In Situ Regeneration and Deactivation of Co-Zn/H-Beta Catalysts in Catalytic Reduction of NOx with Propane
Abstract
:1. Introduction
2. Results and Discussion
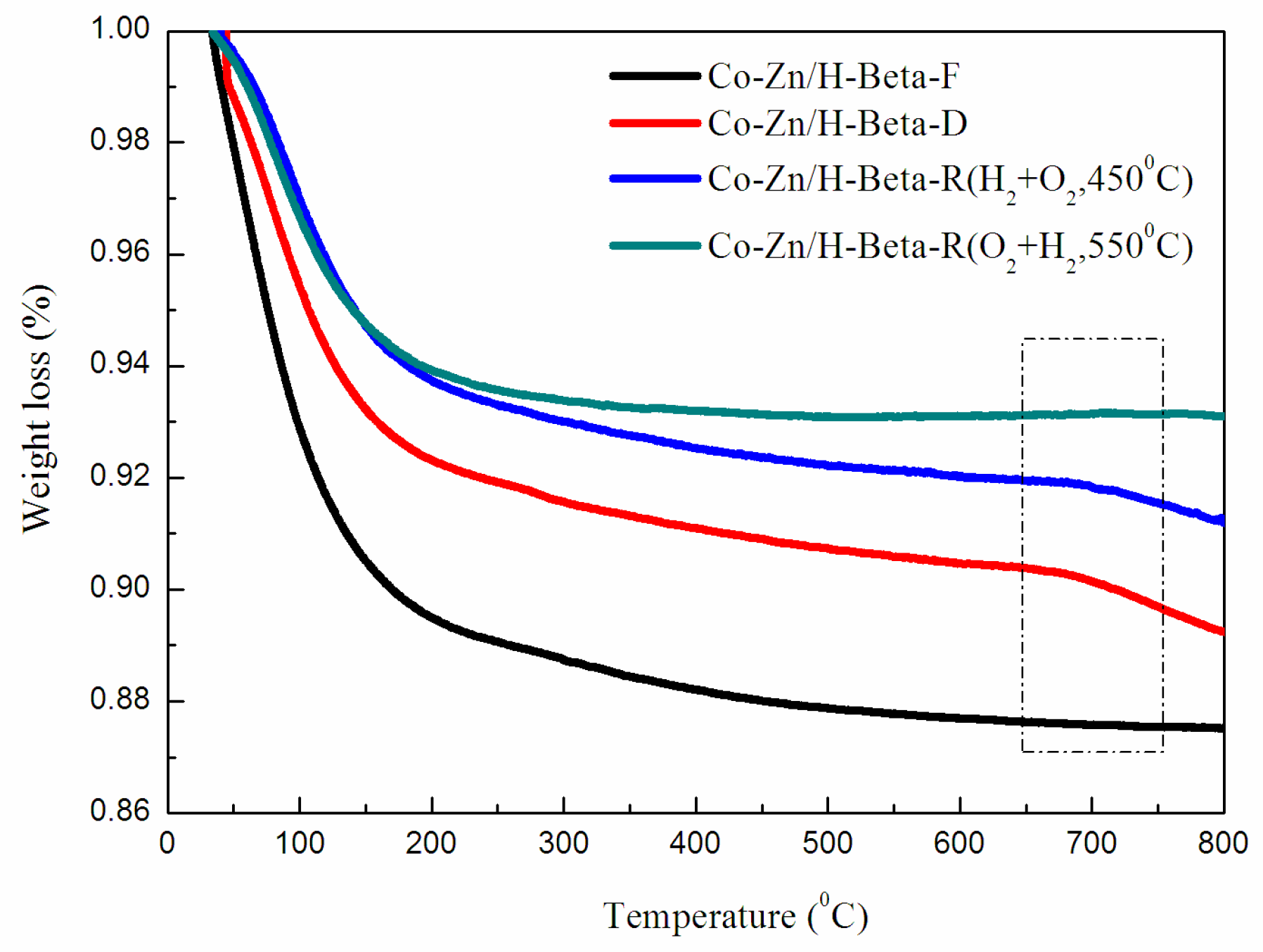
2.1. Stability of Catalyst
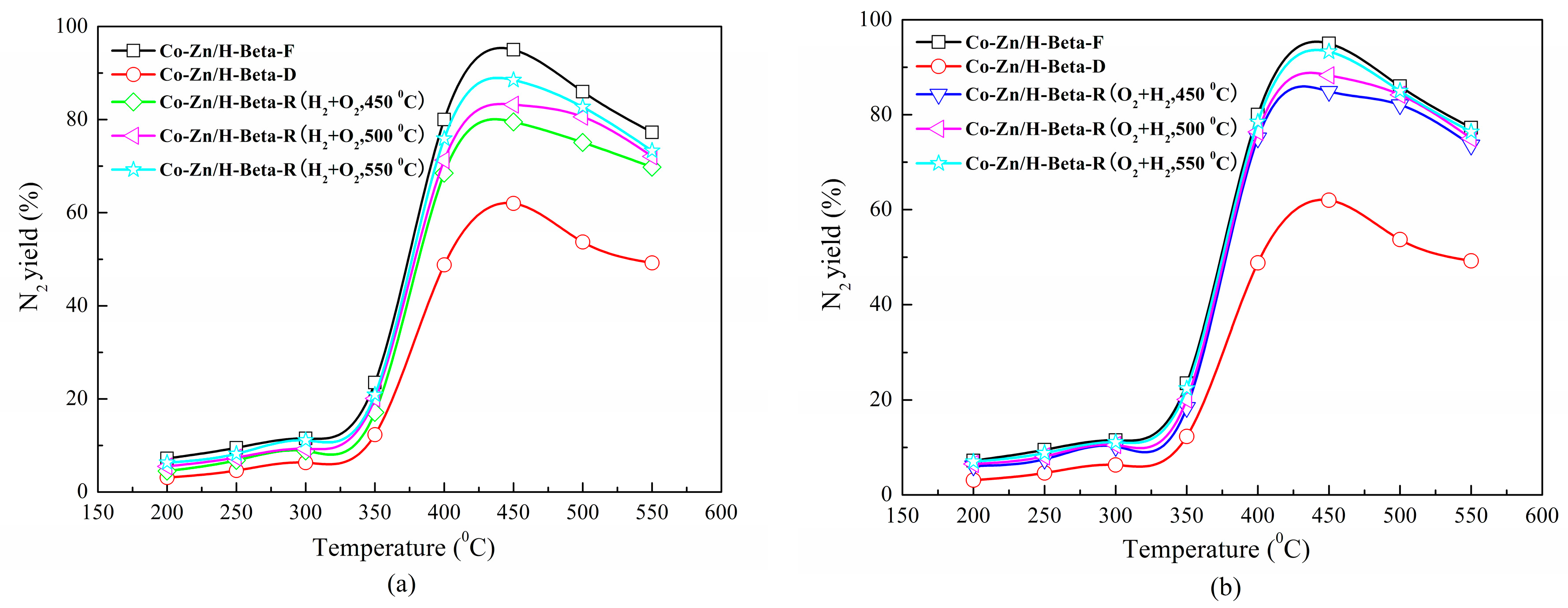
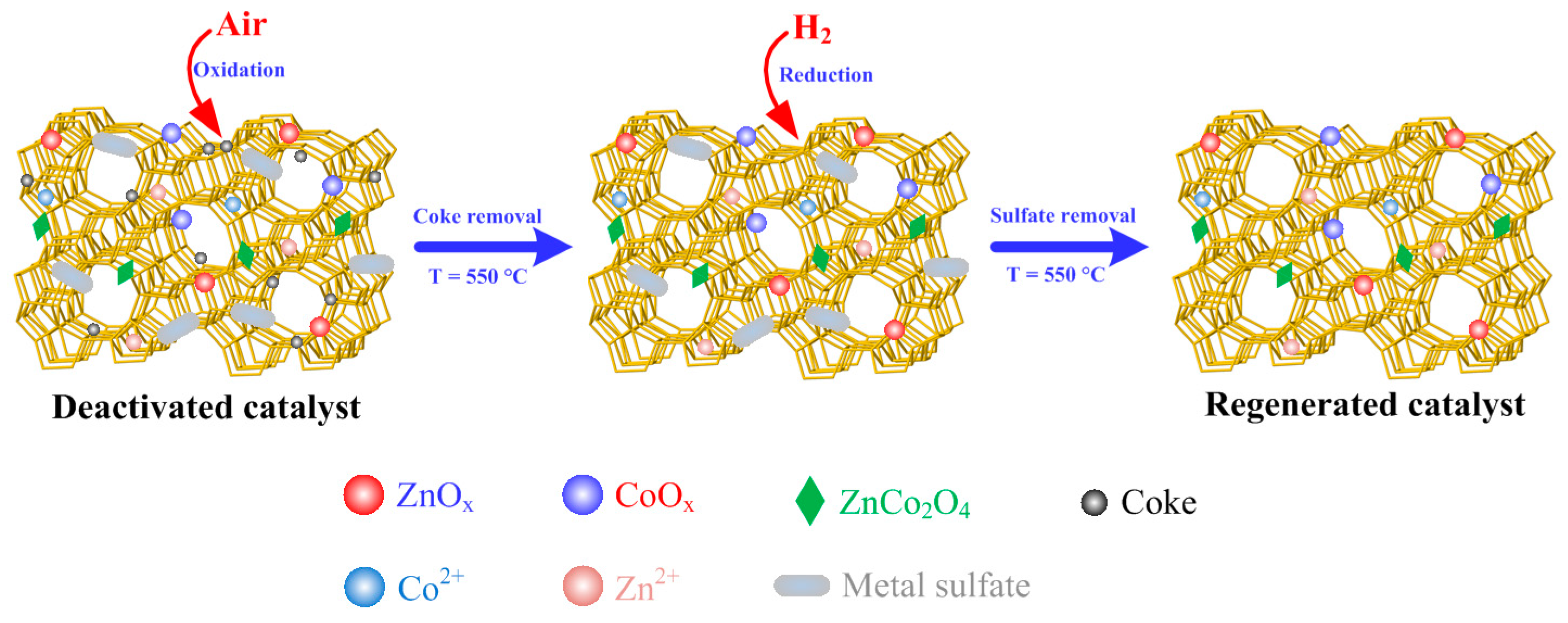
2.2. Regeneration Performance
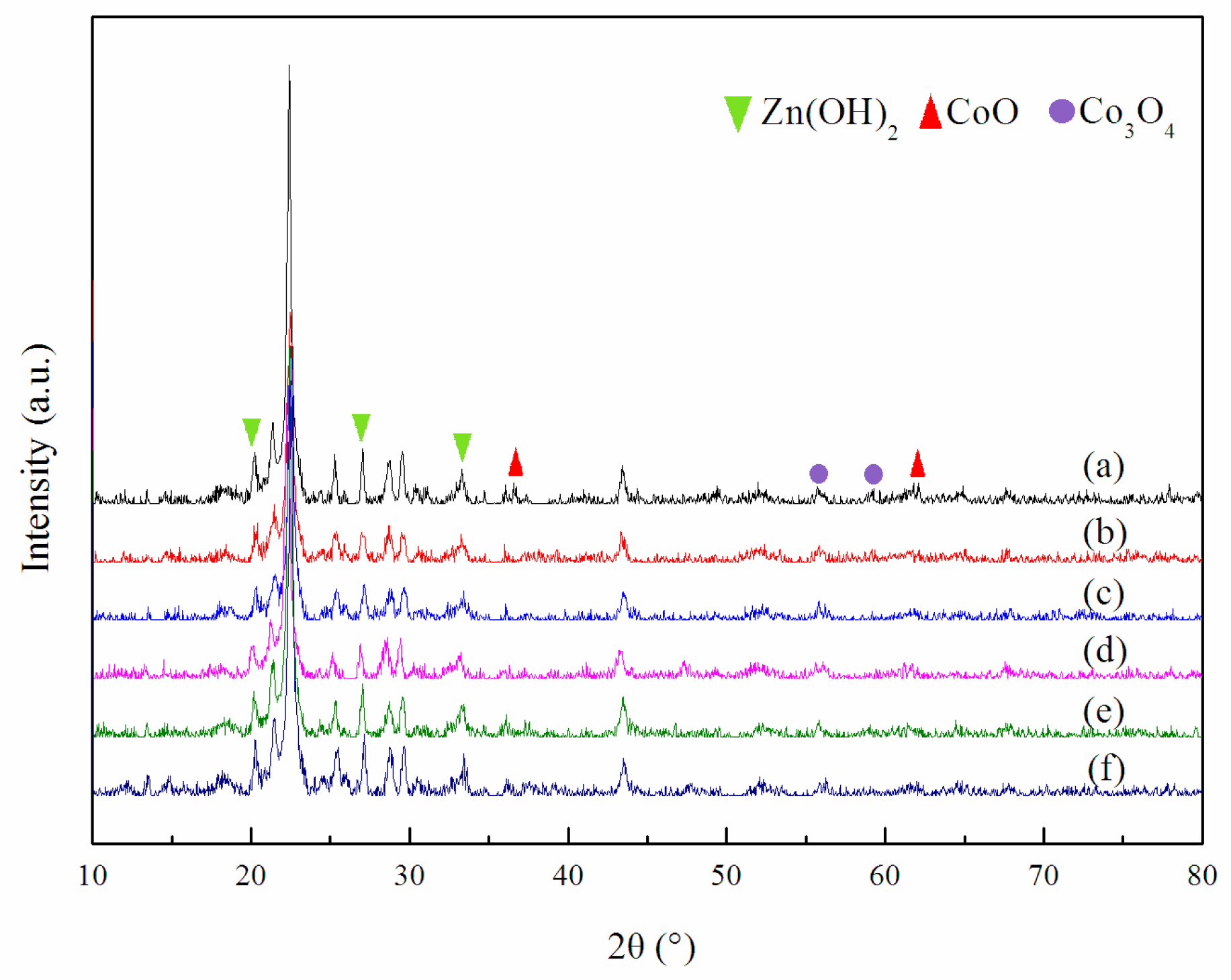
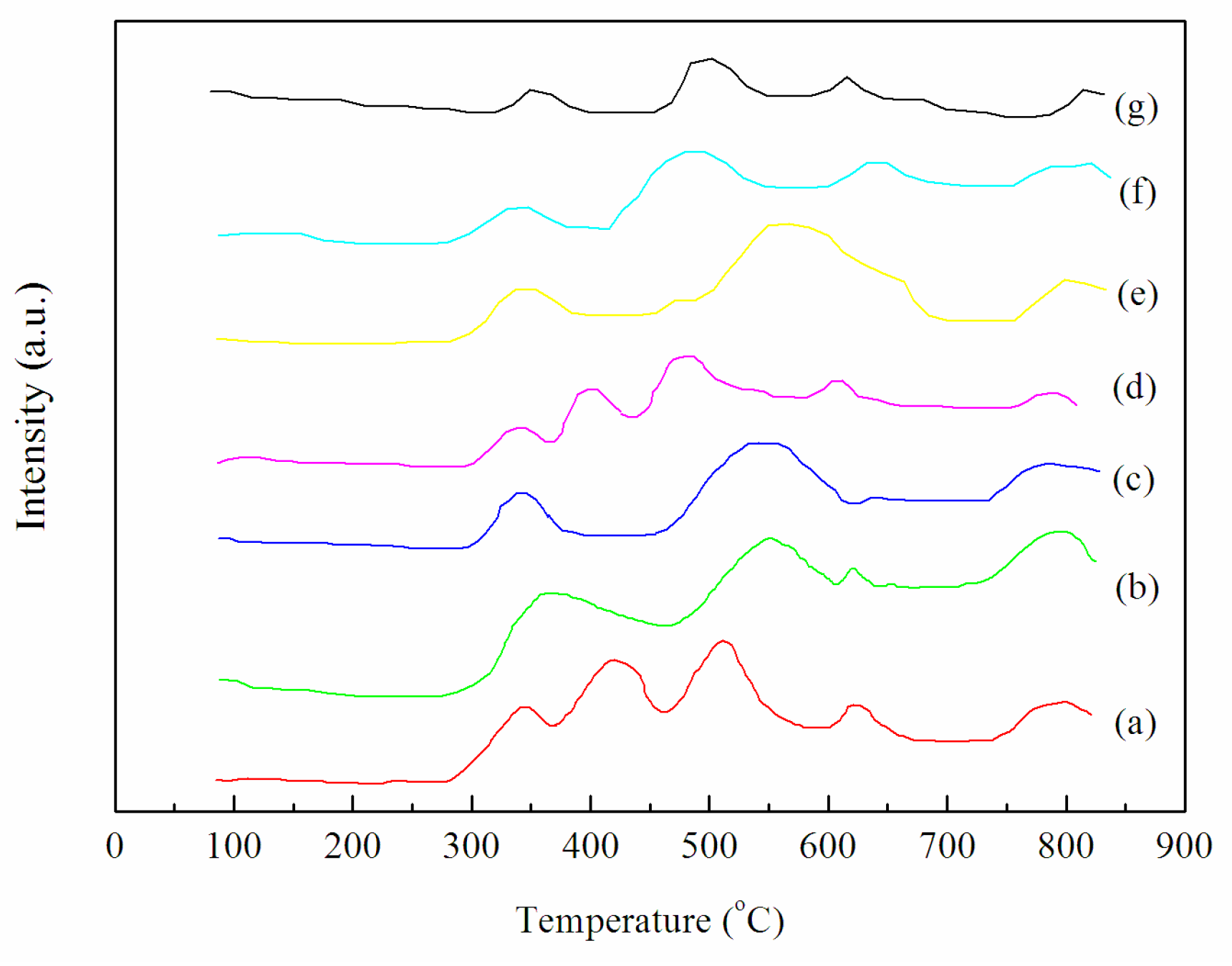
2.3. Structural and Textural Properties of Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Deactivation and Regeneration of Catalysts
3.3. Catalytic Activity Measurement
3.4. Catalyst Characterizations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mendes, A.N.; Ribeiro, M.F.; Henriques, C.; Da Costa, P. On