Structural Evolution of Highly Active Multicomponent Catalysts for Selective Propylene Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of Flame-Made Multicomponent Catalysts
2.2. Catalytic Activity and On-Stream Behaviour
2.3. Structural Characteristics after Long-Term Time-on-Stream Tests
2.4. Tracking Individual Metals: X-Ray Absorption Spectroscopy
2.5. Spatially Resolved 3D Imaging of the Structural Evolution of the Catalyst
2.6. Addressing Catalyst Complexity in a Multimodal, Multiscale Approach
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
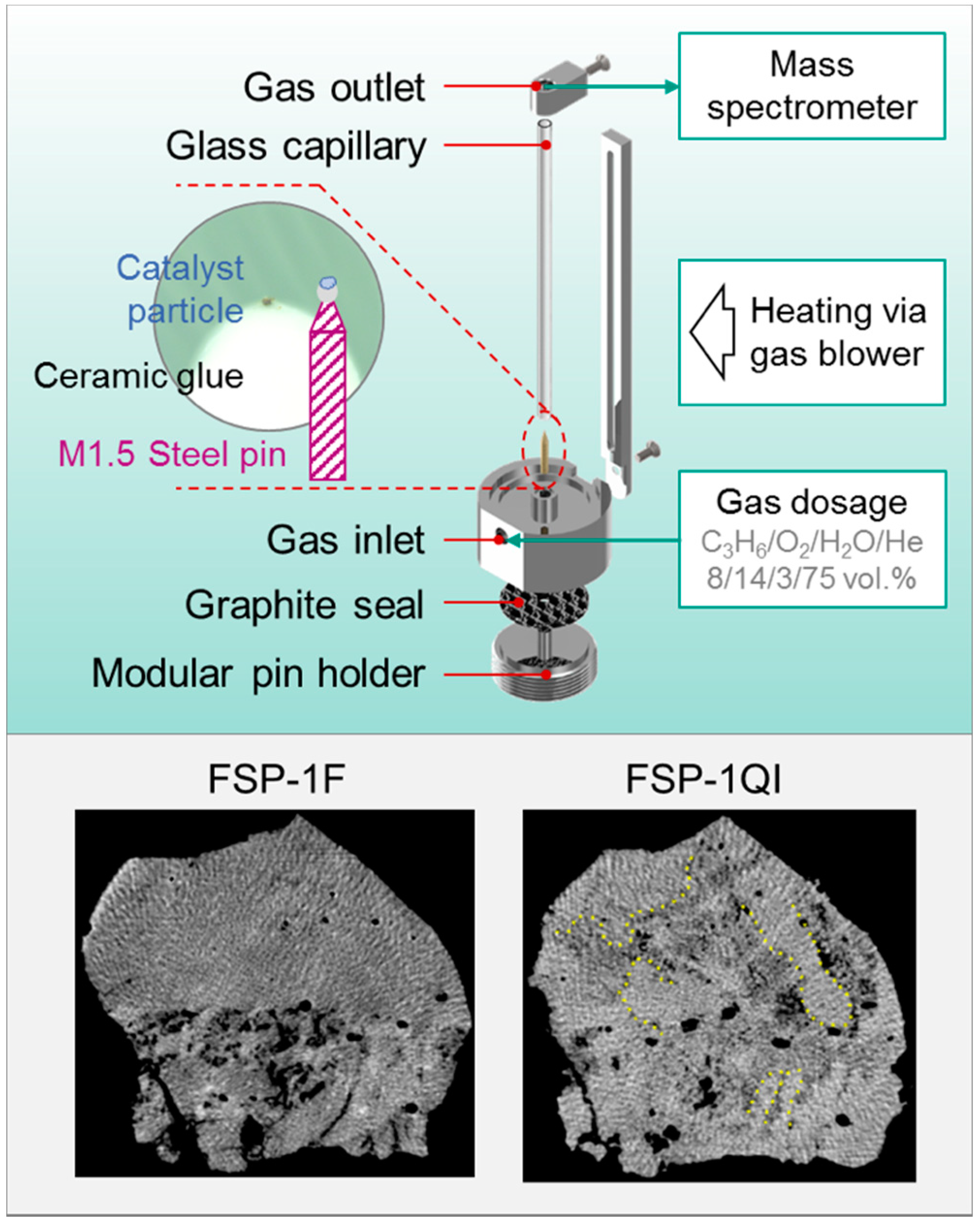
3.4. X-ray Nanotomography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arntz, D.; Fischer, A.; Höpp, M.; Jacobi, S.; Sauer, J.; Ohara, T.; Sato, T.; Shimizu, N.; Schwind, H. Acrolein and methacrolein. In Ullmann’s Encyclopedia of Industrial Chemistry; Weinheim; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; Volume 1, pp. 329–346. [Google Scholar]
- Høj, M.; Linde, K.; Hansen, T.K.; Brorson, M.; Jensen, A.D.; Grunwaldt, J.-D. Flame spray synthesis of CoMo/Al2O3 hydrotreating catalysts. Appl. Catal. A 2011, 397, 201–208. [Google Scholar] [CrossRef]
- Callahan, J.L.; Grasselli, R.K.; Milberger, E.C.; Strecker, H.A. Oxidation and ammoxidation of propylene over bismuth molybdate catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 134–142. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Tenhover, M.A. Ammoxidation. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; Volume 1, pp. 3489–3517. [Google Scholar]
- Sprenger, P.; Kleist, W.; Grunwaldt, J.-D. Recent advances in selective propylene oxidation over bismuth molybdate based catalysts: Synthetic, spectroscopic and theoretical approaches. ACS Catal. 2017, 7, 5628–5642. [Google Scholar] [CrossRef]
- Moro-Oka, Y.; Ueda, W. Multicomponent bismuth molybdate catalyst. Adv. Catal. 1994, 40, 233–273. [Google Scholar]
- Schuh, K.; Kleist, W.; Høj, M.; Trouillet, V.; Beato, P.; Jensen, A.; Grunwaldt, J.-D. Bismuth molybdate catalysts prepared by mild hydrothermal synthesis: Influence of pH on the selective oxidation of propylene. Catalysts 2015, 5, 1554–1573. [Google Scholar] [CrossRef] [Green Version]
