N-Doped K3Ti5NbO14@TiO2 Core-Shell Structure for Enhanced Visible-Light-Driven Photocatalytic Activity in Environmental Remediation
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. Morphology
2.3. XPS Studies
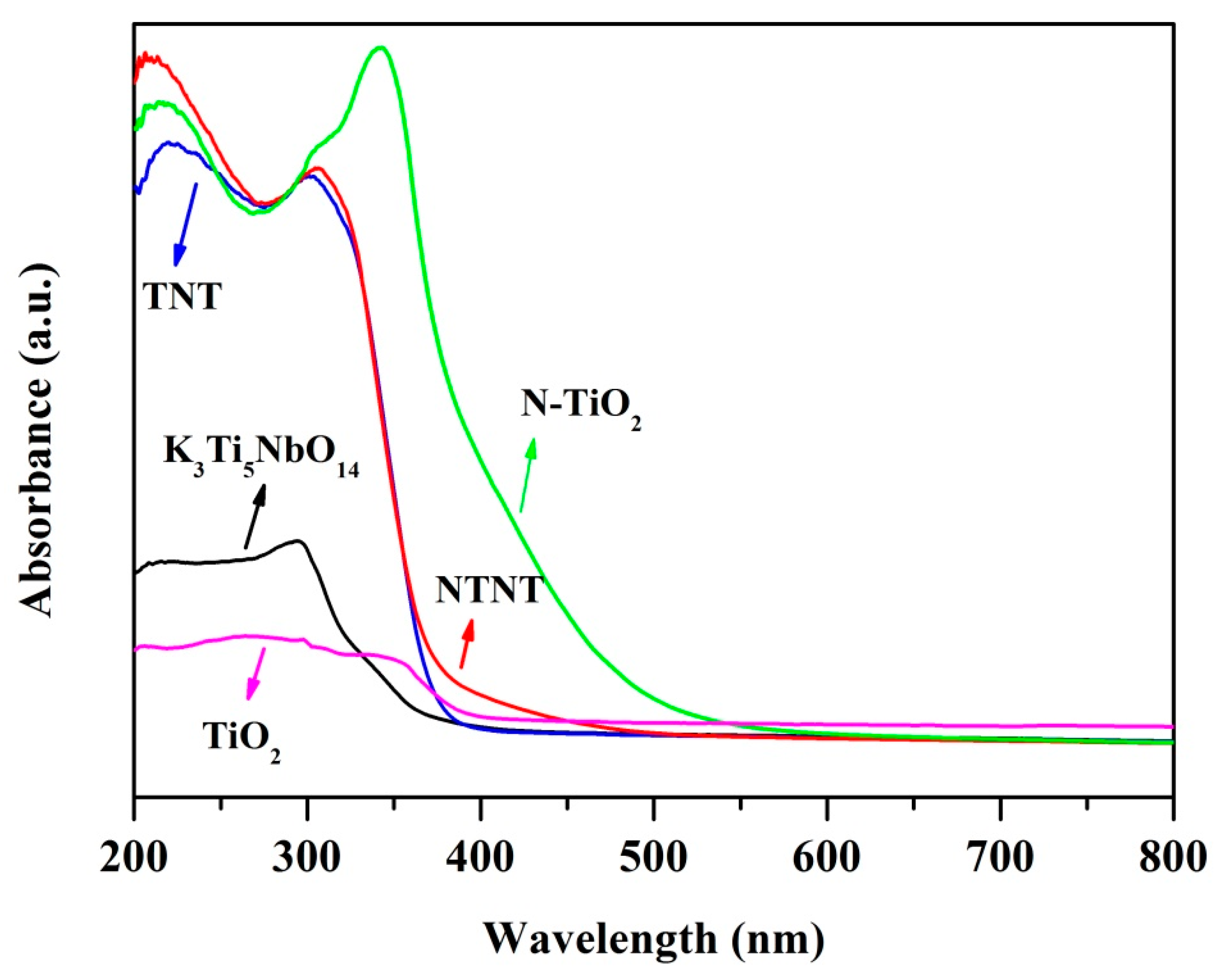
2.4. UV–Vis Diffuse Reflectance Spectra
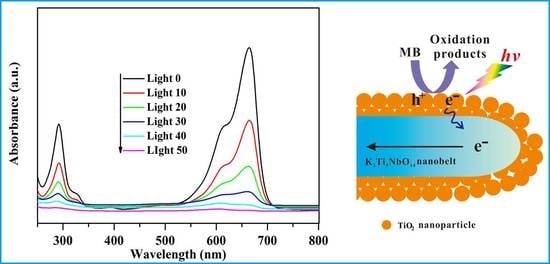
2.5. Photocatalytic Performance
2.6. Photocatalytic Mechanism Discussion
3. Materials and Methods
3.1. Preparation of N-doped K3Ti5NbO14@TiO2 Nanocomposite
3.2. Characterization
3.3. Photocatalytic Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stolarczyk, J.K.; Bhattacharyya, S.; Polavarapu, L.; Feldmann, J. Challenges and prospects in solar water splitting and CO2 reduction with inorganic and hybrid nanostructures. ACS Catal. 2018, 8, 3602–3635. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lai, C.; Zhang, C.; Zeng, G.; Huang, D.; Cheng, M.; Hu, L.; Xiong, W.; Chen, M.; Wang, J.; et al. Semiconductor/boron nitride composites: Synthesis, properties, and photocatalysis applications. Appl. Catal. B Environ. 2018, 238, 6–18. [Google Scholar] [CrossRef]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial activity of photocatalysts: Fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B Environ. 2018, 225, 51–75. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Zhai, Z.; Hu, C.H.; Yang, X.Y.; Zhang, L.H.; Liu, C.; Fan, Y.N.; Hou, W.H. Nitrogen-doped mesoporous nanohybrids of TiO2 nanoparticles and HTiNbO5 nanosheets with a high visible-light photocatalytic activity and a good biocompatibility. J. Mater. Chem. 2012, 22, 19122–19131. [Google Scholar] [CrossRef]
- Liu, C.; Liang, J.Y.; Han, R.R.; Wang, Y.Z.; Zhao, J.; Huang, Q.J.; Chen, J.; Hou, W.H. S-doped Na2Ti6O13@TiO2 core-shell nanorods with enhanced visible light photocatalytic performance. Phys. Chem. Chem. Phys. 2015, 17, 15165–15172. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Wang, W.; Chen, J.J.; Liu, C.; Li, Q.; Zhang, X.; Li, W.W.; Si, Y.; Yu, H.Q. Epitaxial facet junction on TiO2 single crystals for efficient photocatalytic water splitting. Energy Environ. Sci. 2018, 11, 6. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zeng, L.; Song, W.; Qin, Z.; Zeng, D.; Xie, C. In situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal. B Environ. 2017, 202, 489–499. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Ferraro, S.; Seghetti, C.; Minicucci, M.; Gunnella, R.; Cicco, A.D. Enhancement of visible-light photoactivity by polypropylene coated plasmonic Au/TiO2 for dye degradation in water solution. Appl. Surf. Sci. 2018, 441, 575–587. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.M.; Jo, Y.K.; Kim, I.Y.; Hwang, S.J. A facile exfoliation-crystal growth route to multicomponent Ag2CO3/Ag-Ti5NbO14 nanohybrids with improved visible light photocatalytic activity. Dalton. Trans. 2014, 43, 10566–10573. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, H.; Dong, P.Y.; Hou, H.; Xu, Q.; Chen, X.; Xi, X.; Hou, W. Ordered layered N-doped KTiNbO5/g-C3N4 heterojunction with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2018, 228, 54–63. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Wang, J.; Xu, Q.; Chen, X.; Wang, C.; Xi, X.; Hou, W. N-doped CsTi2NbO7@g-C3N4 core-shell nanobelts with enhanced visible light photocatalytic activity. Mater. Lett. 2018, 217, 235–238. [Google Scholar] [CrossRef]
- Liu, C.; Han, R.; Ji, H.; Sun, T.; Zhao, J.; Chen, N.; Chen, J.; Guo, X.; Hou, W.; Ding, W. S-doped mesoporous nanocomposite of HTiNbO5 nanosheets and TiO2 nanoparticles with enhanced visible light photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 801–810. [Google Scholar] [CrossRef]
- Liu, C.; Wu, L.; Chen, J.; Liang, J.Y.; Li, C.S.; Ji, H.M.; Hou, W.H. The nanocomposite of polyaniline and nitrogen-doped layered HTiNbO5 with excellent visible-light photocatalytic performance. Phys. Chem. Chem. Phys. 2014, 16, 13409–13417. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Q.; Ji, M.; Zhu, H.; Hou, H.; Yang, Q.; Jiang, C.; Wang, J.; Tian, L.; Chen, J.; et al. Constructing Z-scheme charge separation in 2D layered porous BiOBr/graphitic C3N4 nanosheets nanojunction with enhanced photocatalytic activity. J. Alloy Compd. 2017, 723, 1121–1131. [Google Scholar] [CrossRef]
- Liu, C.; Xu, G.; Zhu, Y.; Xu, Q.; Yu, G.; Hou, H.; Xu, Q.; Xi, X.; Hou, W. In situ construction of layered K3Ti5NbO14/g-C3N4 composite for improving visible-light-driven photocatalytic performance. J. Mater. Sci. Mater. Electron. 2018, 29, 15859–15868. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Carbajo, J.; Bahamonde, A.; Crippa, M.; Polizzi, S.; Scotti, R.; Wahba, L.; Morazzoni, F. Photogenerated defects in shape-controlled TiO2 anatase nanocrystals: A probe to evaluate the role of crystal facets in photocatalytic processes. J. Am. Chem. Soc. 2011, 133, 17652–17661. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, Z.; Liu, J.; Li, Y.; Wu, M.; Tendeloo, G.V.; Su, B.-L. Blue-edge slow photons promoting visible-light hydrogen production on gradient ternary 3DOM TiO2-Au-CdS photonic crystals. Nano Energy 2018, 47, 266–274. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Zhu, B.; Yu, J.; Xu, J. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal. B Environ. 2018, 230, 194–202. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B.; Kim, H.; Lee, S.M.; Kim, H.J.; Lee, J. Influence of visible-light irradiation on physicochemical and photocatalytic properties of nitrogen-doped three-dimensional (3D) titanium dioxide. J. Hazard. Mater. 2013, 258–259, 10–18. [Google Scholar] [CrossRef]