the Effect of Preparation Methods of PdCe-MOR Catalysts as NOx CH4-SCR System for Natural Gas Vehicles Application. Catalysts 2015, 5, 1815–1830. [Google Scholar] [CrossRef]
- Tabata, T.; Ohtsuka, H.; Sabatino, L.M.F.; Bellussi, G. Selective catalytic reduction of NOx by propane on Co-loaded zeolites. Microporous Mesoporous Mater. 1998, 21, 517–524. [Google Scholar] [CrossRef]
- Tabata, T.; Kokitsu, M.; Ohtsuka, H.; Okada, O.; Sabatino, L.M.F.; Bellussi, G. Study on catalysts of selective reduction of NOx using hydrocarbons for natural gas engines. Catal. Today 1996, 27, 91–98. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Tabata, T.; Okada, O.; Sabatino, L.M.F.; Bellussi, G. A study on selective reduction of NOx by propane on Co-Beta. Catal. Lett. 1997, 44, 265–270. [Google Scholar] [CrossRef]
- Chen, H.H.; Shen, S.C.; Chen, X.Y.; Kawi, S. Selective catalytic reduction of NO over Co/beta-zeolite: Effects of synthesis condition of beta-zeolites, Co precursor, Co loading method and reductant. Appl. Catal. B Environ. 2004, 50, 37–47. [Google Scholar] [CrossRef]
- Čapek, L.; Dědeček, J.; Sazama, P.; Wichterlová, B. The decisive role of the distribution of Al in the framework of beta zeolites on the structure and activity of Co ion species in propane-SCR–NOx in the presence of water vapour. J. Catal. 2010, 272, 44–54. [Google Scholar] [CrossRef]
