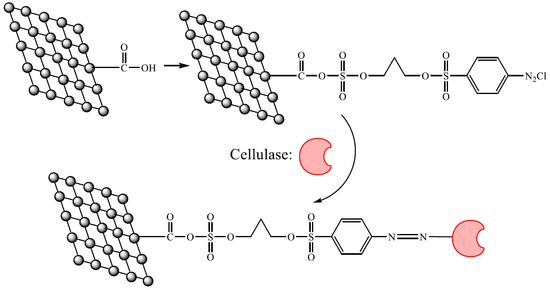
Rapid Immobilization of Cellulase onto Graphene Oxide with a Hydrophobic Spacer
Abstract
:1. Introduction
2. Results
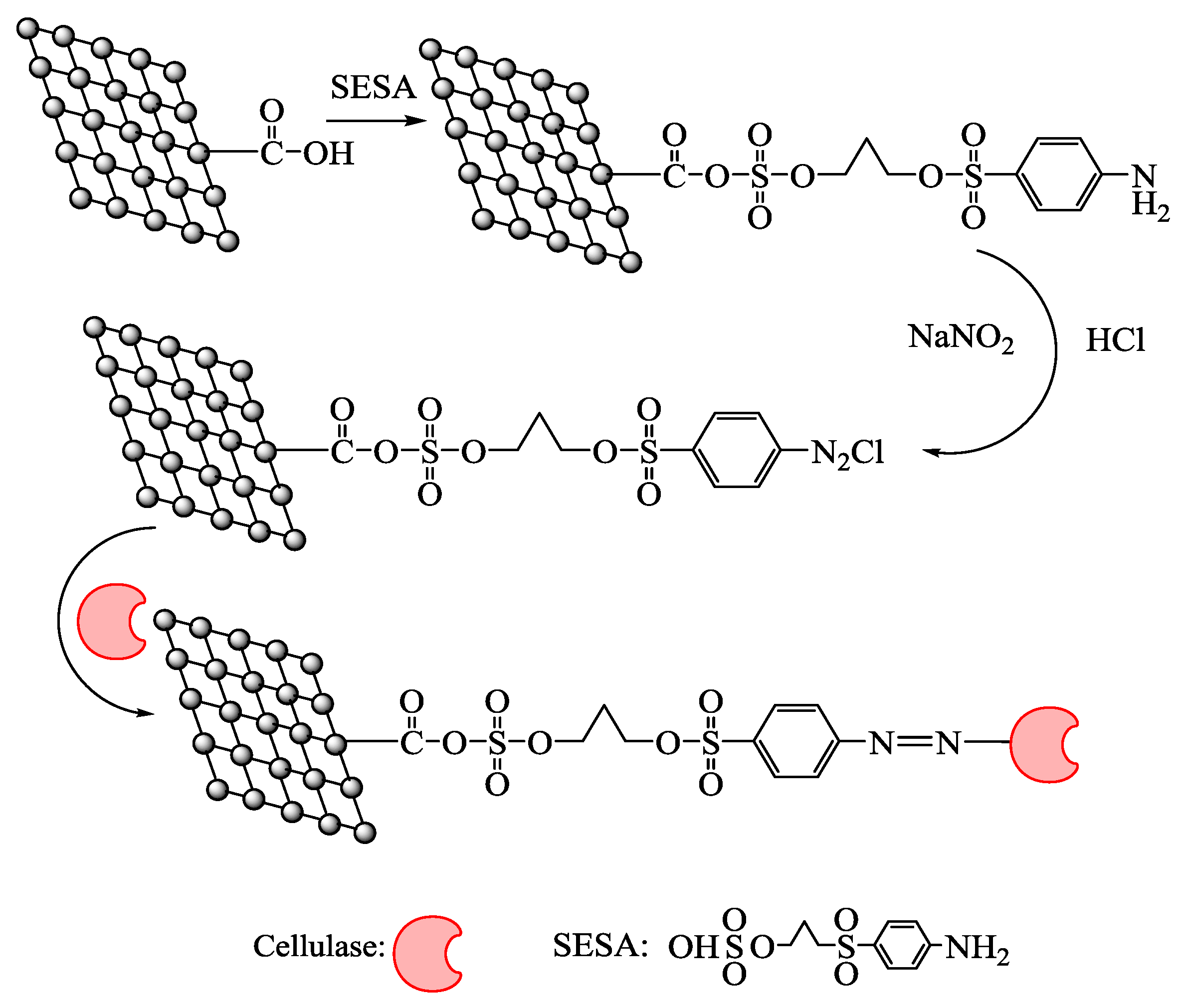
2.1. Characterization of Activated Graphene Oxide
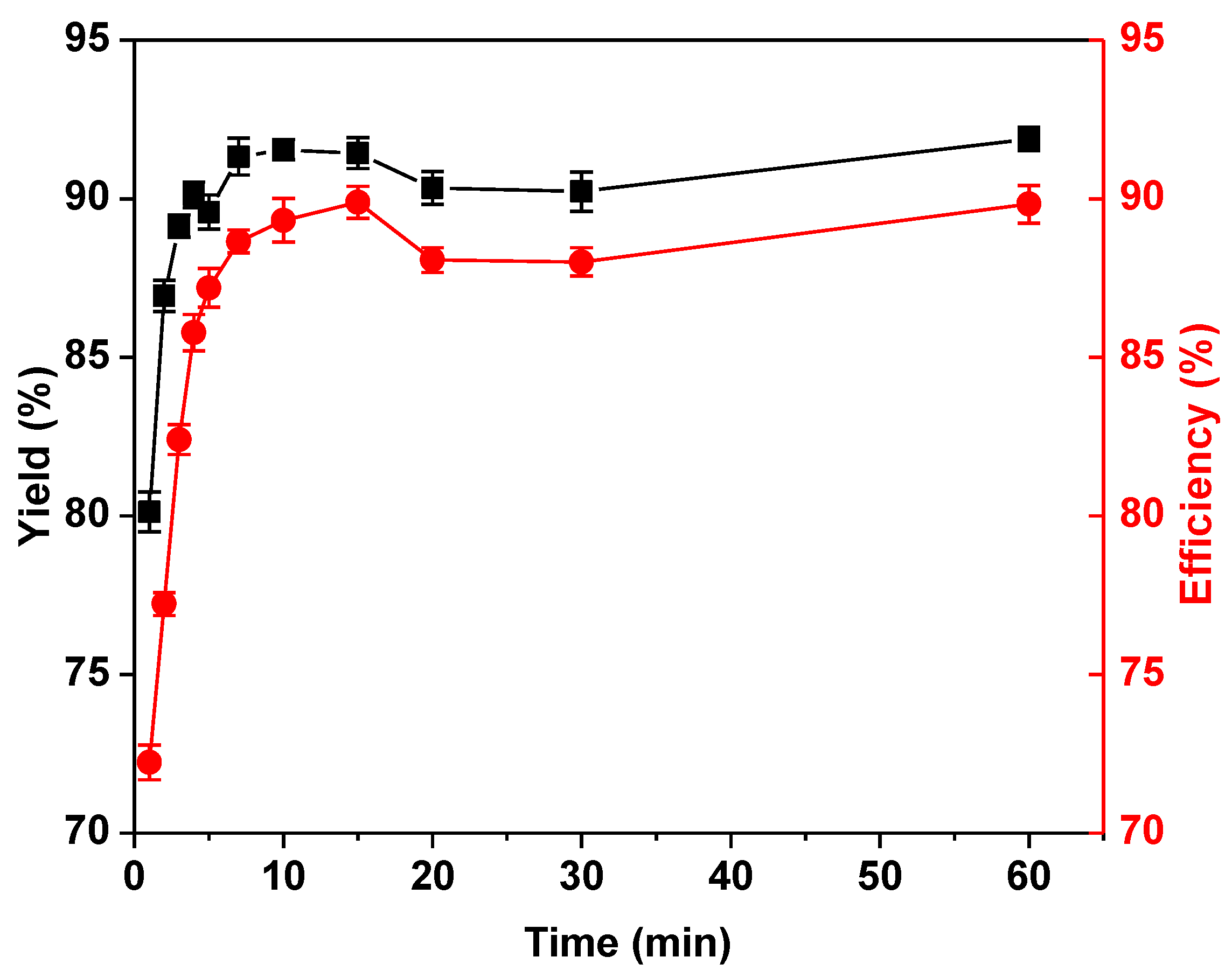
2.2. Optimization of the Immobilization Conditions
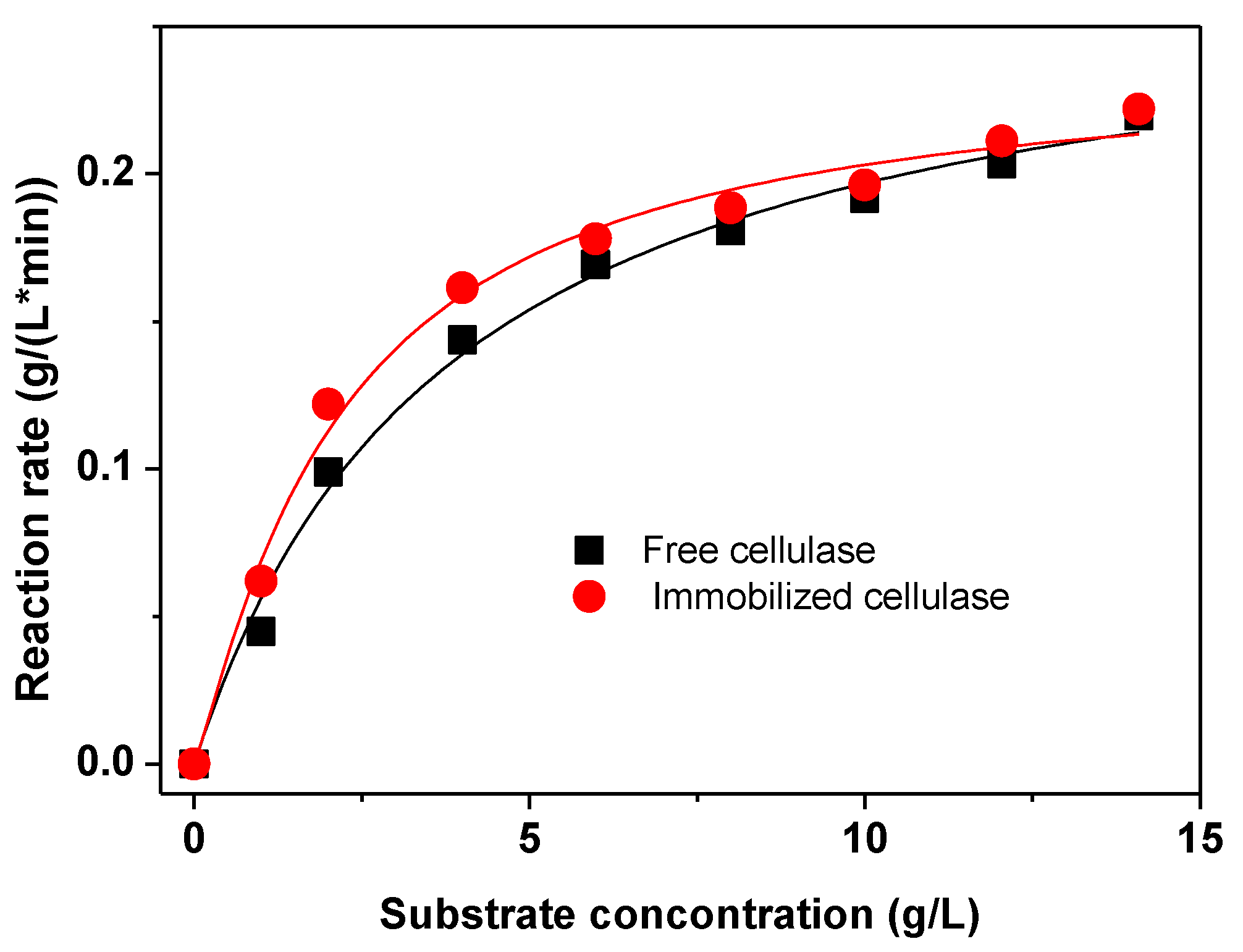
2.3. Characterization of the Immobilized Cellulase
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Functional Graphene Oxide
4.3. Immobilization of Cellulase
4.4. Enzyme Assay
4.5. Optimization of Immobilization Conditions
4.6. Characterization of the Immobilized Cellulase
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hamid, S.B.A.; Islam, M.M.; Das, R. Cellulase biocatalysis: Key influencing factors and mode of action. Cellulose 2015, 22, 2157–2182. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.-Y.; Jiang, X.-P.; Ye, J.-J.; Zhang, Y.-W.; Zhang, X.-Y. Fabrication of graphene oxide decorated with Fe3O4@SiO2 for immobilization of cellulase. J. Nanopart. Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Wong, J.R.; Tan, K.W.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J. Mol. Catal. B Enzym. 2014, 107, 124–131. [Google Scholar] [CrossRef]
- Zang, L.; Qiu, J.; Wu, X.; Zhang, W.; Sakai, E.; Wei, Y. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. Res. 2014, 53, 3448–3454. [Google Scholar] [CrossRef]
- Ince, A.; Bayramoglu, G.; Karagoz, B.; Altintas, B.; Bicak, N.; Arica, M.Y. A method for fabrication of polyaniline coated polymer microspheres and its application for cellulase immobilization. Chem. Eng. J. 2012, 189–190, 404–412. [Google Scholar] [CrossRef]
