Mn Modified Ni/Bentonite for CO2 Methanation
Abstract
:1. Introduction
2. Results and Discussion
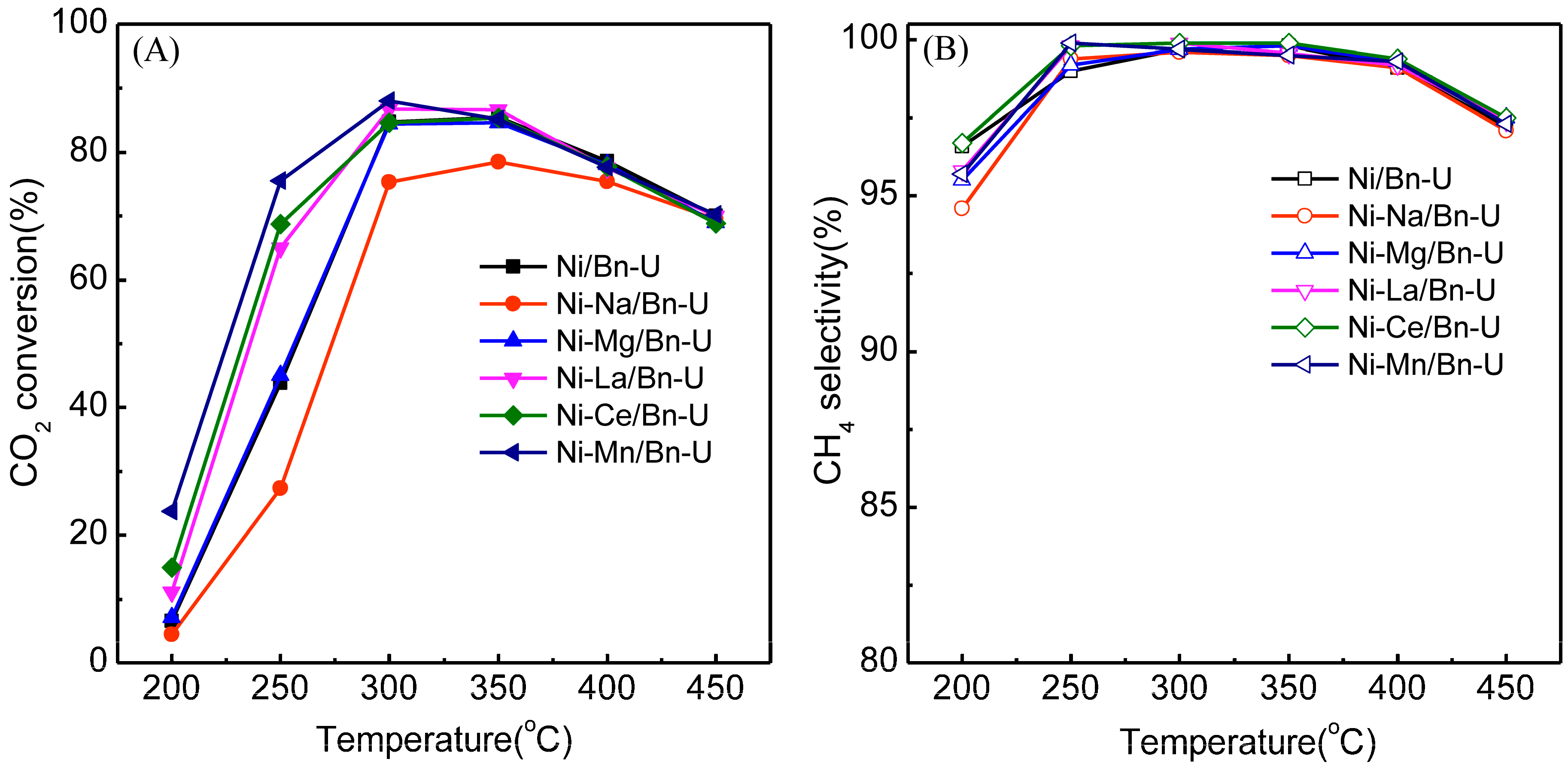
2.1. Effects of Metal Modification on CO2 Methanation on Ni/Bentonite
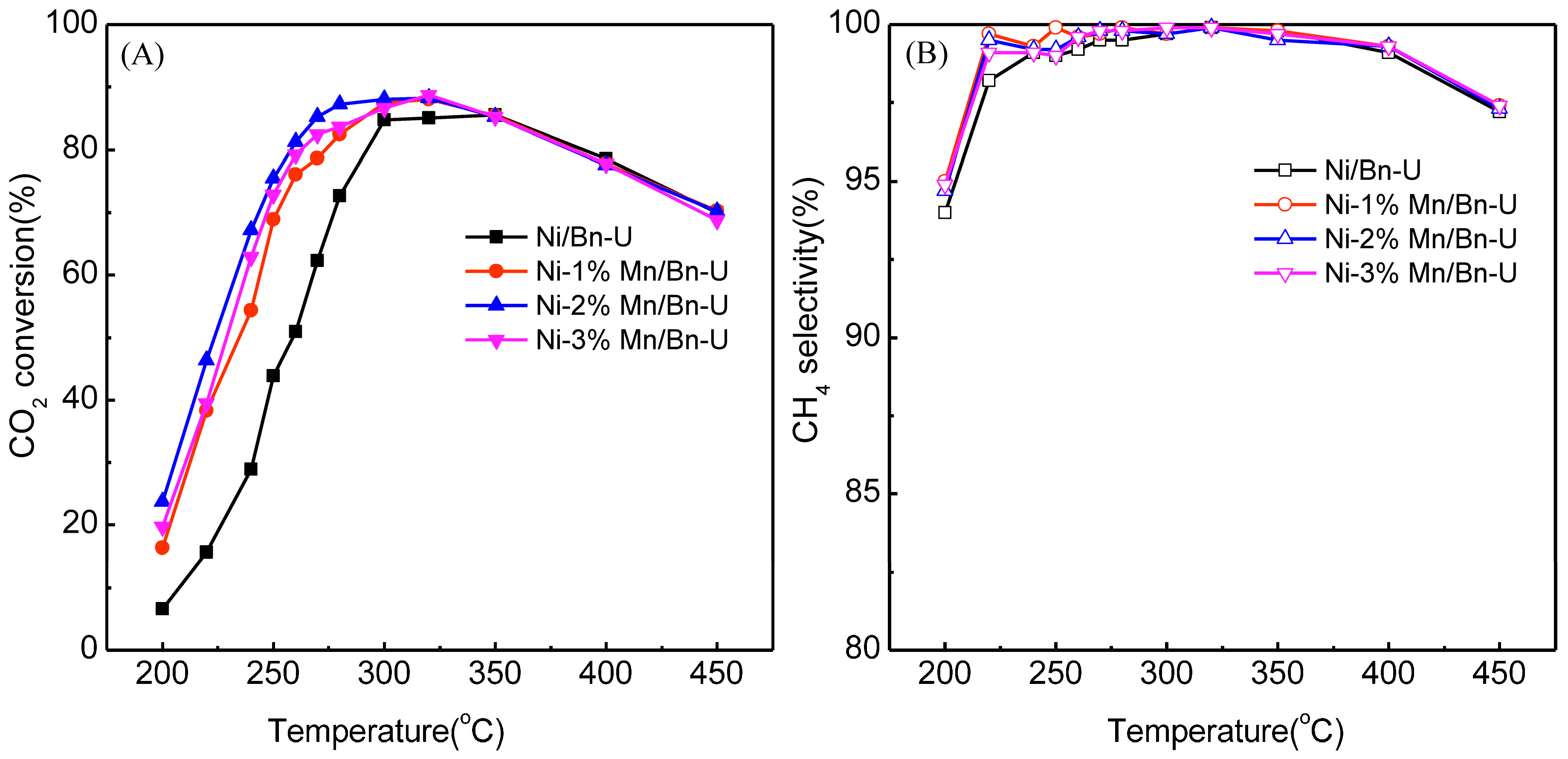