- Schuh, K.; Kleist, W.; Høj, M.; Trouillet, V.; Beato, P.; Jensen, A.D.; Patzke, G.R.; Grunwaldt, J.-D. Selective oxidation of propylene to acrolein by hydrothermally synthesized bismuth molybdates. Appl. Catal. A 2014, 482, 145–156. [Google Scholar] [CrossRef]
- Nell, A.; Getsoian, A.B.; Werner, S.; Kiwi-Minsker, L.; Bell, A.T. Preparation and characterization of high-surface-area Bi(1−x)/3V1−xMoxO4 catalysts. Langmuir 2014, 30, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Farin, B.; Monteverde Videla, A.H.A.; Specchia, S.; Gaigneaux, E.M. Bismuth molybdates prepared by solution combustion synthesis for the partial oxidation of propene. Catal. Today 2015, 257, 11–17. [Google Scholar] [CrossRef]
- Schuh, K.; Kleist, W.; Høj, M.; Trouillet, V.; Jensen, A.D.; Grunwaldt, J.-D. One-step synthesis of bismuth molybdate catalysts via flame spray pyrolysis for the selective oxidation of propylene to acrolein. Chem. Commun. 2014, 50, 15404–15406. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Aouine, M.; Massin, L.; Belliere Baca, V.; Millet, J.M.M. Selective oxidation of propene to acrolein on femoteo catalysts: Determination of active phase and enhancement of catalytic activity and stability. Catal. Sci. Technol. 2017, 7, 4629–4639. [Google Scholar] [CrossRef]
- Sprenger, P.; Stehle, M.; Gaur, A.; Gänzler, A.M.; Gashnikova, D.; Kleist, W.; Grunwaldt, J.-D. Reactivity of bismuth molybdates for selective oxidation of propylene probed by correlative operando spectroscopies. ACS Catal. 2018, 8, 6462–6475. [Google Scholar] [CrossRef]
- Mädler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by fame spray pyrolysis. J. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef] [PubMed]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of catalytic materials in flames: Opportunities and challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, A.J.; Pratsinis, S.E.; Wegner, K. Fluid-particle dynamics during combustion spray aerosol synthesis of ZrO2. Chem. Eng. J. 2012, 191, 491–502. [Google Scholar] [CrossRef]
- Grasselli, R.K. Site isolation and phase cooperation: Two important concepts in selective oxidation catalysis: A retrospective. Catal. Today 2014, 238, 10–27. [Google Scholar] [CrossRef]