- Cho, E.J.; Jung, S.; Kim, H.J.; Lee, Y.G.; Nam, K.C.; Lee, H.-J.; Bae, H.-J. Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose. Chem. Commun. 2011, 48, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Cao, X. Preparation of a pH-sensitive polyacrylate amphiphilic copolymer and its application in cellulase immobilization. Bioresour. Technol. 2012, 116, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.-Y.; Zhang, R.-Z.; Zhang, X.-Y.; Liu, W.; Xu, X.-M.; Zhang, Y.-W. Molecular Imprinting and Immobilization of Cellulase onto Magnetic Fe3O4@SiO2 Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.-M.; Wang, X.-Y.; Ma, P.; Yang, Y.; Qin, J.-M.; Zhang, X.-J.; Zhang, Y.-W. Covalent immobilization of penicillin G acylase onto Fe3O4@Chitosan magnetic nanoparticles. J. Microbiol. Biotechnol. 2016, 26, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.-L.; Li, Y.; Shi, Y.; Liu, R.-J.; Zhang, Y.-W.; Guo, J. Application of molecular imprinted magnetic Fe3O4@SiO2 nanoparticles for selective immobilization of cellulase. J. Nanosci. Nanotechnol. 2016, 16, 6055–6060. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.-Y.; Zhou, Q.-L.; Wang, X.-Y.; Zhang, J.-X.; Xue, L.; Wang, R.; Zhang, J.-X.; Zhang, Y.-W. Immobilization of lipase onto dopamine functionalized magnetic nanoparticles. Nanosci. Nanotechnol. Lett. 2016, 8, 251–254. [Google Scholar] [CrossRef]
- Zhuang, M.-Y.; Jiang, X.-P.; Ling, X.-M.; Xu, M.-Q.; Zhu, Y.-H.; Zhang, Y.-W. Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration. J. Chem. Technol. Biotechnol. 2018, 93. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Jiang, X.-P.; Li, Y.; Zeng, S.; Zhang, Y.-W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.-Y.; Wang, C.; Xu, M.-Q.; Ling, X.-M.; Shen, J.-J.; Zhang, Y.-W. Using concanavalinA as a spacer for immobilization of E. coli onto magnetic nanoparticles. Int. J. Biol. Macromol. 