2.2. The Effects of the Mn on the CO2 Methanation on Ni/bentonite Catalysts
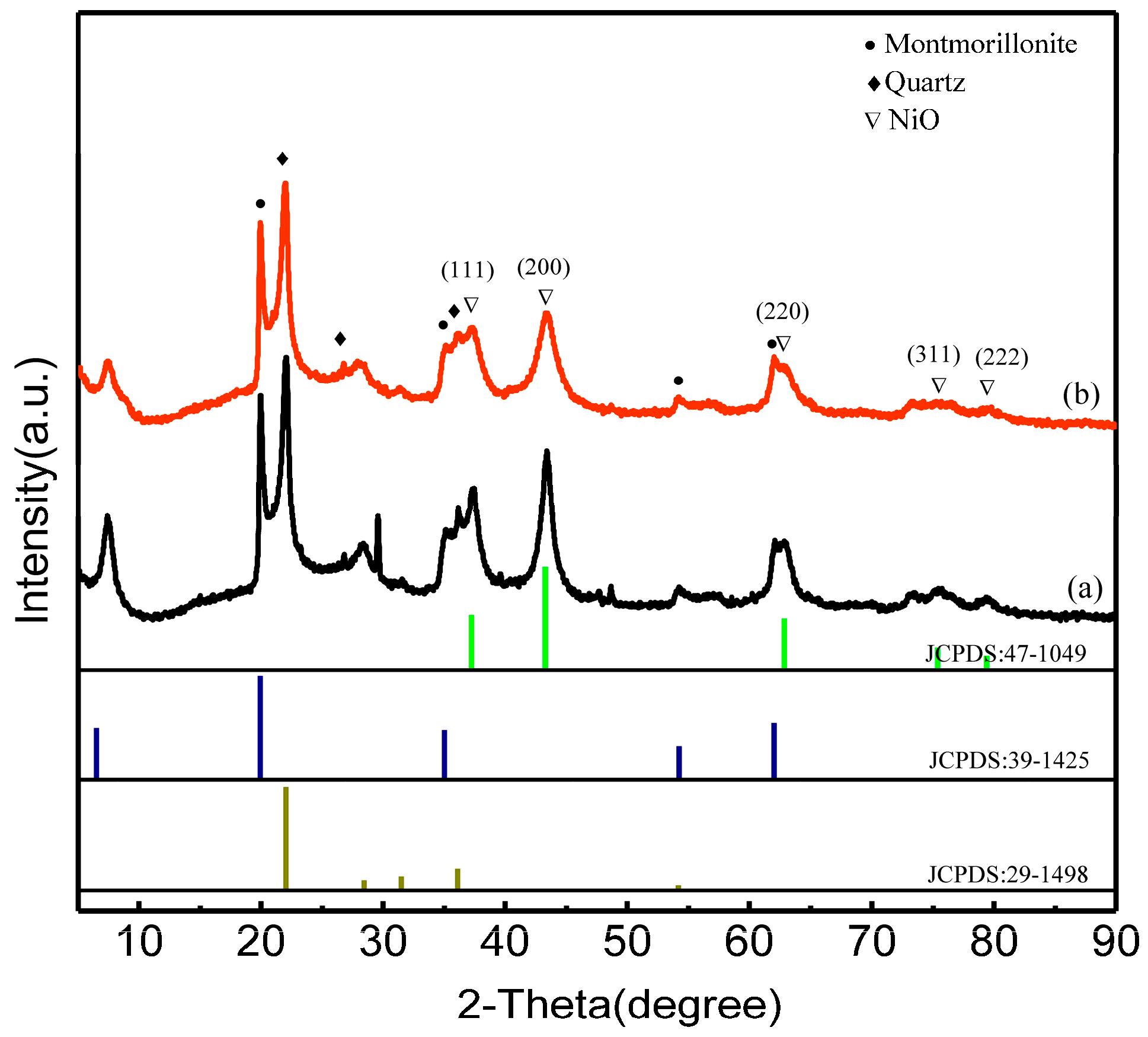
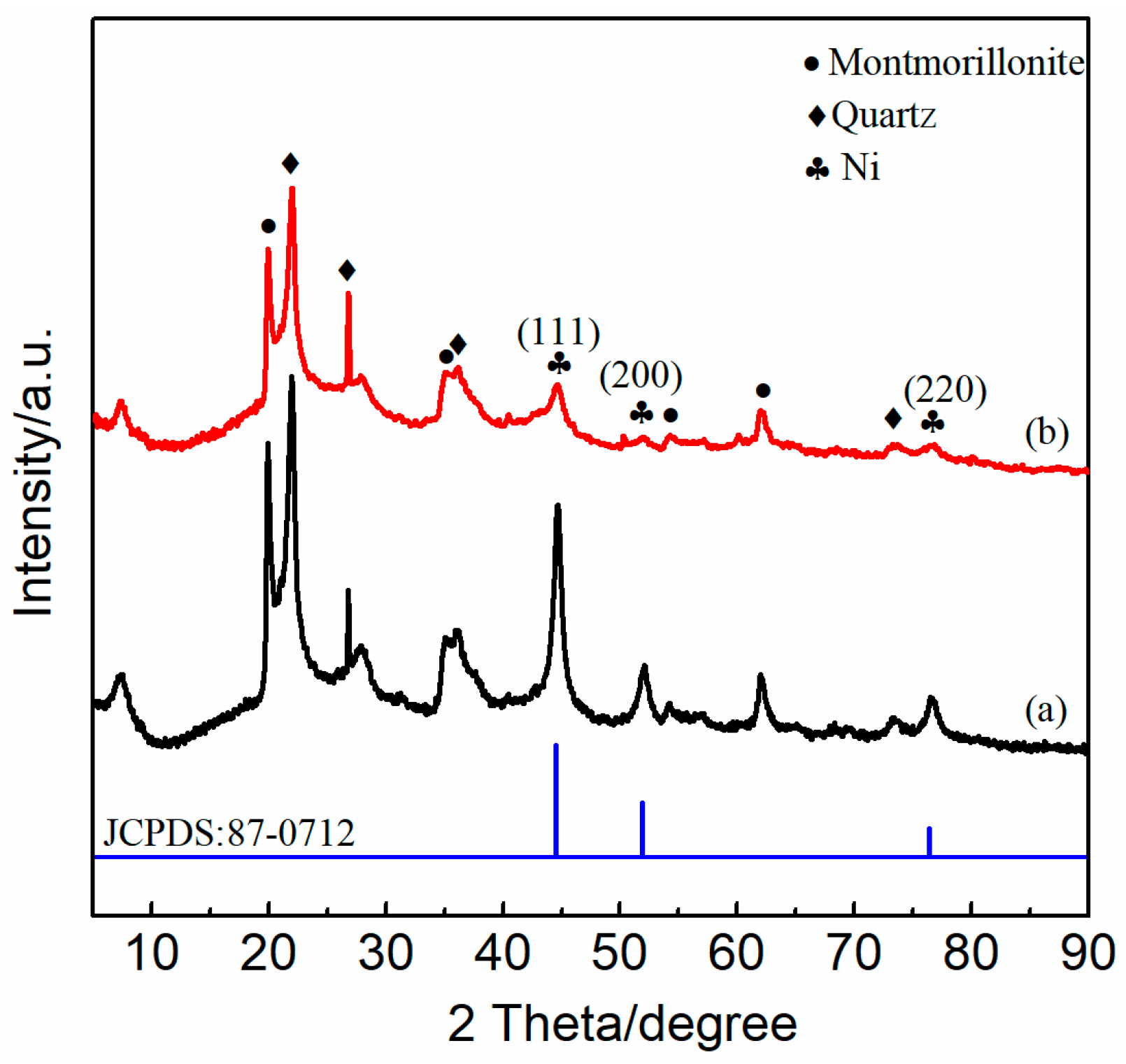
2.3. XRD Analysis
2.4. SEM Analysis
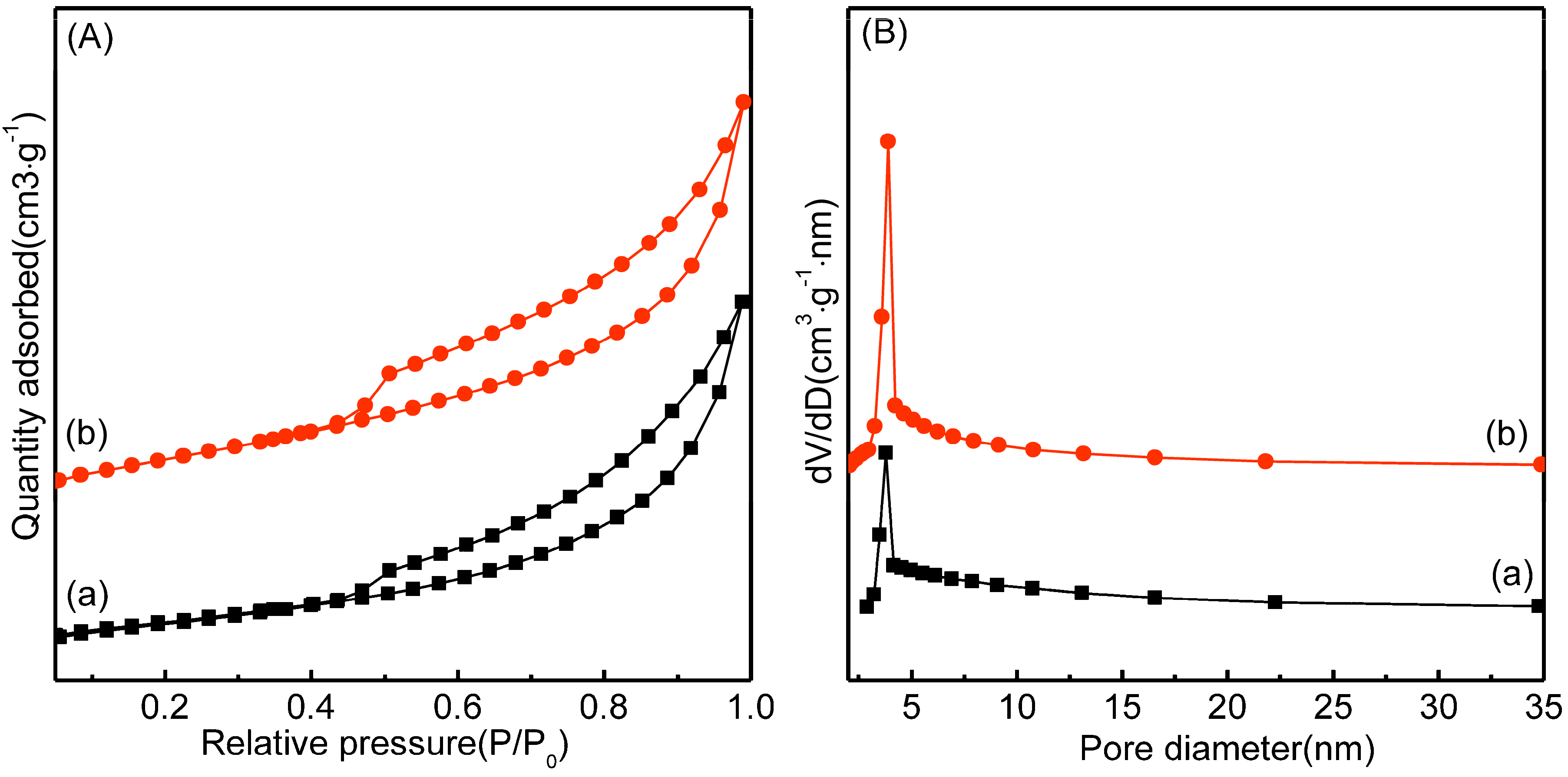
2.5. N2 Adsorption-Desorption Analysis
2.6. XPS Analysis
