The Effects of Coordinated Molecules of Two Gly-Schiff Base Copper Complexes on Their Oxygen Reduction Reaction Performance
Abstract
:1. Introduction
2. Discussion
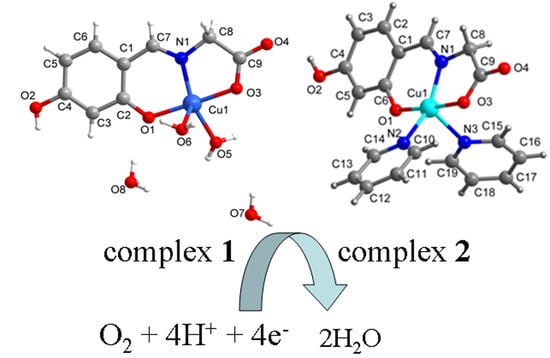
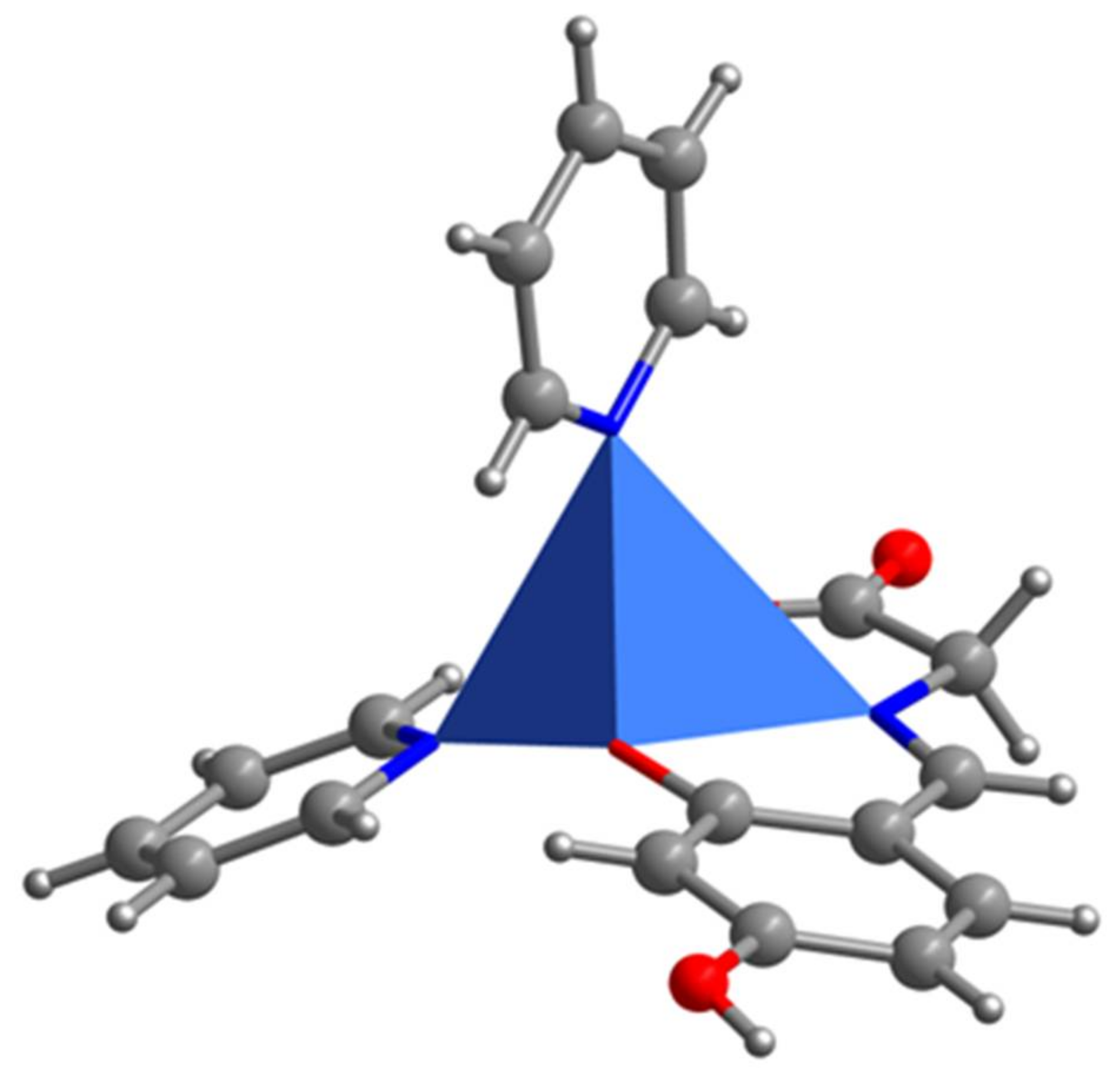
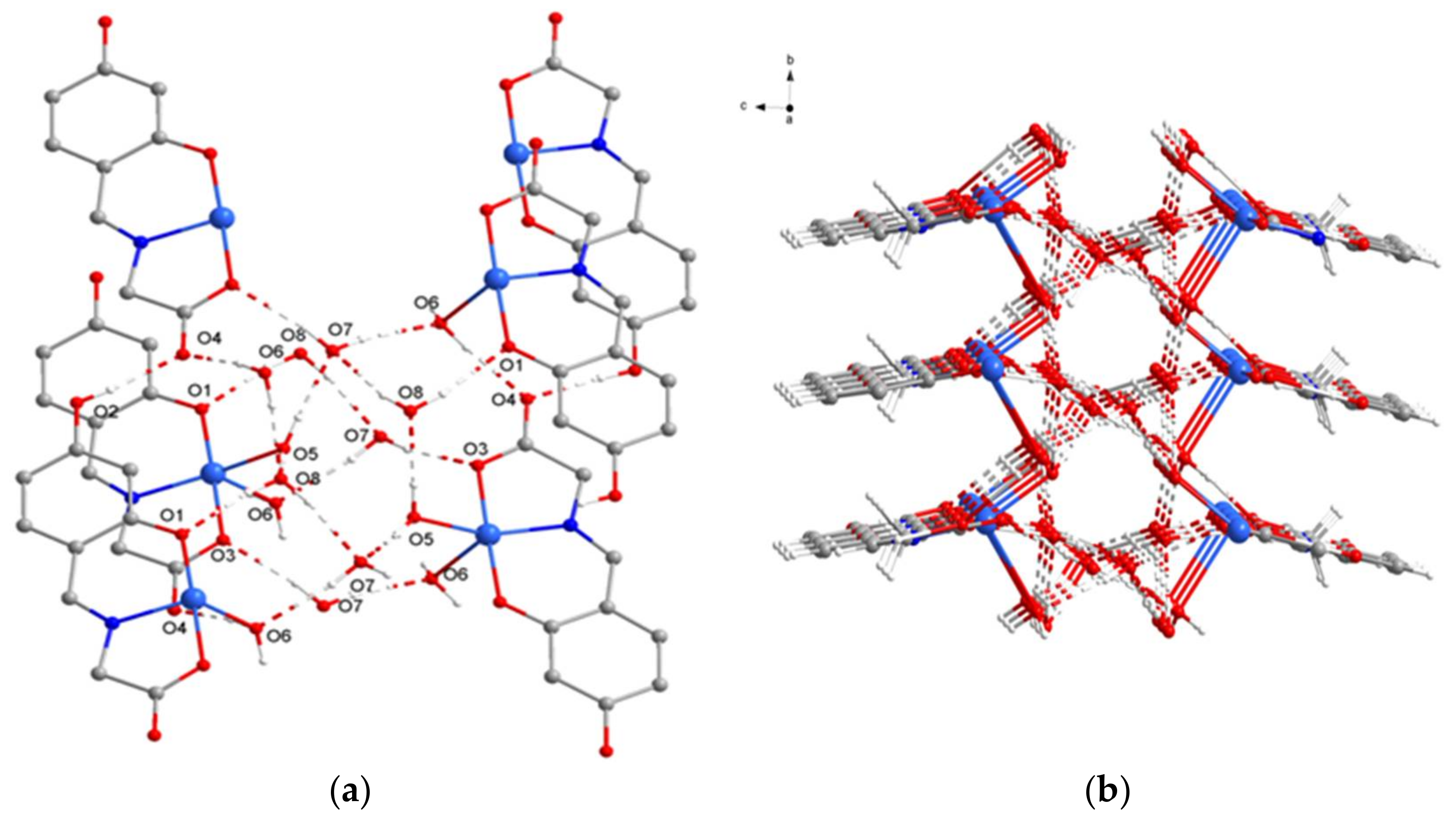
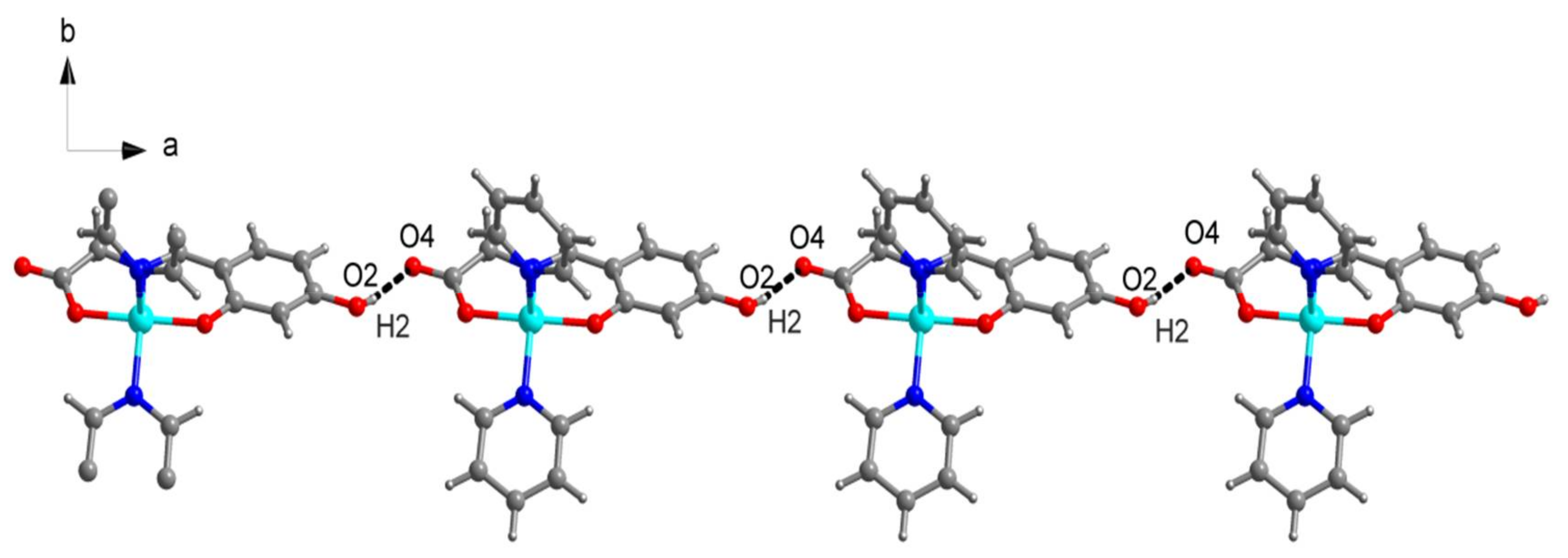
2.1. SXRD Analysis
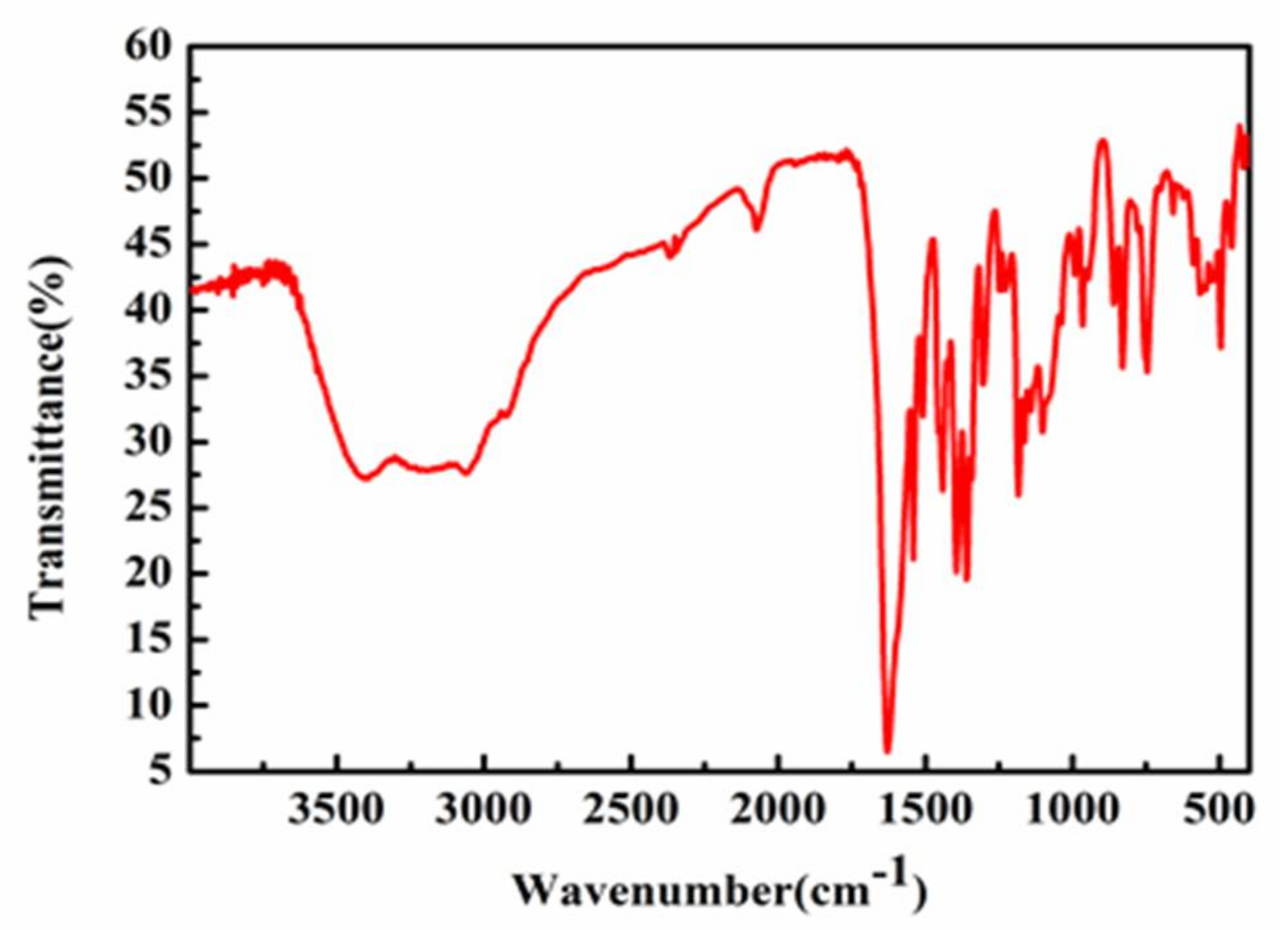
2.2. Infrared Spectroscopy (IR) Analysis
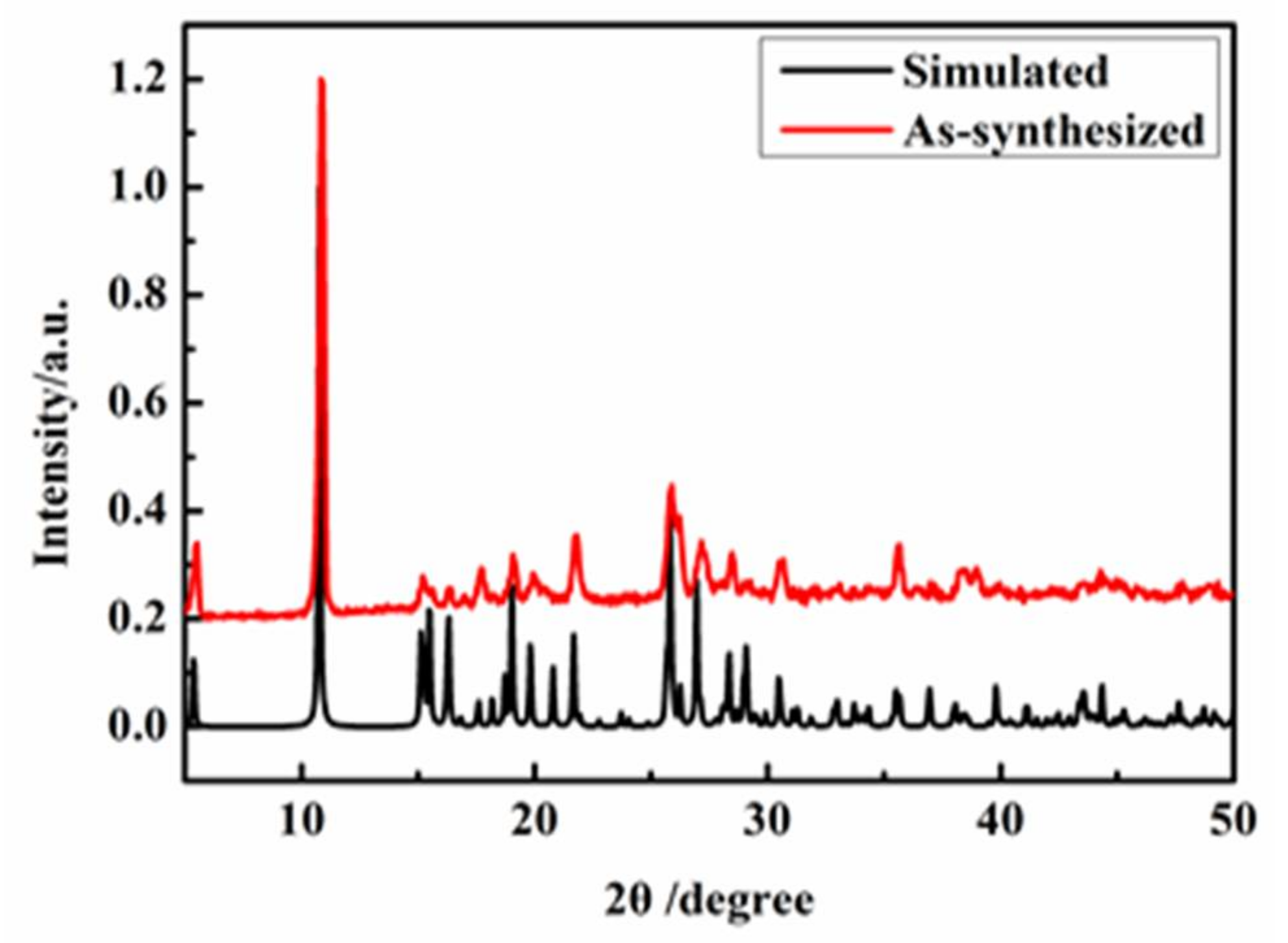
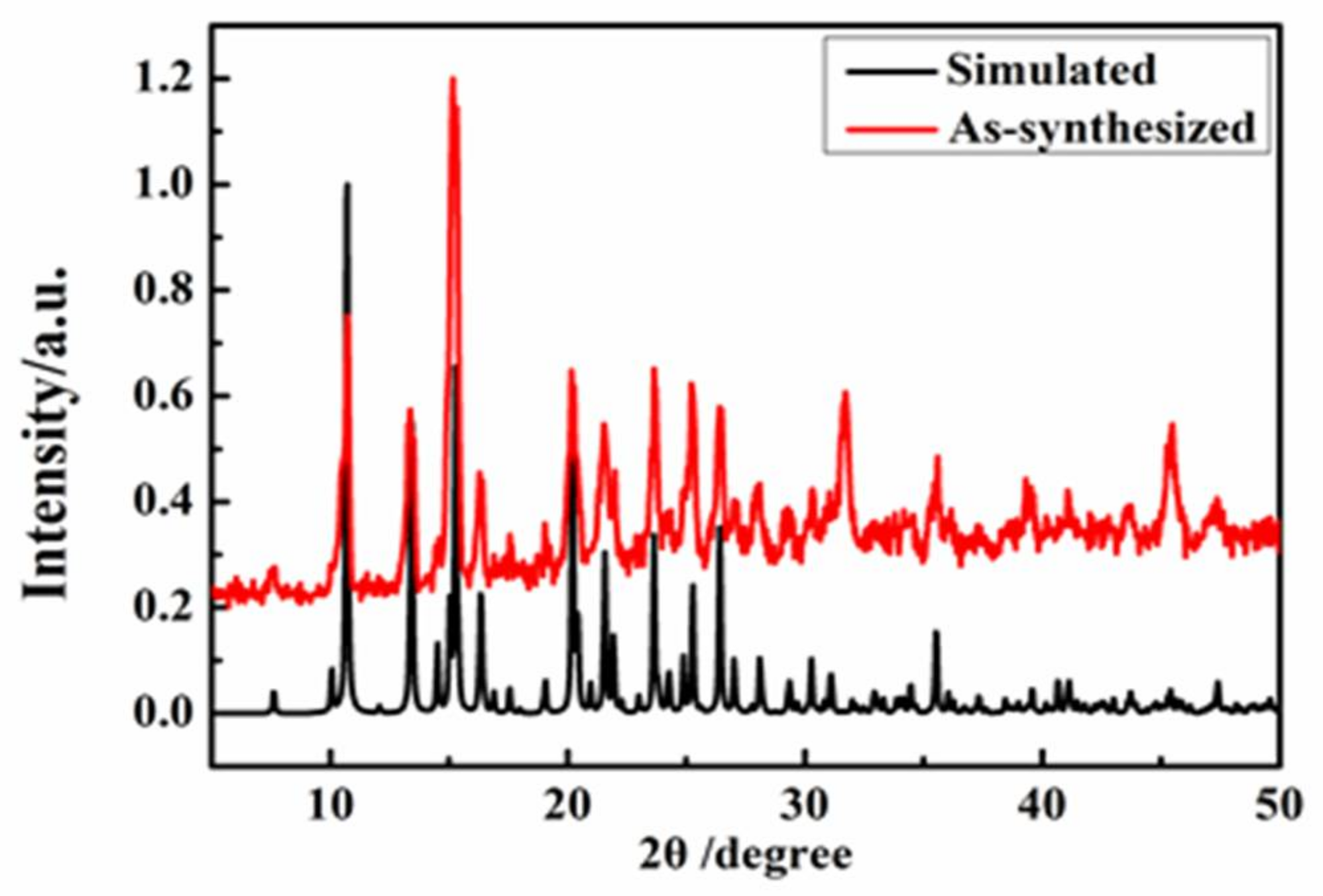
2.3. Polycrystal Powder X-ray Diffraction (PXRD) Analysis
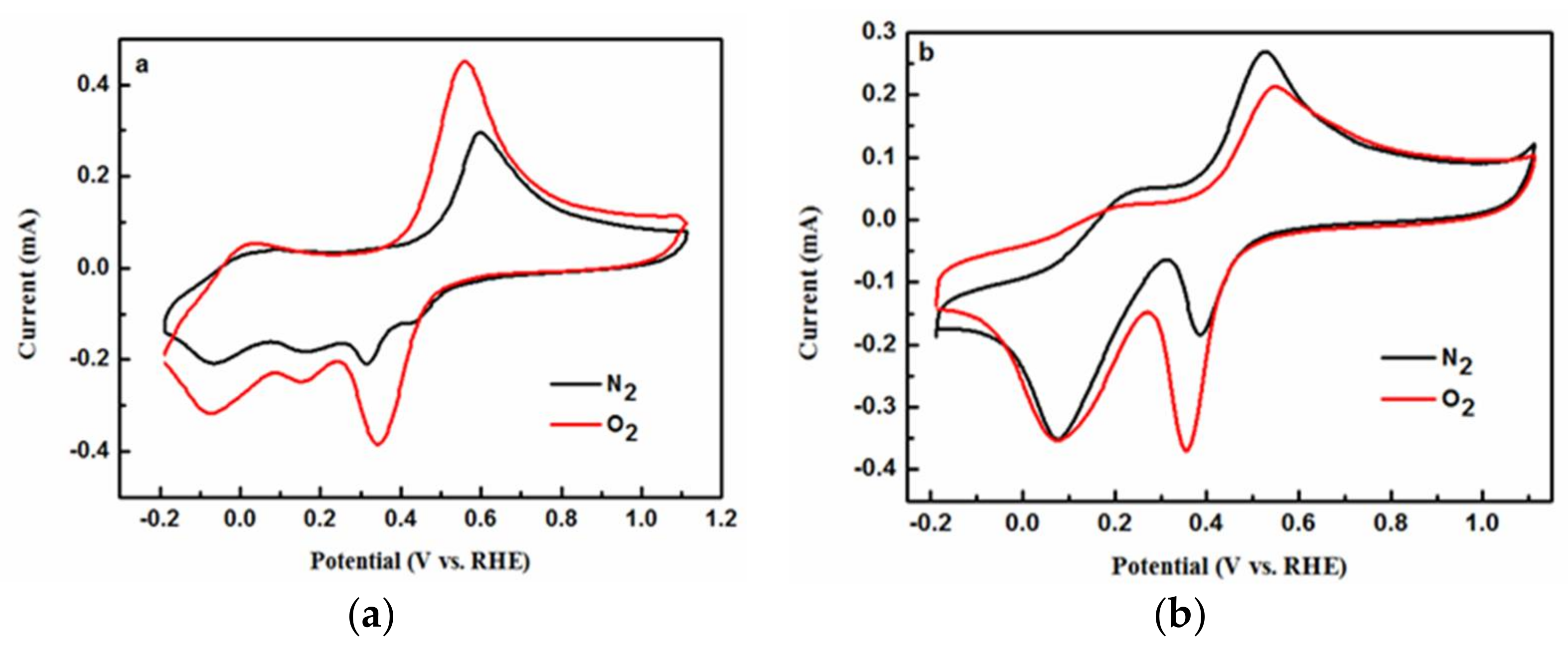
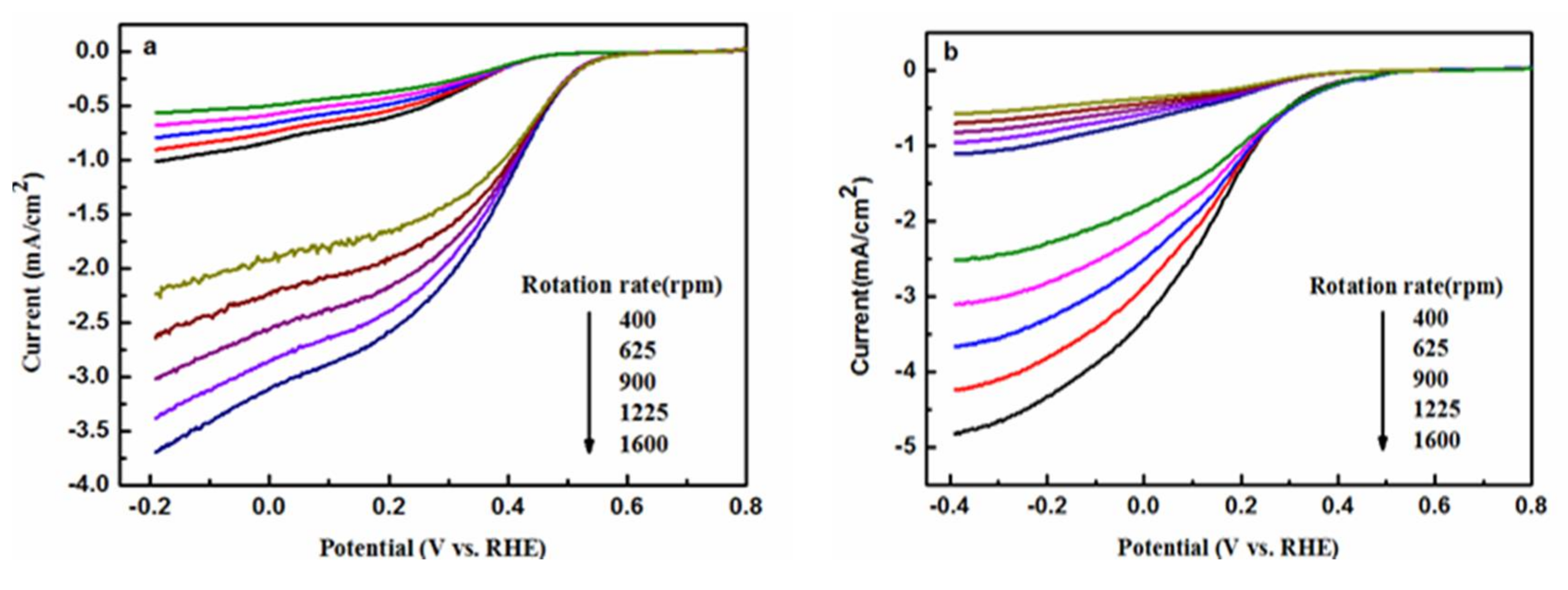
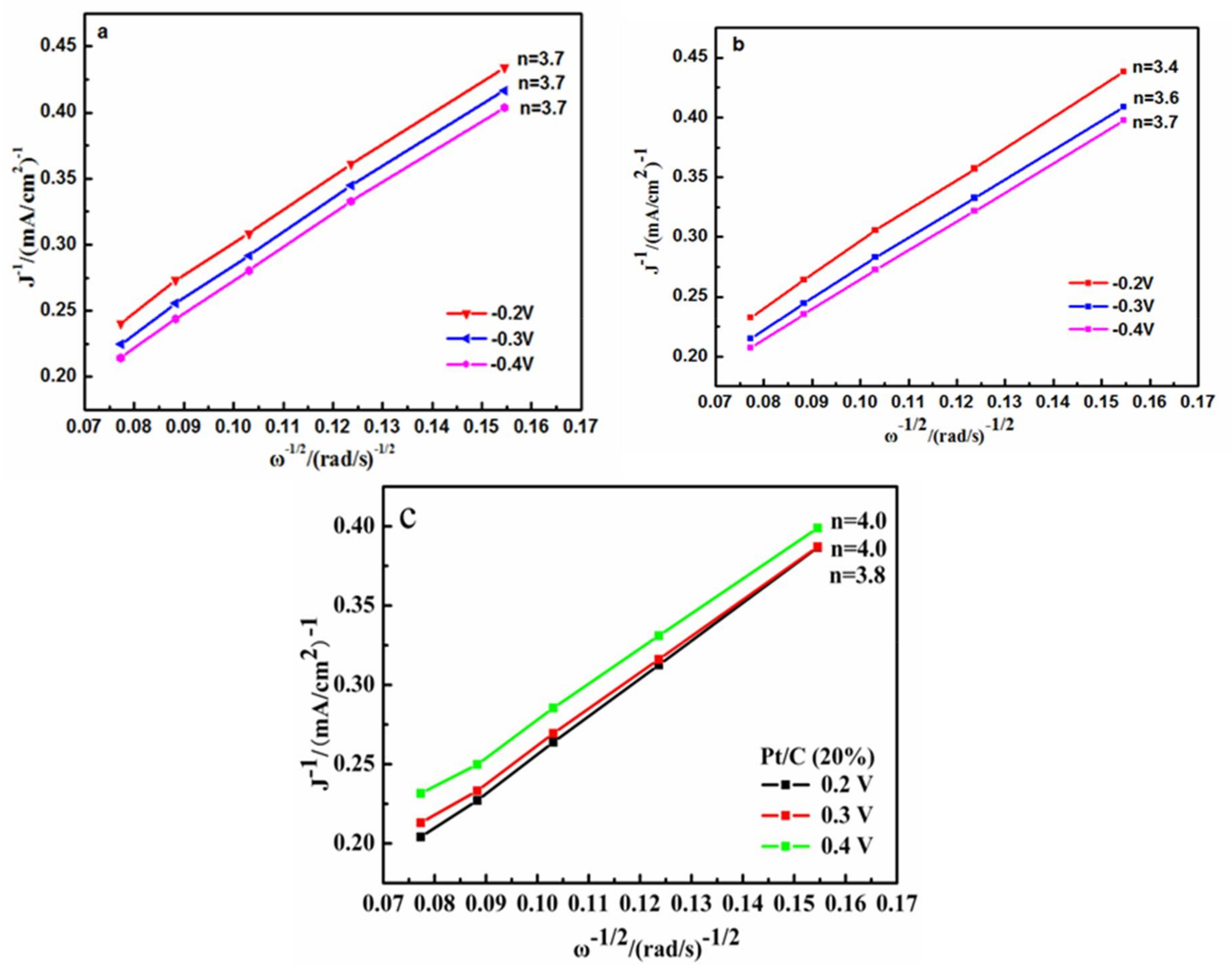
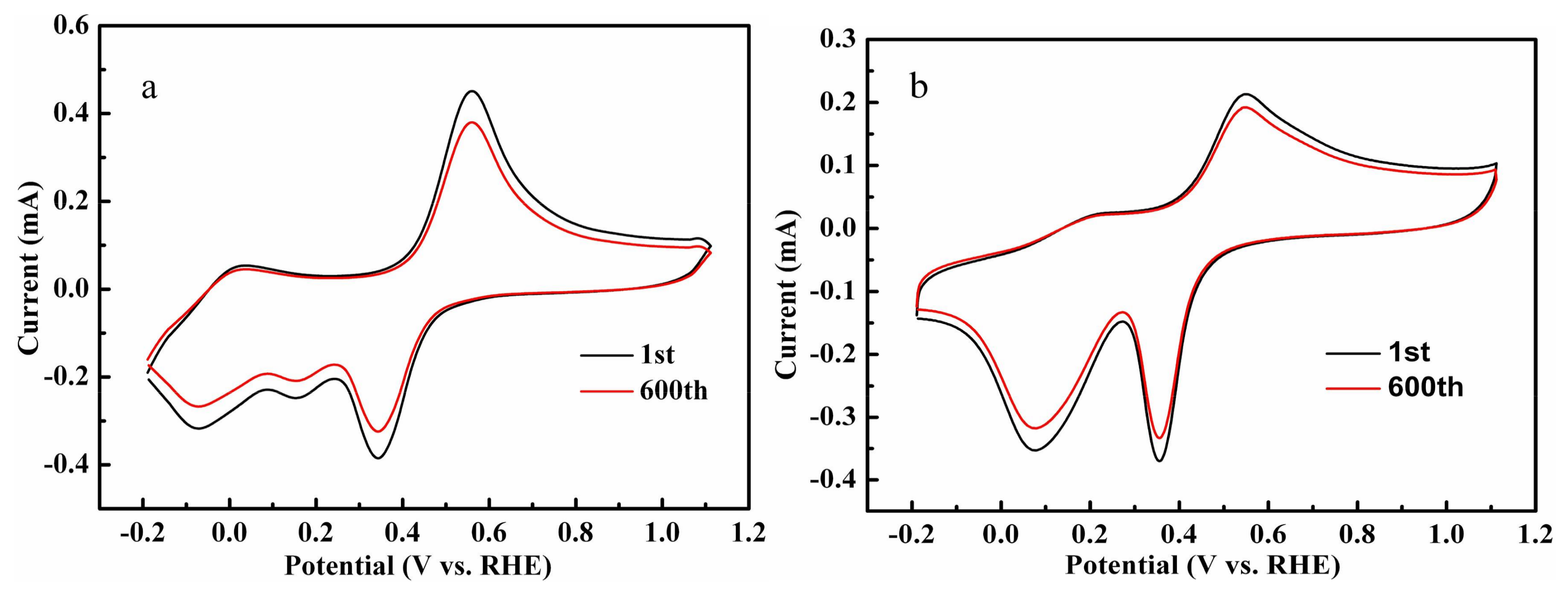
2.4. Electrochemical Analysis
3. Materials and Experimental
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis Procedure of Complex 1
3.2.2. Synthesis Procedure of Complex 2
3.3. Physical Characterization of the Crystals
3.4. Preparation of the Catalysts Modified Electrode
3.5. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumar, R.; Yadav, A.; Mahiya, K.; Mathur, P. Copper (II) complexes with box or flower type morphology: Sustainability versus perishability upon catalytic recycling. Inorg. Chim. Acta 2016, 450, 279–284. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yuan, S.; Day, G.; Wang, X.; Yang, X.Y.; Zhou, H.C. Luminescent sensors based on metal-organic frameworks. Coodination Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Fan, T.Y.; Yin, F.X.; Wang, H.; He, X.B.; Li, G.R. A metal-organic-framework/carbon composite with enhanced bifunctional electrocatalytic activities towards oxygen reduction/evolution reactions. Int. J. Hydrogen Energy 2017, 42, 17376–17385. [Google Scholar] [CrossRef]