- Ou, H.H.; Lo, S.L.; Liao, C.H. N-Doped TiO2 Prepared from microwave-assisted titanate nanotubes (NaxH2−xTi3O7): The effect of microwave irradiation during TNT synthesis on the visible light photoactivity of N-doped TiO2. J. Phys. Chem. C. 2011, 115, 4000–4007. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Huo, Y. Highly active TiO2N photocatalysts prepared by treating TiO2 precursors in NH3/ethanol fluid under supercritical conditions. J. Phys. Chem. B. 2006, 110, 1559–1565. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Photocatalytic degradation of Rhodamine B on C-, S-, N-, and Fe-doped TiO2 under visible-light irradiation. Appl. Catal. B Environ. 2009, 91, 657–662. [Google Scholar] [CrossRef]
- Pulsipher, D.J.V.; Martin, I.T.; Fisher, E.R. Controlled nitrogen doping and film colorimetrics in porous TiO2 materials using plasma processing. ACS. Appl. Mater. Interfaces 2010, 2, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Schafranek, R.; Payan, S.; Maglione, M.; Klein, A. Barrier height at (Ba, Sr)TiO3/Pt interfaces studied by photoemission. Phys. Rev. 2008, 77, 195310. [Google Scholar] [CrossRef]
- Jiang, D.; Wen, B.; Zhang, Y.; Jin, Y.; Li, D.; Chen, M. MoS2/SnNb2O6 2D/2D nanosheet heterojunctions with enhanced interfacial charge separation for boosting photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 536, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Lin, B.Z.; Chen, Y.L.; Zhang, K.Z.; Li, B.; Zhu, H. Pillaring and photocatalytic properties of mesoporous α-Fe2O3/titanate nanocomposites via an exfoliation and restacking route. J. Phys. Chem. Solids 2010, 71, 841–847. [Google Scholar] [CrossRef]
- Tian, L.; Sun, K.; Rui, Y.; Cui, W.; An, W. Facile synthesis of an Ag@AgBr nanoparticle-decorated K4Nb6O17 photocatalyst with improved photocatalytic properties. RSC Adv. 2018, 8, 29309–29320. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.L.; Shi, Y.X.; Xu, G.L.; Zhang, E.P.; Wang, H.B.; Kong, Z.; Xi, J.H.; Ji, Z.G. Anatase TiO2 nanosheets with coexposed {101} and {001} facets coupled with ultrathin SnS2 nanosheets as a face-to-face n-p-n dual heterojunction photocatalyst for enhancing photocatalytic activity. Appl. Surf. Sci. 2017, 420, 839–848. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, G.; Li, J.; Wu, X. 0D Bi nanodots/2D Bi3NbO7 nanosheets heterojunctions for efficient visible light photocatalytic degradation of antibiotics: Enhanced molecular oxygen activation and mechanism insight. Appl. Catal. B Environ. 2019, 240, 39–49. [Google Scholar] [CrossRef]