2.7. H2-TPR Analysis
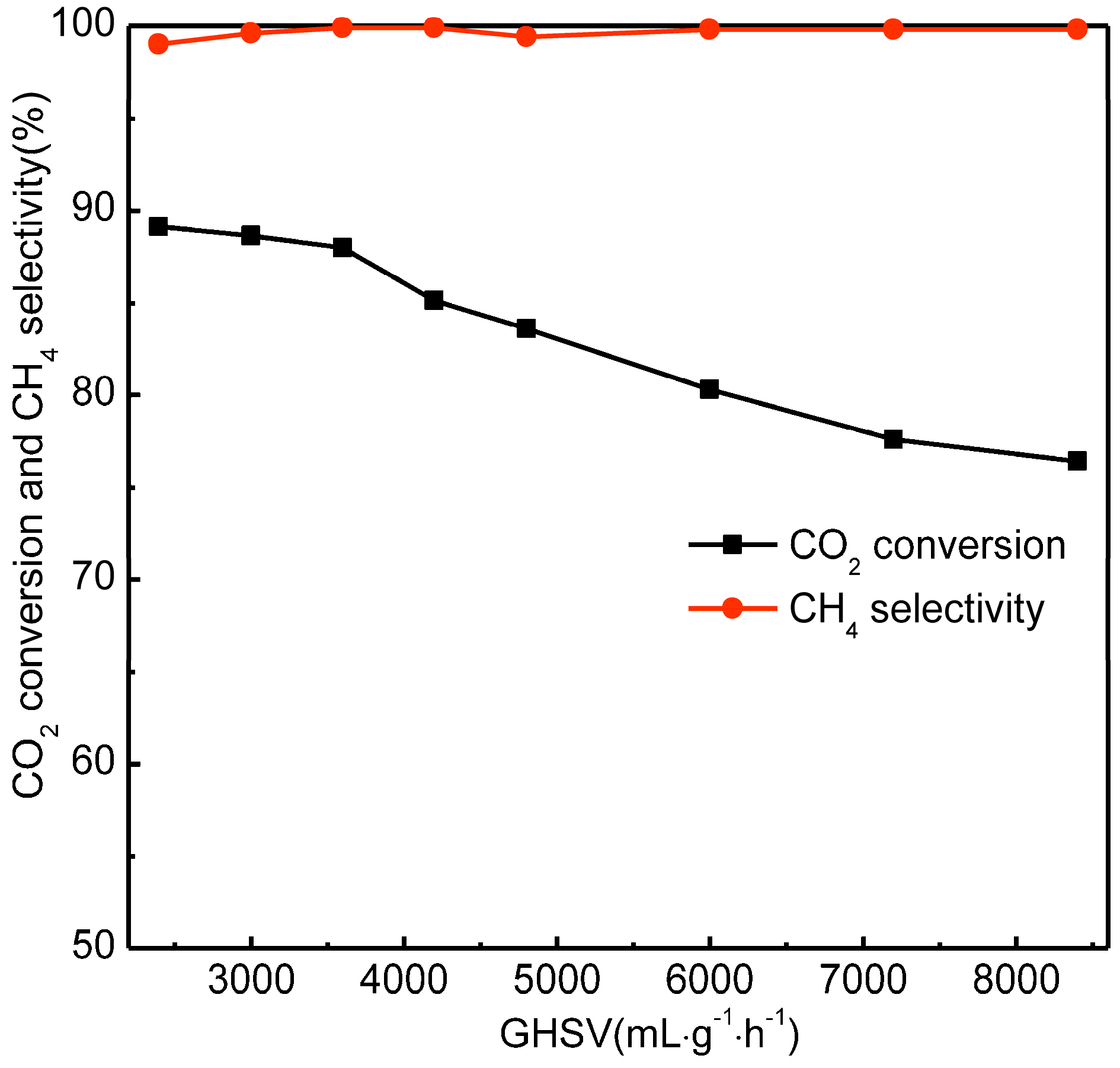
2.8. Effect of Gas Hourly Space Velocity
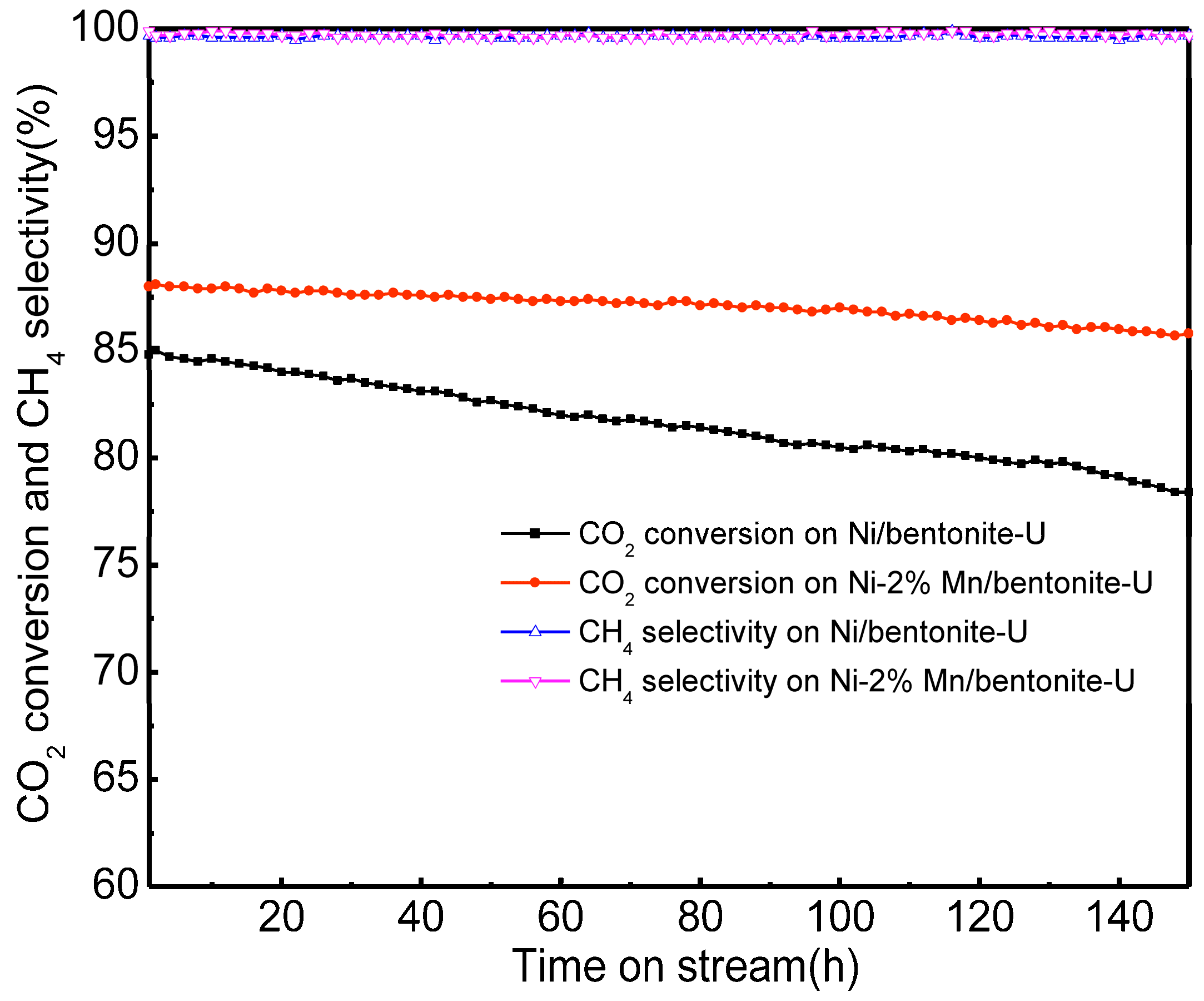
2.9. Stability of Catalysts
2.10. Catalyst Characterization after the Reaction
3. Experiments
3.1. Catalysts Preparation
3.2. Catalytic Hydrogenation of CO2
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, L.; Wang, F.; Chen, M.; Zhang, J.; Yuan, K.; Wang, L.; Wu, K.; Xu, G.; Chen, W. CO2 methanation over a Ni based ordered mesoporous catalyst for the production of synthetic natural gas. RSC Adv. 2016, 6, 28489–28499. [Google Scholar] [CrossRef]
- IEA. Global Energy & CO2 Status Report: The Latest Trends in Energy and Emissions in 2017. Available online: https://www.iea.org/publications/freepublications/publication/GECO2017.pdf (accessed on 3 December 2018).