- Levy, N.; Mahammed, A.; Friedman, A.; Gavriel, B.; Gross, Z.; Elbaz, L. Metallocorroles as non-precious metal electrocatalysts for highly efficient oxygen reduction in alkaline media. ChemCatChem 2016, 8, 2832–2837. [Google Scholar] [CrossRef]
- Hossain, M.D.; Sato, T.; Higuchi, M. A green copper-based metallo-supramolecular polymer: Synthesis, structure, and electrochromic properties. Chem. Asian J. 2013, 8, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Thorseth, M.A.; Tornow, C.E.; Tse, E.C.M.; Gewirth, A.A. Cu complexes that catalyze the oxygen reduction reaction. Coordination Chem. Rev. 2013, 257, 130–139. [Google Scholar] [CrossRef]
- Burkitt, R.; Whiffen, T.R.; Yu, E.H. Iron phthalocyanine and MnOx composite catalysts for microbial fuel cell applications. Appl. Catal. B Environ. 2016, 181, 279–288. [Google Scholar] [CrossRef]
- Liu, B.C.; Brückner, C.; Lei, Y.; Cheng, Y.; Santoro, C.; Li, B.K. Cobalt porphyrin-based material as methanol tolerant cathode in single chamber microbial fuel cells. J. Power Source 2014, 257, 246–253. [Google Scholar] [CrossRef]
- Arul, A.; Pak, H.; Moon, K.U.; Christy, M.; Oh, M.Y.; Nahm, K.S. Metallomacrocyclic-carbon complex: A study of bifunctional electrocatalytic activity for oxygen reduction and oxygen evolution reactions and their lithium-oxygen battery applications. Appl. Catal. B Environ. 2018, 220, 488–496. [Google Scholar] [CrossRef]
- Mahammed, A.; Gross, Z. Metallocorroles as electrocatalysts for the oxygen reduction reaction (ORR). Isr. J. Chem. 2016, 56, 756–762. [Google Scholar] [CrossRef]