- Ponceblanc, H.; Millet, J.M.M.; Coudurier, G.; Védrine, J.C. Synergy effect of multicomponent Co, Fe, and Bi molybdates in propene partial oxidation. In Catalytic Selective Oxidation; American Chemical Society: Washington, DC, USA, 1993; Volume 523, pp. 262–272. [Google Scholar]
- Grasselli, R.K. Advances and future trends in oxidation and ammoxidation catalysis. Catal. Today 1999, 49, 141–153. [Google Scholar] [CrossRef]
- Moro-Oka, Y.; Ueda, W.; Lee, K.-H. The role of bulk oxide ion in the catalytic oxidation reaction over metal oxide catalyst. J. Mol. Catal. A Chem. 2003, 199, 139–148. [Google Scholar] [CrossRef]
- Zhai, Z.; Getsoian, A.B.; Bell, A.T. The kinetics of selective oxidation of propene on bismuth vanadium molybdenum oxide catalysts. J. Catal. 2013, 308, 25–36. [Google Scholar] [CrossRef]
- Bañares, M.A. Operando methodology: Combination of in situ spectroscopy and simultaneous activity measurements under catalytic reaction conditions. Catal. Today 2005, 100, 71–77. [Google Scholar] [CrossRef]
- Buurmans, I.L.; Weckhuysen, B.M. Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy. Nat. Chem. 2012, 4, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Grunwaldt, J.-D.; Wagner, J.B.; Dunin-Borkowski, R.E. Imaging catalysts at work: A hierarchical approach from the macro- to the meso- and nano-scale. ChemCatChem 2013, 5, 62–80. [Google Scholar] [CrossRef]
- Gross, E.; Somorjai, G.A. Molecular catalysis science: Nanoparticle synthesis and instrument development for studies under reaction conditions. J. Catal. 2015, 328, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, A.I.; Rodriguez, J.A.; Chen, J.G. Synchrotron techniques for in situ catalytic studies: Capabilities, challenges, and opportunities. ACS Catal. 2012, 2, 2269–2280. [Google Scholar] [CrossRef]
- Borfecchia, E.; Mino, L.; Groppo, E.; Bordiga, S.; Bugaev, A.L.; Budnyk, A.; Lomachenko, K.A.; Guda, A.A.; Soldatov, M.A.; Soldatov, A.V.; et al. Spectroscopic methods in catalysis and their application in well-defined nanocatalysts. Stud. Surf. Sci. Catal. 2017, 177, 221–284. [Google Scholar]