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Su, T.; Tian, H.; Qin, Z.; Ji, H. Preparation and characterization of Cu modified BiYO3 for carbon dioxide reduction to formic acid. Appl. Catal. B 2017, 202, 364–373. [Google Scholar] [CrossRef]
- Su, T.; Zhou, X.; Qin, Z.; Ji, H. Intrinsic Kinetics for Dimethyl Ether Synthesis from Plasma Activation CO2 Hydrogenation over Cu-Fe-Ce/HZSM-5. ChemPhysChem 2017, 18, 299–309. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Qin, Z.; Xie, Q.; Ji, H. Influence of Zr, Ce, and La on Co3O4 catalyst for CO2 methanation at low temperature. Chin. J. Chem. Eng. 2018, 26, 768–774. [Google Scholar] [CrossRef]
- Wei, W.; Jinlong, G. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 Methanation: The Effect of Catalysts and Reaction Conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. CO2 hydrogenation at low temperature over Rh/γ-Al2O3 catalysts: Effect of the metal particle size on catalytic performances and reaction mechanism. Appl. Catal. B 2012, 113–114, 237–249. [Google Scholar] [CrossRef]
- Garbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrogen Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Yang Lim, J.; McGregor, J.; Sederman, A.J.; Dennis, J.S. Kinetic studies of CO2 methanation over a Ni/γ-Al2O3 catalyst using a batch reactor. Chem. Eng. Sci. 2016, 141, 28–45. [Google Scholar] [CrossRef]
- Hu, L.; Urakawa, A. Continuous CO2 capture and reduction in one process: CO2 methanation over unpromoted and promoted Ni/ZrO2. J. CO2 Util. 2018, 25, 323–329. [Google Scholar] [CrossRef]
- Romero-Sáez, M.; Dongil, A.B.; Benito, N.; Espinoza-González, R.; Escalona, N.; Gracia, F. CO2 methanation over nickel-ZrO2 catalyst supported on carbon nanotubes: A comparison between two impregnation strategies. Appl. Catal. B 2018, 237, 817–825. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Yu, Y.; Chan, Y.M.; Bian, Z.; Song, F.; Wang, J.; Zhong, Q.; Kawi, S. Enhanced performance and selectivity of CO2 methanation over g-C3N4 assisted synthesis of NiCeO2 catalyst: Kinetics and DRIFTS studies. Int. J. Hydrogen Energy 2018, 43, 15191–15204. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Bértolo, R.; Graça, I.; Lopes, J.M.; Henriques, C. The effect of the compensating cation on the catalytic performances of Ni/USY zeolites towards CO2 methanation. J. CO2 Util. 2017, 21, 280–291. [Google Scholar] [CrossRef]
- Azzolina-Jury, F.; Thibault-Starzyk, F. Mechanism of Low Pressure Plasma-Assisted CO2 Hydrogenation Over Ni-USY by Microsecond Time-resolved FTIR Spectroscopy. Top. Catal. 2017, 60, 1709–1721. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Zhao, J.; Chou, L. Methanation of Carbon Dioxide over a Highly Dispersed Ni/La2O3 Catalyst. Chin. J. Catal. 2010, 31, 21–23. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiwi-Minsker, L.; Roger, A.-C. Effect of Ce/Zr composition and noble metal promotion on nickel based CexZr1−xO2 catalysts for carbon dioxide methanation. Appl. Catal. A Gen. 2011, 392, 36–44. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal. Sci. Technol. 2013, 3, 2627–2633. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.; Dong, L.; Qin, Z.; Ji, H. Ni/bentonite catalysts prepared by solution combustion method for low-temperature CO2 methanation. Chin. J. Chem. Eng. 2018, 26, 2361–2367. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni-Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, P.; Qin, Z.; Yu, C.; Li, W.; Qin, Q.; Li, B.; Fan, M.; Liang, X.; Dong, L. Low temperature CO oxidation catalysed by flower-like Ni–Co–O: How physicochemical properties influence catalytic performance. RSC Adv. 2018, 8, 7110–7122. [Google Scholar] [CrossRef]
- Mu, J.; Li, X.; Sun, W.; Fan, S.; Wang, X.; Wang, L.; Qin, M.; Gan, G.; Yin, Z.; Zhang, D. Enhancement of Low-Temperature Catalytic Activity over a Highly Dispersed Fe–Mn/Ti Catalyst for Selective Catalytic Reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 10159–10169. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. La and Mn Promotion of Ni/Al2O3 Catalysts for Syngas Methanation. Energy Source Part A Recover. Util. Environ. Eff. 2014, 36, 1049–1056. [Google Scholar] [CrossRef]
- Song, H.; Hu, F.; Peng, Y.; Li, K.; Bai, S.; Li, J. Non-thermal plasma catalysis for chlorobenzene removal over CoMn/TiO2 and CeMn/TiO2: Synergistic effect of chemical catalysis and dielectric constant. Chem. Eng. J. 2018, 347, 447–454. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Qin, Z.; Ji, H. Preparation of Ni/bentonite catalyst and its applications in the catalytic hydrogenation of nitrobenzene to aniline. Chin. J. Chem. Eng. 2016, 24, 1195–1200. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, T.; Xu, Y.; Li, X.; Qin, Z.; Ji, H. Anti-Coke Properties of Acid-Treated Bentonite-Supported Nickel-Boron Catalyst. Chem. Eng. Technol. 2018, 41, 175–181. [Google Scholar] [CrossRef]