2017, 104, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, F.; Zhang, X.-Y.; Li, Y.; Wang, X.-Y.; Xu, X.-M.; Zhang, Y.-W. Preparation of magnetic Fe3O4@SiO2 nanoparticles for immobilization of lipase. J. Nanosci. Nanotechnol. 2014, 14, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Balasubramanian, R. Graphene/semiconductor nanocomposites (GSNs) for heterogeneous photocatalytic decolorization of wastewaters contaminated with synthetic dyes: A review. Appl. Catal. B Environ. 2014, 160–161, 307–324. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, H.K.; Han, J.; Chung, H.-S.; Jang, S.-H.; Lee, C. Increased thermal stability of cold-adapted esterase at ambient temperatures by immobilization on graphene oxide. Bioresour. Technol. 2013, 148, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Devasenathipathy, R.; Chen, S.-M.; Huang, S.-T.; Vasantha, V.S. Immobilization of glucose oxidase on graphene and cobalt phthalocyanine composite and its application for the determination of glucose. Enzyme Microb. Technol. 2014, 66, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.A.; Lu, J.; Lee, I. Immobilization of cellulase on magnetoresponsive graphene nano-supports. J. Mol. Catal. B Enzym. 2013, 90, 76–86. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Wang, J.; Wang, E. Mussel-inspired biopolymer modified 3D graphene foam for enzyme immobilization and high performance biosensor. Electrochim. Acta 2015, 161, 17–22. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Jing, L.; Peng, X.; Li, Y. Facile self-assembly of magnetite nanoparticles on three-dimensional graphene oxide-chitosan composite for lipase immobilization. Biochem. Eng. J. 2015, 98, 75–83. [Google Scholar] [CrossRef]