- Choi, Y.K.; Park, J.K.; Jeon, S. Electrocatalytic reduction of dioxygen at carbon electrodes modified with Co.(II)-Schiff base complexes in various pH solutions. Electroanalysis 1999, 11, 134–138. [Google Scholar] [CrossRef]
- Ghasemi, M.; Wan Daud, W.R.; Rahimnejad, M.; Rezayi, M.; Fatemi, A.; Jafari, Y.; Somalu, M.R.; Manzour, A. Copper-phthalocyanine and nickel nanoparticles as novel cathode catalysts in microbial fuel cells. Int. J. Hydrogen Energy 2013, 38, 9533–9540. [Google Scholar] [CrossRef]
- Pal, S.; Barik, A.K.; Gupta, S.; Hazra, A.; Kar, S.K.; Peng, S.M. Copper(II) mediated anion dependent formation of Schiff base complexes. Inorg. Chem. 2005, 44, 3880–3889. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, M.J.; Park, M.K.; Thompson, L.K. Coordination compounds of Schiff-base ligands derived from diaminomaleonitrile (dmn): Mononuclear, dinuclear, and macrocyclic derivatives. Inorg. Chem. 1996, 35, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Palaniandavar, M.; Halcrow, M.A. Copper (II) complexes of sterically hindered Schiff base ligands: Synthesis, structure, spectra and electrochemistry. Polyhedron 2006, 25, 1077–1088. [Google Scholar] [CrossRef]
- Roy, P.; Manassero, M. Tetranuclear copper (II)-Schiff-base complexes as active catalysts for oxidation of cyclohexane and toluene. Dalton Trans. 2010, 39, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Roy, A.S.; Mondal, P.; Mubarak, M.; Mondal, S.; Hossain, D. Synthesis, catalytic oxidation and antimicrobial activity of copper (II) Schiff base complex. J. Mol. Catal. A Chem. 2011, 336, 106–114. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Samanta, B.; Chakraborty, J.; Choudhury, C.R.; Dey, S.K.; Dey, D.K.; Batten, S.R. New Cu(II) complexes with polydentate chelating Schiff base ligands: Synthesis, structures, characterisations and biochemical activity studies. Chin. J. Struct. Chem. 2007, 18, 33–41. [Google Scholar] [CrossRef]
- Sundaravel, K.; Suresh, E.; Palaniandavar, M. Synthesis, structures, spectral and electrochemical properties of copper (II) complexes of sterically hindered Schiff base ligands. Inorg. Chim. Acta 2009, 362, 199–207. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.L.; Wang, M.; Kong, L.Q; Zhao, J.S. 1,10-phenanthroline metal complex covalently bonding to poly-(pyrrole-3-carboxylic acid)-coated carbon: An efficient electrocatalyst for oxygen reduction. Electrochim. Acta 2015, 180, 86–95. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Ottenwaelder, X.; Stack, T.D.P.; Chidsey, C.E.D. Kinetic and mechanistic studies of the electrocatalytic reduction of O2 to H2O with mononuclear Cu complexes of substituted 1,10-phenanthrolines. J. Phys. Chem. A 2007, 111, 12641–12650. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.B.; Anson, F.C. Mechanistic aspects of the electroreduction of dioxygen as catalyzed by copper-phenathroline complexes adsorbed on graphite electrode. Inorg. Chem. 1994, 33, 5003–5009. [Google Scholar] [CrossRef]
- Schatz, M.; Raab, V.; Foxon, S.P.; Brehm, G.; Schneider, S.; Reiher, M.; Holthausen, M.C.; Sundermeyer, J.; Schindler, S. Combined spectroscopic and theoretical evidence for a persistent end-on copper superoxo complex. Angew. Chem. Int. Ed. 2004, 43, 4360–4363. [Google Scholar] [CrossRef] [PubMed]