- Beale, A.M.; Jacques, S.D.; Weckhuysen, B.M. Chemical imaging of catalytic solids with synchrotron radiation. Chem. Soc. Rev. 2010, 39, 4656–4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weckhuysen, B.M. Chemical imaging of spatial heterogeneities in catalytic solids at different length and time scales. Angew. Chem. Int. Ed. 2009, 48, 4910–4943. [Google Scholar] [CrossRef] [PubMed]
- Grunwaldt, J.-D.; Schroer, C.G. Hard and soft X-ray microscopy and tomography in catalysis: Bridging the different time and length scales. Chem. Soc. Rev. 2010, 39, 4741–4753. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, T.L.; Price, S.W.T.; Benzi, F.; Baier, S.; Klumpp, M.; Dittmeyer, R.; Schwieger, W.; Grunwaldt, J.D. In situ multimodal 3D chemical imaging of a hierarchically structured core@shell catalyst. J. Am. Chem. Soc. 2017, 139, 7855–7863. [Google Scholar] [CrossRef] [PubMed]
- Price, S.W.; Martin, D.J.; Parsons, A.D.; Slawinski, W.A.; Vamvakeros, A.; Keylock, S.J.; Beale, A.M.; Mosselmans, J.F. Chemical imaging of Fischer-Tropsch catalysts under operating conditions. Sci. Adv. 2017, 3, e1602838. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.; Rochet, A.; Ogel, E.; Casapu, M.; Ritter, S.; Ogurreck, M.; Grunwaldt, J.-D. Aging of a Pt/Al2O3 exhaust gas catalyst monitored by quasi in situ X-ray micro computed tomography. RSC Adv. 2015, 5, 6893–6905. [Google Scholar] [CrossRef]
- Bare, S.R.; Charochak, M.E.; Kelly, S.D.; Lai, B.; Wang, J.; Chen-Wiegart, Y.-C.K. Characterization of a fluidized catalytic cracking catalyst on ensemble and individual particle level by X-ray micro- and nanotomography, micro-X-ray fluorescence, and micro-X-ray diffraction. ChemCatChem 2014, 6, 1427–1437. [Google Scholar] [CrossRef]
- Da Silva, J.C.; Mader, K.; Holler, M.; Haberthur, D.; Diaz, A.; Guizar-Sicairos, M.; Cheng, W.C.; Shu, Y.; Raabe, J.; Menzel, A.; et al. Assessment of the 3D pore structure and individual components of preshaped catalyst bodies by X-ray imaging. ChemCatChem 2015, 7, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Ihli, J.; Jacob, R.R.; Holler, M.; Guizar-Sicairos, M.; Diaz, A.; da Silva, J.C.; Ferreira Sanchez, D.; Krumeich, F.; Grolimund, D.; Taddei, M.; et al. A three-dimensional view of structural changes caused by deactivation of fluid catalytic cracking catalysts. Nat. Commun. 