- Liu, C.-H.; Li, X.-Q.; Jiang, X.-P.; Zhuang, M.-Y.; Zhang, J.-X.; Bao, C.-H.; Zhang, Y.-W. Preparation of functionalized graphene oxide nanocomposites for covalent immobilization of NADH oxidase. Nanosci. Nanotechnol. Lett. 2016, 8, 164–167. [Google Scholar] [CrossRef]
- Andre, J.; Saleh, D.; Syldatk, C.; Hausmann, R. Effect of spacer modification on enzymatic synthetic and hydrolytic activities of immobilized trypsin. J. Mol. Catal. B Enzym. 2016, 125, 88–96. [Google Scholar] [CrossRef]
- Nouaimi, M.; Moschel, K.; Bisswanger, H. Immobilization of trypsin on polyester fleece via different spacers. Enzyme Microb. Technol. 2001, 29, 567–574. [Google Scholar] [CrossRef]
- Deere, J.; De Oliveira, R.F.; Tomaszewski, B.; Millar, S.; Lalaouni, A.; Solares, L.F.; Flitsch, S.L.; Halling, P.J. Kinetics of enzyme attack on substrates covalently attached to solid surfaces: Influence of spacer chain length, immobilized substrate surface concentration and surface charge. Langmuir 2008, 24, 11762–11769. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Liu, B.; Hou, X.; Zhang, B.; Du, C. Covalent immobilization of glucose oxidase onto Poly(St-GMA-NaSS) monodisperse microspheres via BSA as spacer arm. Appl. Surf. Sci. 2009, 255, 7937–7941. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, K.; Zhang, Q.; Li, F.; Wu, T.; Niu, L. Decorated graphene sheets for label-free DNA impedance biosensing. Biomaterials 2012, 33, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Hu, K.; Yu, H.; Zhou, L.; Song, L.; Zhang, Y.; Shan, X.; Liu, J.; Gu, N. A functional iron oxide nanoparticles modified with PLA-PEG-DG as tumor-targeted MRI contrast agent. Pharm. Res. 2017, 34, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Xu, Y.; Shen, H.; Shen, S.; Ge, Y.; Xie, J. Negative-charge-functionalized mesoporous silica nanoparticles as drug vehicles targeting hepatocellular carcinoma. Int. J. Pharmaceut. 