- Aboelella, N.W.; Lewis, E.A.; Reynolds, A.M.; Brennessel, W.W.; Cramer, C.J.; Tolman, W.B. Snapshots of dioxygen activation by copper: The structure of a 1:1 Cu/O2 adduct and its use in syntheses of asymmetric bis (μ-oxo) complexes. J. Am. Chem. Soc. 2002, 124, 10660–10661. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Xu, A.W. Noble-metal-free Fe-N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; Wang, M.; Zhao, J.S.; Zhang, Y. Poly(10,12-bis(4-hexylthiophen-2-yl)thieno[3′,4′:5,6]pyrazino[2,3-f][1,10]-phenanthroline)-copper(II) complex(II) complex as an efficient electrocatalyst for oxygen reduction. Chem. Eng. J. 2017, 316, 680–691. [Google Scholar] [CrossRef]
- Yang, T.T.; Wang, M.; Ju, X.P.; Zhao, J.S.; Fu, C.G. The efficient oxygen reduction catalysts based on the Non-Noble metal and conducting polymers. Int. J. Electrochem. Sci. 2017, 12, 12125–12139. [Google Scholar] [CrossRef]
- Thorseth, M.A.; Letko, C.S.; Tse, E.C.M.; Rauchfuss, T.B.; Gewirth, A.A. Ligand effects on the overpotential for dioxygen reduction by tris(2-pyridylmethyl) amine derivatives. Inorg. Chem. 2013, 52, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Iwase, K.; Yoshioka, T.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Copper-modified covalent trizazine frameworks as non-noble-metal electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 11068–11072. [Google Scholar] [CrossRef] [PubMed]