- Huang, C.P.; Richardson, J.T. Alkali promotion of nickel catalysts for carbon monoxide methanation. J. Catal. 1978, 51, 1–8. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Magnesium as Promoter of CO2 Methanation on Ni-Based USY Zeolites. Energy Fuels 2017, 31, 9776–9789. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active Site Dependent Reaction Mechanism over Ru/CeO2 Catalyst toward CO2 Methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef] [PubMed]
- Konishcheva, M.V.; Potemkin, D.I.; Snytnikov, P.V.; Stonkus, O.A.; Belyaev, V.D.; Sobyanin, V.A. The insights into chlorine doping effect on performance of ceria supported nickel catalysts for selective CO methanation. Appl. Catal. B 2018, 221, 413–421. [Google Scholar] [CrossRef]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef] [Green Version]
- Konishcheva, M.V.; Potemkin, D.I.; Badmaev, S.D.; Snytnikov, P.V.; Paukshtis, E.A.; Sobyanin, V.A.; Parmon, V.N. On the Mechanism of CO and CO2 Methanation Over Ni/CeO2 Catalysts. Top. Catal. 2016, 59, 1424–1430. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Monforte, G.; Bonura, G.; Ferraro, M.; Dispenza, G.; Antonucci, V.; Aricò, A.S.; Antonucci, P.L. The role of Gadolinia Doped Ceria support on the promotion of CO2 methanation over Ni and NiFe catalysts. Int. J. Hydrogen Energy 2017, 42, 26828–26842. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; El Amarti, A.; Cifredo, G.; Fitian, L.; Galtayries, A.; Martín, J.; Pintado, J.M. Surface basicity of ceria-supported lanthana. Influence of the calcination temperature. Surf. Interface Anal. 2006, 38, 229–233. [Google Scholar] [CrossRef]
- McFarland, E.W.; Metiu, H. Catalysis by Doped Oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Z.; Gao, J.; Zheng, X. DRIFTS Study on Adsorption and Activation of CH4 and CO2 over Ni/SiO2 Catalyst with Various Ni Particle Sizes. Chin. J. Catal. 2007, 28, 22–26. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, H.; Jin, F.; Zhang, H.; Ying, W. Mn and Mg dual promoters modified Ni/α-Al2O3 catalysts for high temperature syngas methanation. Fuel Process. Technol. 2018, 172, 225–232. [Google Scholar] [CrossRef]
- Miao, G.; Zan, Y.; Sun, Y.; Wang, H.; Li, S.; Liu, C.; Li, S.; Kong, L.; Sun, Y. Mn-promoted hydrogenation of microalgae (Chlorococcum sp.) to 1,2-propanediol and ethylene glycol over Ni-ZnO catalysts. Appl. Catal. A 2018, 565, 34–45. [Google Scholar] [CrossRef]
- Mendoza de la Cruz, J.L.; Castellanos-Ramírez, I.V.; Ortiz-Tapia, A.; Buenrostro-González, E.; Durán-Valencia, C.D.L.A.; López-Ramírez, S. Study of monolayer to multilayer adsorption of asphaltenes on reservoir rock minerals. Colloids Surf. Physicochem. Eng. Asp. 2009, 340, 149–154. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Fan, C.Q.; Yin, R. Electrochemical hydrogenation of coal on Ni-based catalysts. Fuel 2014, 122, 54–59. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Nickel Catalysts Supported on Barium Hexaaluminate for Enhanced CO Methanation. Ind. Eng. Chem. Res. 2012, 51, 10345–10353. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, S.; Guan, Q.; Li, W.; Du, J. A high anticorrosive chromium-free conversion coating prepared with an alkaline conversion bath on electroless Ni–P coating. Appl. Surf. Sci. 2015, 349, 108–115. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Gladky, A.Y.; Prosvirin, I.P.; Saraev, A.A.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Bukhtiyarov, V.I. In situ XPS study of self-sustained oscillations in catalytic oxidation of propane over nickel. Surf. Sci. 2013, 609, 113–118. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Li, J.; Zhang, M.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Ni/Al2O3 catalysts for CO methanation: Effect of Al2O3 supports calcined at different temperatures. J. Energy Chem. 2013, 22, 919–927. [Google Scholar] [CrossRef]
- Qin, Z.; Ren, J.; Miao, M.; Li, Z.; Lin, J.; Xie, K. The catalytic methanation of coke oven gas over Ni-Ce/Al2O3 catalysts prepared by microwave heating: Effect of amorphous NiO formation. Appl. Catal. B 2015, 164, 18–30. [Google Scholar] [CrossRef]