- Zhu, Y.; Marianov, A.; Xu, H.; Lang, C.; Jiang, Y. Bimetallic Ag–Cu supported on graphitic carbon nitride nanotubes for improved visible-light photocatalytic hydrogen production. ACS Appl. Mater. Interfaces 2018, 10, 9468–9477. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, S.; Liu, Y.; Liu, Q.; Zhang, Y. In situ preparation of highly stable polyaniline/W18O49 hybrid nanocomposite as efficient visible light photocatalyst for aqueous Cr(VI) reduction. J. Hazard. Mater. 2018, 353, 466–475. [Google Scholar] [CrossRef]
- Ye, F.; Li, H.; Yu, H.; Chen, S.; Quan, X. Constructing BiVO4-Au@CdS photocatalyst with energic charge-carrier-separation capacity derived from facet induction and Z-scheme bridge for degradation of organic pollutants. Appl. Catal. B Environ. 2018, 227, 258–265. [Google Scholar] [CrossRef]
- Rommozzi, E.; Zannotti, M.; Giovannetti, R.; D’Amato, C.A.; Ferraro, S.; Minicucci, M.; Gunnella, R.; Cicco, A.D. Reduced graphene oxide/TiO2 nanocomposite: From synthesis to characterization for efficient visible light photocatalytic applications. Catalysts 2018, 8, 598. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Minicucci, M.; Gunnella, R.; Cicco, A.D. Band gap implications on nano-TiO2 surface modification with ascorbic acid for visible light-active polypropylene coated photocatalyst. Nanomaterials 2018, 8, 599. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Wang, C.; Xu, Q.; Lv, H.; Chen, T.; Liu, C.; Xi, X. N-Doped K3Ti5NbO14@TiO2 Core-Shell Structure for Enhanced Visible-Light-Driven Photocatalytic Activity in Environmental Remediation. Catalysts 2019, 9, 106. https://doi.org/10.3390/catal9010106
Gao X, Wang C, Xu Q, Lv H, Chen T, Liu C, Xi X. N-Doped K3Ti5NbO14@TiO2 Core-Shell Structure for Enhanced Visible-Light-Driven Photocatalytic Activity in Environmental Remediation. Catalysts. 2019; 9(1):106. https://doi.org/10.3390/catal9010106
Chicago/Turabian StyleGao, Xin, Chen Wang, Qixiang Xu, Hongjie Lv, Ting Chen, Chao Liu, and Xinguo Xi. 2019. "N-Doped K3Ti5NbO14@TiO2 Core-Shell Structure for Enhanced Visible-Light-Driven Photocatalytic Activity in Environmental Remediation" Catalysts 9, no. 1: 106. https://doi.org/10.3390/catal9010106
APA StyleGao, X., Wang, C., Xu, Q., Lv, H., Chen, T., Liu, C., & Xi, X. (2019). N-Doped K3Ti5NbO14@TiO2 Core-Shell Structure for Enhanced Visible-Light-Driven Photocatalytic Activity in Environmental Remediation. Catalysts, 9(1), 106. https://doi.org/10.3390/catal9010106