- Thorum, M.S.; Yadav, J.; Gewirth, A.A. Oxygen reduction activity of a copper complex of 3,5-diamino-1,2,4-triazole supported on carbon black. Angew. Chem. Int. Ed. 2009, 48, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Oyaizu, N.; Shimazu, K.; Yagi, I. Oxygen reduction reaction catalyzed by self-assembled monolayers of copper-based electrocatalysts on a polycrystalline gold surface. J. Phys. Chem. C 2016, 120, 15814–15822. [Google Scholar] [CrossRef]
- Li, C.J.; Yan, C.X.; Yang, X.X.; Ren, Y.H.; Xue, Z.C.; Cui, C.S. Two 2,4-dihydroxybenzaldehydeglycine Schiff base complexes: Syntheses, structures and selective oxidation catalytic properties for alcohols. Chin. J. Inorg. Chem. 2016, 32, 891–898. [Google Scholar]
- Zhao, Y.Y.; Chu, Y.; Ju, X.P.; Zhao, J.S.; Kong, L.Q.; Zhang, Y. Carbon-supported copper-based nitrogen-containing supramolecule as an efficient oxygen reduction reaction catalyst in neutral medium. Catalysts 2018, 8, 53. [Google Scholar] [CrossRef]
- He, H.Y.; Wang, M.; Zhang, Y.; Zhao, J.S. Soluble conjugated polymer enriched with pyridinic nitrogen and its application as high-performance catalyst for oxygen reduction. J. Solid State Electrochem. 2017, 21, 1639–1651. [Google Scholar] [CrossRef]
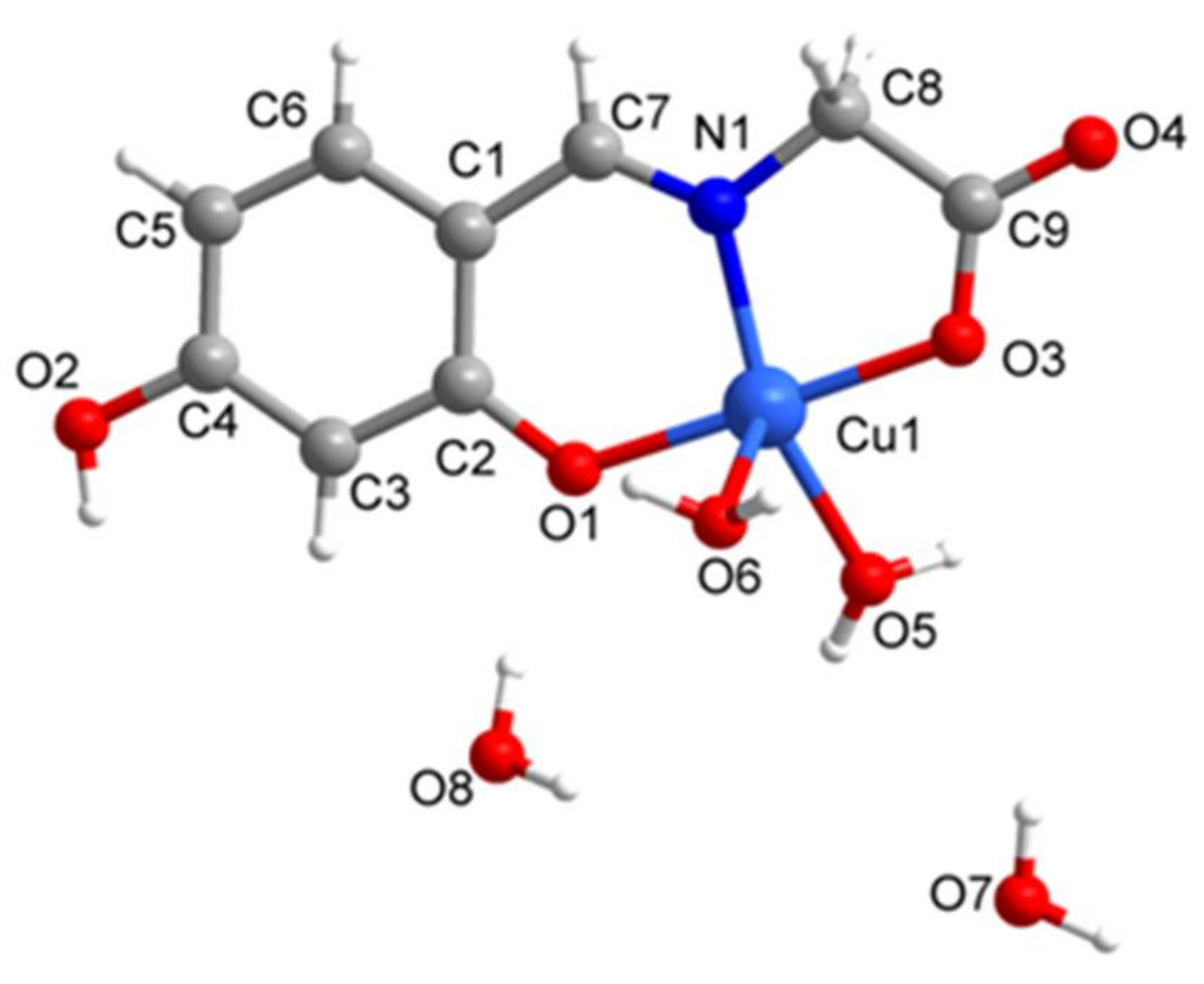
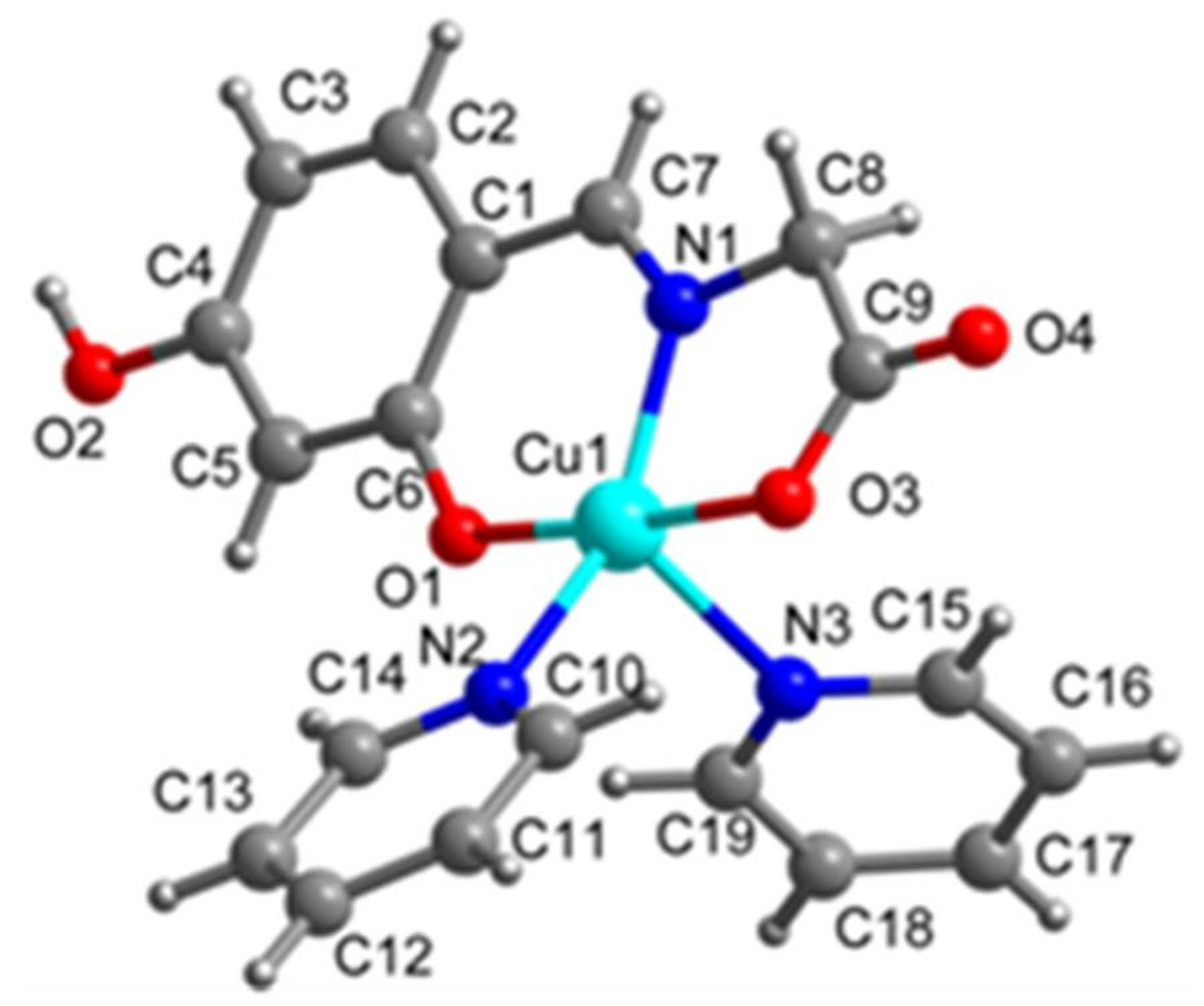


| Complex 1 | Complex 2 | |
|---|---|---|
| Formula | C9H15CuNO8 | C19H17CuN3O4 |
| Mr | 328.76 | 414.90 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P2(1)/c |
| Temperature | 298(2) K | 298(2) K |
| a (Å) | 10.9346(9) | 1.17003(8) |
| b (Å) | 6.9358(7) | 1.17716(9) |
| c (Å) | 32.977(3) | 1.33059(12) |
| α (degree) | 90 | 90 |
| β (degree) | 95.1290(10) | 97.7180(10) |
| γ (degree) | 90 | 90 |
| V (Å3) | 2490.9(4) | 1816.0(2) |