2017, 8, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meirer, F.; Kalirai, S.; Morris, D.; Soparawalla, S.; Liu, Y.; Mesu, G.; Andrews, J.C.; Weckhuysen, B.M. Life and death of a single catalytic cracking particle. Sci. Adv. 2015, 1, e1400199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meirer, F.; Krest, C.M.; Webb, S.; Weckhuysen, B.M. Relating structure and composition with accessibility of a single catalyst particle using correlative 3-dimensional micro-spectroscopy. Nat. Commun. 2016, 7, 12634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, S.S. Infrared and Raman spectroscopic studies of the polymorphic forms of nickel, cobalt and ferric molybdates. Infrared Phys. 1987, 27, 309–315. [Google Scholar] [CrossRef]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.-C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3 xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Tichý, J. Oxidation of acrolein to acrylic acid over vanadium-molybdenum oxide catalysts. Appl. Catal. A 1997, 157, 363–385. [Google Scholar] [CrossRef]
- Bui, L.; Bhan, A. Mechanisms for C–C bond cleavage and formation during acrolein production on a mixed metal oxide catalyst. Appl. Catal. A 2017, 546, 87–95. [Google Scholar] [CrossRef]
- Panov, G.I.; Starokon, E.V.; Parfenov, M.V.; Wei, B.; Sobolev, V.I.; Pirutko, L.V. Quasi-catalytic identification of intermediates in the oxidation of propene to acrolein over a multicomponent Bi–Mo catalyst. ACS Catal. 2018, 8, 1173–1177. [Google Scholar] [CrossRef]
- Zhang, W.; Trunschke, A.; Schlögl, R.; Su, D. Real-space observation of surface termination of a complex metal oxide catalyst. Angew. Chem. Int. Ed. 2010, 49, 6084–6089. [Google Scholar] [CrossRef] [PubMed]
- Engeldinger, J.; Radnik, J.; Kreyenschulte, C.; Devred, F.; Gaigneaux, E.M.; Fischer, A.; Zanthoff, H.-W.; Bentrup, U. Probing the structural changes and redox behavior of mixed molybdate catalysts under ammoxidation conditions: An operando Raman spectroscopy study. ChemCatChem 2016, 8, 976–983. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. Molecular structure of molybdenum oxide in bismuth molybdates by Raman spectroscopy. J. Phys. Chem. 1991, 95, 10763–10772. [Google Scholar] [CrossRef]