2014, 474, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, L.; Liu, J.; Xie, M.; Shen, H.; Qi, X.; Yan, Y.; Ge, Y.; Jin, Y. Core–shell structured Fe3O4@TiO2-doxorubicin nanoparticles for targeted chemo-sonodynamic therapy of cancer. Int. J. Pharmaceut. 2015, 486, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Tao, Z.; Jia, L.; Luo, Y.-F.; Xu, J.; Chen, R.-H.; Ge, Z.-J.; Ma, T.-L.; Chen, H. Multifunctional nanocomposite based on halloysite nanotubes for efficient luminescent bioimaging and magnetic resonance imaging. Int. J. Nanomed. 2016, 11, 4765–4776. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Morsy, R.; El-Zawawy, N.; Fareed, M.; Bedaiwy, M. Synthesized zinc peroxide nanoparticles (ZnO2-NPs): a novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int. J. Nanomed. 2017, 12, 6059–6073. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, S.; Cao, J.; Ji, L.; He, Y.; Xi, J. Preparation and evaluation of a novel bioactive glass/lysozyme/PLGA composite microsphere. Drug Dev. Ind. Pharm. 2015, 41, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Wang, N.; Tain, L. Immobilization and stabilization of pectinase by multipoint attachment onto an activated agar-gel support. Food Chem. 2008, 109, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Fan, H.; Ai, S.; Han, R.; Qiu, Y. Hemoglobin (Hb) immobilized on amino-modified magnetic nanoparticles for the catalytic removal of bisphenol A. Chemosphere 2011, 83, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, P.; Ding, F.; Yang, Y. Immobilization of cellulase on polyamidoamine dendrimer-grafted silica. J. Mol. Catal. B-Enzym. 2013, 89, 35–40. [Google Scholar] [CrossRef]