| Z | 8 | 4 |
| Dcalc.(g cm−3) | 1.753 | 1.517 |
| F (000) | 1352 | 852 |
| R1 [I > 2σ(I)] | 0.0945 | 0.0391 |
| wR2 [I > 2σ(I)] | 0.2214 | 0.0958 |
| R1 (all data) | 0.1093 | 0.0687 |
| wR2 (all data) | 0.2286 | 0.1110 |
| GOOF | 1.114 | 1.027 |
| CCDC No. | 1446565 | 1062408 |
| Complex 1 | |||
| Cu1-O1 1.916(5) | Cu1-N1 1.923(7) | Cu1-O3 1.964(6) | Cu1-O5 1.971(6) |
| Cu1-O6 2.378(7) | N1-C7 1.295(11) | N1-C8 1.468(10) | O1-C2 1.318(10) |
| O2-C4 1.353(10) | O3-C9 1.271(11) | O4-C9 1.254(10) | C1-C6 1.397(13) |
| O1-Cu1-N1 95.3(3) | O1-Cu1-O3 178.6(2) | N1-Cu1-O3 83.3(3) | O1-Cu1-O5 95.5(2) |
| N1-Cu1-O5 155.0(3) | O3-Cu1-O5 85.8(3) | O1-Cu1-O6 88.0(3) | N1-Cu1-O6 112.3(3) |
| O3-Cu1-O6 92.5(3) | O5-Cu1-O6 90.5(3) | C7-N1-C8 119.6(7) | C7-N1-Cu1 125.6(6) |
| Complex 2 | |||
| Cu1-O1 1.906(2) | Cu1-N1 1.936(3) | Cu1-O3 1.968(2) | Cu1-N2 2.068(3) |
| Cu1-N3 2.282(3) | O1-C6 1.315(4) | N1-C7 1.284(4) | N1-C8 1.462(4) |
| O1-Cu1-N1 93.24(9) | O1-Cu1-O3 176.03(10) | N1-Cu1-O3 83.21(10) | N1-Cu1-N2 150.98(11) |
| O3-Cu1-N2 91.13(10) | O1-Cu1-N3 91.05(10) | N1-Cu1-N3 107.47(11) | O3-Cu1-N3 91.70(11) |
| N2-Cu1- N3 101.11(10) | C6-O1-Cu1 127.3(2) | C7-N1-Cu1 126.6(2) | C8-N1-Cu1 113.33(19) |
| Catalyst | Eonset (V) | E1/2 (V) | ETN | Electrolyte | Reference Electrode | References |
|---|---|---|---|---|---|---|
| Complex 1/C | 0.45 | - | 3.7 | PBS (pH = 7) | RHE | This study |
| Complex 2/C | 0.58 | - | 3.6 | PBS (pH = 7) | RHE | This study |
| Pt/C (20%) | 0.89 | 0.63 | 4.0 | PBS (pH = 7) | RHE | This study |
| [Cu(TPA)(L)]2+/C | 0.69 | 0.23 | 3.8 | Britton–Robinson buffer (pH = 7) | RHE | [29] |