- Antonio, M.R.; Teller, R.G.; Sandstrom, D.R.; Mehicic, M.; Brazdil, J.F. Structural characterization of bismuth molybdates by X-ray absorption spectroscopy and powder neutron diffraction profile analysis. J. Phys. Chem. 1988, 92, 2939–2944. [Google Scholar] [CrossRef]
- Li, H.; Li, K.; Wang, H. Hydrothermal synthesis and photocatalytic properties of bismuth molybdate materials. Mater. Chem. Phys. 2009, 116, 134–142. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Chaturvedi, S.; Hanson, J.C.; Albornoz, A.; Brito, J.L. Electronic properties and phase transformations in CoMoO4 and NiMoO4: XANES and time-resolved synchrotron XRD studies. J. Phys. Chem. B 1998, 102, 1347–1355. [Google Scholar] [CrossRef]
- Beale, A.M.; Jacques, S.D.M.; Sacaliuc-Parvalescu, E.; O’Brien, M.G.; Barnes, P.; Weckhuysen, B.M. An iron molybdate catalyst for methanol to formaldehyde conversion prepared by a hydrothermal method and its characterization. Appl. Catal. A 2009, 363, 143–152. [Google Scholar] [CrossRef]
- Price, S.W.; Ignatyev, K.; Geraki, K.; Basham, M.; Filik, J.; Vo, N.T.; Witte, P.T.; Beale, A.M.; Mosselmans, J.F. Chemical imaging of single catalyst particles with scanning μ-XANES-CT and μ-XRF-CT. Phys. Chem. Chem. Phys. 2015, 17, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, S.D.; Di Michiel, M.; Beale, A.M.; Sochi, T.; O’Brien, M.G.; Espinosa-Alonso, L.; Weckhuysen, B.M.; Barnes, P. Dynamic X-ray diffraction computed tomography reveals real-time insight into catalyst active phase evolution. Angew. Chem. Int. Ed. 2011, 50, 10148–10152. [Google Scholar] [CrossRef] [PubMed]
- Briois, V.; Fontaine, C.L.; Belin, S.; Barthe, L.; Th, M.; Pinty, V.; Carcy, A.; Girardot, R.; Fonda, E. Rock: The new Quick-EXAFS beamline at SOLEIL. J. Phys. Conf. Ser. 2016, 712, 012149. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Athena, artemis, hephaestus: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Criado, G.; Villanova, J.; Tucoulou, R.; Salomon, D.; Suuronen, J.P.; Laboure, S.; Guilloud, C.; Valls, V.; Barrett, R.; Gagliardini, E.; et al. ID16B: A hard X-ray nanoprobe beamline at the ESRF for nano-analysis. J. Synchrotron Radiat. 2016, 23, 344–352. [Google Scholar] [CrossRef] [PubMed]






