly Synthesis of Alkyl Carbamates from Urea and Alcohols with Silica Gel Supported Catalysts" Catalysts 8, no. 12: 579. https://doi.org/10.3390/catal8120579