- Kesavan, J.K.; Luisetto, I.; Tuti, S.; Meneghini, C.; Battocchio, C.; Iucci, G. Ni supported on YSZ: XAS and XPS characterization and catalytic activity for CO2 methanation. J. Mater. Sci. 2017, 52, 10331–10340. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Zhang, L. Promotion effect of additive Fe on Al2O3 supported Ni catalyst for CO2 methanation. Appl. Organomet. Chem. 2018, 32, e4328. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z.; Liu, Y.; Zhang, X. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Ding, W.; Chen, Y.; Fu, X. Oxidative coupling of methane over Ce4+-doped Ba3WO6 catalysts: Investigation on oxygen species responsible for catalytic performance. Catal. Lett. 1994, 23, 69–78. [Google Scholar] [CrossRef]
- Baran, R.; Valentin, L.; Dzwigaj, S. Incorporation of Mn into the vacant T-atom sites of a BEA zeolite as isolated, mononuclear Mn: FTIR, XPS, EPR and DR UV-Vis studies. Phys. Chem. Chem. Phys. 2016, 18, 12050–12057. [Google Scholar] [CrossRef] [PubMed]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B 2015, 176–177, 532–541. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Jin, X.; Ge, Q.; Li, W. Characterizations and activities of the nano-sized Ni/Al2O3 and Ni/La–Al2O3 catalysts for NH3 decomposition. Appl. Catal. A 2005, 290, 87–96. [Google Scholar] [CrossRef]
- Lu, X.; Gu, F.; Liu, Q.; Gao, J.; Liu, Y.; Li, H.; Jia, L.; Xu, G.; Zhong, Z.; Su, F. VOx promoted Ni catalysts supported on the modified bentonite for CO and CO2 methanation. Fuel Process. Technol. 2015, 135, 34–46. [Google Scholar] [CrossRef]
- Mile, B.; Stirling, D.; Zammitt, M.A.; Lovell, A.; Webb, M. TPR studies of the effects of preparation conditions on supported nickel catalysts. J. Mol. Catal. 1990, 62, 179–198. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Ge, Q.; Xu, H.; Li, W. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen. Appl. Catal. B 2008, 80, 98–105. [Google Scholar] [CrossRef]
- Tao, X.; Bai, M.; Li, X.; Long, H.; Shang, S.; Yin, Y.; Dai, X. CH4–CO2 reforming by plasma—Challenges and opportunities. Prog. Energy Combust. Sci. 2011, 37, 113–124. [Google Scholar] [CrossRef]
- Sehested, J. Sintering of nickel steam-reforming catalysts. J. Catal. 2003, 217, 417–426. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Hongfang, M.; Fang, D. Ni/Al2O3 catalysts for syngas methanation: Effect of Mn promoter. J. Nat. Gas Chem. 2012, 21, 170–177. [Google Scholar] [CrossRef]




| Catalysts | SBET1 (m2·g−1) | Average Pore Diameter (nm) | Pore Volume (mL·g−1) | Ni Dispersion 2 (%) |
|---|---|---|---|---|
| Bentonite 3 | 75.7 | 10.6 | 0.200 | - |
| Ni/bentonite-U3 | 79.8 | 11.4 | 0.228 | 18.9 |
| Ni-2wt%Mn/bentonite-U | 92.6 | 8.8 | 0.203 | 22.1 |
| XPS Spectra | Element Valence | Binding Energy (eV) (Percent of Valence State, %) | |
|---|---|---|---|
| Ni/Bentonite-U | Ni-2wt%Mn/Bentonite-U | ||
| Ni 2p | Ni2+(Ni 2p1/2) | 879.4(14.43) | 879.6(14.87) |
| Ni2+(Ni 2p1/2) | 873.0(16.94) | 873.2(16.83) | |
| Ni2+(Ni 2p3/2) | 861.4(32.79) | 861.7(31.18) | |
| Ni2+(Ni 2p3/2) | 856.2(22.24) | 856.3(24.54) | |
| Ni2+(Ni 2p3/2) | 854.2(13.61) | 854.5(12.57) | |
| Mn 2p | Mn4+(Mn 2p1/2) | - | 654.0(4.26) |
| Mn4+(Mn 2p3/2) | - | 642.1(95.74) | |
| O 1s | O− | 532.4(74.85) | 532.3(83.55) |
| OH- | 531.4(13.32) | 531.2(7.70) | |
| O2− | 529.9(13.32) | 529.6.0(8.76) | |
| Catalysts | Tmax Ni Species Reduction (°C) | Peak Area 1 (a.u.) | Total Area (a.u.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| θ | α | β | γ | δ | θ | α | β | γ | δ | ||
| Ni/bentonite-U | - | 340 | 393 | 493 | 696 | - | 4381 | 8887 | 1729 | 431 | 15,428 |
| Ni-2wt%Mn/bentonite-U | 224 | 321 | 389 | 504 | 679 | 1140 | 1130 | 13,740 | 3395 | 848 | 20,253 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Huang, T.; Dong, L.; Su, T.; Li, B.; Luo, X.; Xie, X.; Qin, Z.; Xu, C.; Ji, H. Mn Modified Ni/Bentonite for CO2 Methanation. Catalysts 2018, 8, 646. https://doi.org/10.3390/catal8120646
Jiang Y, Huang T, Dong L, Su T, Li B, Luo X, Xie X, Qin Z, Xu C, Ji H. Mn Modified Ni/Bentonite for CO2 Methanation. Catalysts. 2018; 8(12):646. https://doi.org/10.3390/catal8120646
Chicago/Turabian StyleJiang, Yuexiu, Tongxia Huang, Lihui Dong, Tongming Su, Bin Li, Xuan Luo, Xinling Xie, Zuzeng Qin, Cuixia Xu, and Hongbing Ji. 2018. "Mn Modified Ni/Bentonite for CO2 Methanation" Catalysts 8, no. 12: 646. https://doi.org/10.3390/catal8120646
APA StyleJiang, Y., Huang, T., Dong, L., Su, T., Li, B., Luo, X., Xie, X., Qin, Z., Xu, C., & Ji, H. (2018). Mn Modified Ni/Bentonite for CO2 Methanation. Catalysts, 8(12), 646. https://doi.org/10.3390/catal8120646