| Sample Notation | Molar Ratio/% | Bi 2-eha * /g | Mo 2-eha * /g | Co 2-eha * /g | Fe(acac)3 * /g | |||
|---|---|---|---|---|---|---|---|---|
| Bi | Mo | Co | Fe | |||||
| FSP-1 | 7.2 | 59.2 | 24.8 | 8.8 | 1.14 | 9.47 | 3.29 | 0.77 |
| FSP-2 | 4.2 | 50.0 | 33.3 | 12.5 | 0.67 | 7.99 | 4.43 | 1.10 |
| Data Type | Characterisation Method | FSP-1F | FSP-1R |
|---|---|---|---|
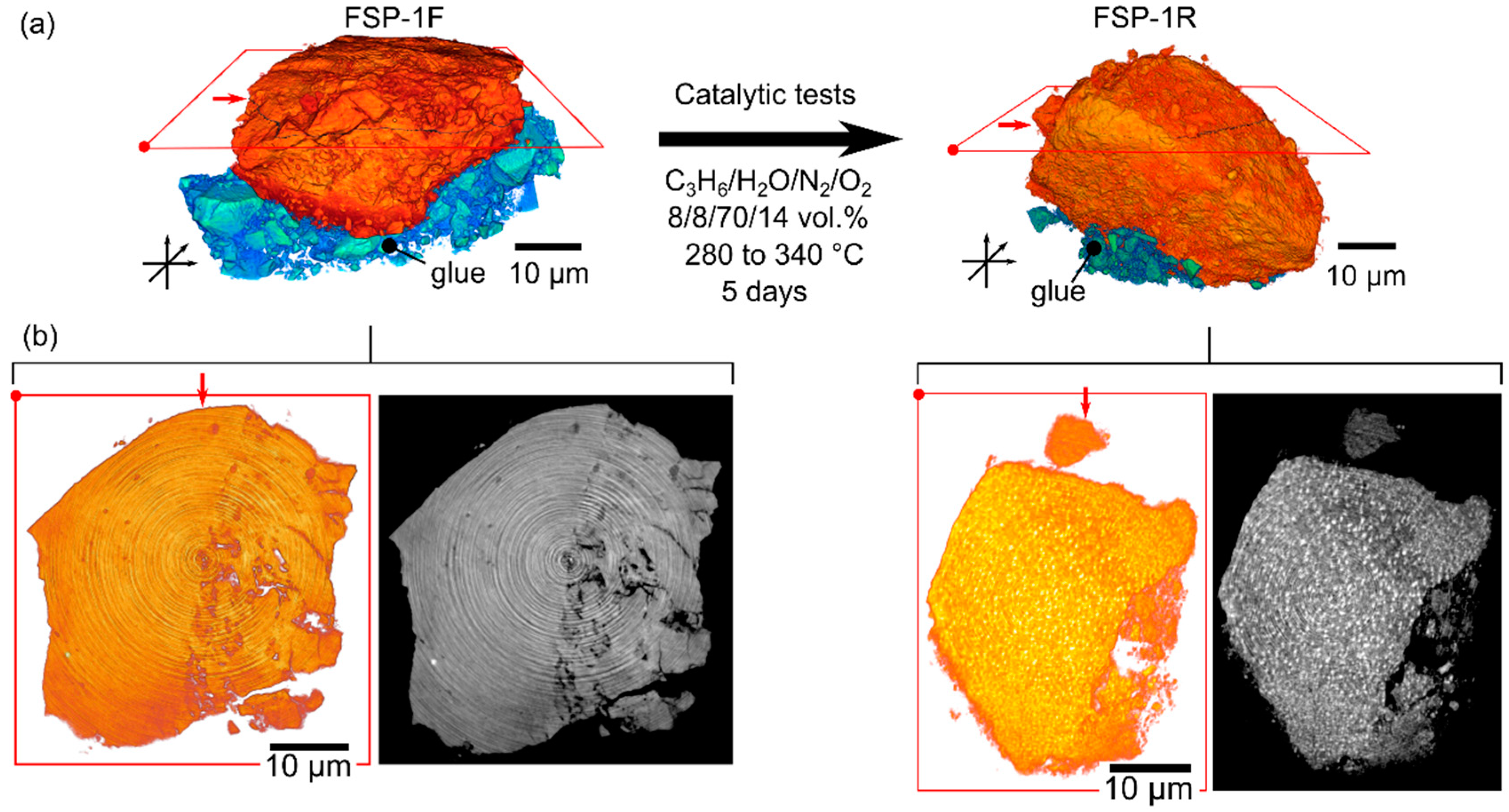
| Integral properties | Structure (TEM, N2-physisorption) | Nanoparticles (5–30 nm), SBET = 52 m2 g–1 | Nanoparticles (50–200 nm), SBET = 8 m2 g–1 |
| Bulk elemental (ICP-OES) | Observed values match expected values; Bi: 6.9; Mo: 58.2; Co: 26.5; Fe: 8.4 mol% | No change observed; Bi: 7.0; Mo: 58.0; Co: 26.5; Fe: 8.5 mol% | |
| Surface elemental (XPS) | Surface enriched in Co and Fe, compared to bulk; Bi: 3.0; Mo: 42.6; Co: 35.9; Fe: 18.6 mol% | Surface enriched in Co and Fe, surface Mo/Bi ratio further increased; Bi: 1.5; Mo: 40.9; Co: 34.3; Fe: 23.2 mol% | |
| Crystalline phases (XRD) | Nanocrystalline/amorphous material; Co0.7Fe0.3MoO4, α-CoMoO4, β-CoMoO4, MoO3 | Increased crystallinity and formation of phases; α-Bi2Mo3O12, Co0.7Fe0.3MoO4, β-CoMoO4, Bi3FeMo2O12, MoO3 | |
| Amorphous and crystalline phases (Raman spectroscopy) | Nanocrystalline/amorphous material; α-CoMoO4, MoO3 | Increased crystallinity, only main phases visible; β-CoMoO4, MoO3 | |
| Local probe: Oxidation state, phase type, metal coordination (XANES) | Mo6+: tetrahedral coord. (as in α-Bi2Mo3O12, β-Bi2Mo2O9, β-CoMoO4) Bi3+ Fe2+: tetrahedral coord. (FeMoO4) Co2+: tetrahedral coord. (β-CoMoO4) | Mo6+: more octahedral coord., inc. crystallinity (γ-Bi2MoO6, α-CoMoO4, MoO3) Bi3+ Fe2+(3+): inc. octahedral coord. (FeMoO4, Fe2Mo3O12) Co2+: Slightly inc. octahedral coord. (α/β-CoMoO4) | |
| Catalytic activity | High conversion (reference catalyst in brackets), e.g., XPropylene = 88% (5%) at 320 °C and weight hourly space velocity (WHSV) = 1.14 h–1, good selectivity at similar conversion, e.g., for FSP-2 SAcrolein = 87% (93%), SAcrylic acid = 0% (2%) at XPropylene = 13% (13%); stable conversion over 5 days | ||