- Wu, L.L.; Yuan, X.Y.; Sheng, J. Immobilization of cellulase in nanofibrous PVA membranes by electrospinning. J. Membr. Sci. 2005, 250, 167–173. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Senkal, B.F.; Arica, M.Y. Preparation of clay–poly(glycidyl methacrylate) composite support for immobilization of cellulase. Appl. Clay Sci. 2013, 85, 88–95. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]







| Carrier | Spacer | Km (g·L−1) | Stability 1 | Immobilization Time | Reference |
|---|---|---|---|---|---|
| Clay composite materials | - | - | 1.4 | 8 h | [39] |
| polyacrylate amphiphilic copolymer | carbodiimide | 120.89 | 1.2 | 4 h | [7] |
| polyamidoamine dendrimer-grafted silica | glutaraldehyde | 0.33 | 2.2 | 2 h | [37] |
| PVA membrane | - | - | - | 1 h | [38] |
| Graphene oxide | SESA | 2.17 | 2.1 | 10 min | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Lu, C.-L.; Wang, Y.; Wang, S.-S.; Shen, J.-J.; Zhang, J.-X.; Zhang, Y.-W. Rapid Immobilization of Cellulase onto Graphene Oxide with a Hydrophobic Spacer. Catalysts 2018, 8, 180. https://doi.org/10.3390/catal8050180
Gao J, Lu C-L, Wang Y, Wang S-S, Shen J-J, Zhang J-X, Zhang Y-W. Rapid Immobilization of Cellulase onto Graphene Oxide with a Hydrophobic Spacer. Catalysts. 2018; 8(5):180. https://doi.org/10.3390/catal8050180
Chicago/Turabian StyleGao, Jian, Chun-Liu Lu, Yue Wang, Shuang-Shuang Wang, Jia-Jia Shen, Jiu-Xun Zhang, and Ye-Wang Zhang. 2018. "Rapid Immobilization of Cellulase onto Graphene Oxide with a Hydrophobic Spacer" Catalysts 8, no. 5: 180. https://doi.org/10.3390/catal8050180
APA StyleGao, J., Lu, C.-L., Wang, Y., Wang, S.-S., Shen, J.-J., Zhang, J.-X., & Zhang, Y.-W. (2018). Rapid Immobilization of Cellulase onto Graphene Oxide with a Hydrophobic Spacer. Catalysts, 8(5), 180. https://doi.org/10.3390/catal8050180