| [Cu(PMEA)(L)]2+/C | 0.69 | 0.37 | 4.0 | Britton–Robinson buffer (pH = 7) | RHE | [29] |
| [Cu(PMAP)(L)]2+/C | 0.69 | 0.42 | 3.6 | Britton–Robinson buffer (pH = 7) | RHE | [29] |
| [Cu(TEPA)(L)]2+/C | 0.69 | 0.52 | 3.7 | Britton–Robinson buffer (pH = 7) | RHE | [29] |
| Cu–CTF/CPS | 0.81 | - | 3.75–3.95 | PBS (pH = 7) | RHE | [30] |
| [Cu(Hdatrz)]/Au | 0.72 | - | 3.9–4.0 | Britton–Robinson buffer (pH = 7) | RHE | [31] |
| Cu–AMT/Au | 0.74 | - | - | Britton–Robinson buffer (pH = 7) | RHE | [32] |
| Cu–HT/Au | 0.74 | - | - | Britton–Robinson buffer (pH = 7) | RHE | [32] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Chu, Y.; Fu, C.; Du, H.; Huang, X.; Zhao, J. The Effects of Coordinated Molecules of Two Gly-Schiff Base Copper Complexes on Their Oxygen Reduction Reaction Performance. Catalysts 2018, 8, 156. https://doi.org/10.3390/catal8040156
Ma Z, Chu Y, Fu C, Du H, Huang X, Zhao J. The Effects of Coordinated Molecules of Two Gly-Schiff Base Copper Complexes on Their Oxygen Reduction Reaction Performance. Catalysts. 2018; 8(4):156. https://doi.org/10.3390/catal8040156
Chicago/Turabian StyleMa, Zhe, Ya Chu, Chonggang Fu, Hongmei Du, Xianqiang Huang, and Jinsheng Zhao. 2018. "The Effects of Coordinated Molecules of Two Gly-Schiff Base Copper Complexes on Their Oxygen Reduction Reaction Performance" Catalysts 8, no. 4: 156. https://doi.org/10.3390/catal8040156
APA StyleMa, Z., Chu, Y., Fu, C., Du, H., Huang, X., & Zhao, J. (2018). The Effects of Coordinated Molecules of Two Gly-Schiff Base Copper Complexes on Their Oxygen Reduction Reaction Performance. Catalysts, 8(4), 156. https://doi.org/10.3390/catal8040156