| Structural fitting | Local probe: Lattice neighbours and phases (EXAFS) | Mo: paths of Mo-O, Mo-Mo, Mo-Fe, and Mo-Co Bi: only Bi-O coord. in first shell, highly amorphous Fe: paths of Fe-O, Fe-Fe, and Fe-Mo, tetrahedral coord. Co: paths of β-CoMoO4 | Mo: inc. crystallinity and long-range order Bi: inc. long-range order, incl. Bi-Fe and/or Bi-Co (Bi3FeMo2O12) Fe: incl. order and Fe-O coord. Co: inc. order, incl. crystallinity, exclusive Co-Mo character (β-CoMoO4 and weak α-CoMoO4) |
| Spatially resolved imaging | 2D-Elemental distribution (TEM–EDX) | Homogeneous distribution, similar to bulk composition | Heterogeneous distribution on nm scale mainly due to Bi segregation; elemental ratios found correspond to α-Bi2Mo3O12, Co0.7Fe0.3MoO4, and MoO3 phases |
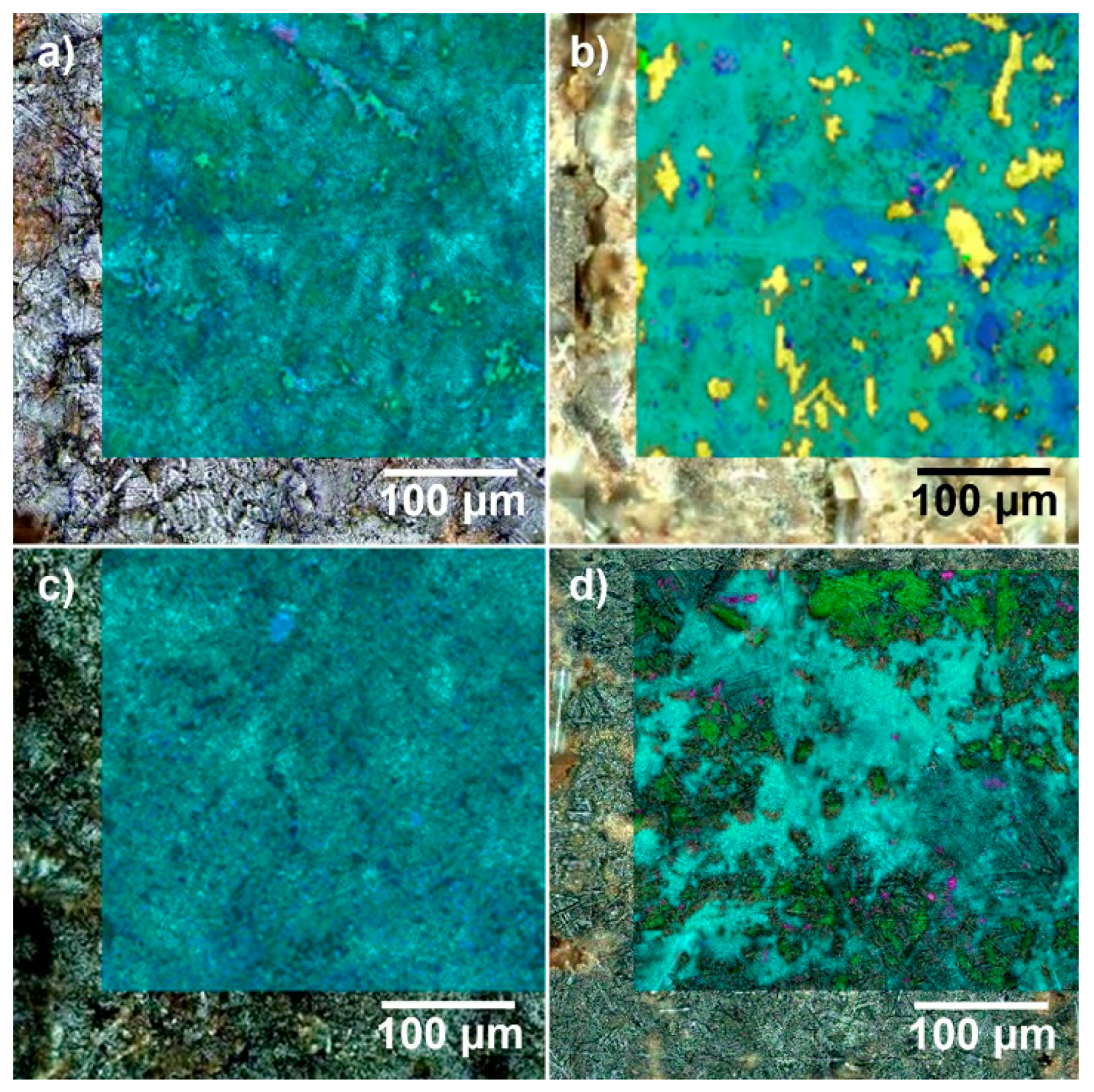
| 2D-Phase distribution (Raman mapping) | Homogeneous distribution of α-CoMoO4 and minor amounts of MoO3 | Heterogeneous phase distribution on µm scale; main (α/β CoMoO4, MoO3) and minor phases (α-Bi2Mo3O12, FeMoO4, Fe2Mo3O12) | |
| 3D-Structure and elemental distribution (Nanotomography) | Homogeneous distribution of metals within catalyst particle, uniform phase contrast | Heterogeneity of metals and phase contrast; aggregation of Bi within whole catalyst volume on nm to µm scale | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprenger, P.; Sheppard, T.L.; Suuronen, J.-P.; Gaur, A.; Benzi, F.; Grunwaldt, J.-D. Structural Evolution of Highly Active Multicomponent Catalysts for Selective Propylene Oxidation. Catalysts 2018, 8, 356. https://doi.org/10.3390/catal8090356
Sprenger P, Sheppard TL, Suuronen J-P, Gaur A, Benzi F, Grunwaldt J-D. Structural Evolution of Highly Active Multicomponent Catalysts for Selective Propylene Oxidation. Catalysts. 2018; 8(9):356. https://doi.org/10.3390/catal8090356
Chicago/Turabian StyleSprenger, Paul, Thomas L Sheppard, Jussi-Petteri Suuronen, Abhijeet Gaur, Federico Benzi, and Jan-Dierk Grunwaldt. 2018. "Structural Evolution of Highly Active Multicomponent Catalysts for Selective Propylene Oxidation" Catalysts 8, no. 9: 356. https://doi.org/10.3390/catal8090356
APA StyleSprenger, P., Sheppard, T. L., Suuronen, J.-P., Gaur, A., Benzi, F., & Grunwaldt, J.-D. (2018). Structural Evolution of Highly Active Multicomponent Catalysts for Selective Propylene Oxidation. Catalysts, 8(9), 356. https://doi.org/10.3390/catal8090356