- Pietrzyk, P.; Dujardin, C.; Gora-Marek, K.; Granger, P.; Sojka, Z. Spectroscopic IR, EPR, and operando DRIFT insights into surface reaction pathways of selective reduction of NO by propene over the Co–BEA zeolite. Phys. Chem. Chem. Phys. 2012, 14, 2203–2215. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Henriques, C.; Ribeiro, M.F.; Ribeiro, F.R. SCR of NO with methane over Co-HBEA and PdCo-HBEA catalysts: The promoting effect of steaming over bimetallic catalyst. Catal. Today 2005, 107–108, 181–191. [Google Scholar] [CrossRef]
- Kubacka, A.; Janas, J.; Sulikowski, B. In/Co-ferrierite: A highly active catalyst for the CH4-SCR NO process under presence of steam. Appl. Catal. B Environ. 2006, 69, 43–48. [Google Scholar] [CrossRef]
- Ren, L.L.; Zhang, T.; Liang, D.B.; Xu, C.H.; Tang, J.W.; Lin, L.W. Effect of addition of Zn on the catalytic activity of a Co/HZSM-5 catalyst for the SCR of NOx with CH4. Appl. Catal. B Environ. 2002, 35, 317–321. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Liu, Y.Y.; Fan, W.B.; He, Y.; Li, R.F. Effect of SO2 on Catalytic Performance of CoH-FBZ for Selective Catalytic Reduction of NO by CH4 in The Presence of O2. Environ. Eng. Sci. 2007, 24, 292–300. [Google Scholar] [CrossRef]
- Chen, S.W.; Yan, X.L.; Wang, Y.; Chen, J.Q.; Pan, D.H.; Ma, J.H.; Li, R.F. Effect of SO2 on Co sites for NO-SCR by CH4 over Co-Beta. Catal. Today 2011, 175, 12–17. [Google Scholar] [CrossRef]
- Krishna, K.; Makkee, M. Coke formation over zeolites and CeO2-zeolites and its influence on selective catalytic reduction of NOx. Appl. Catal. B Environ. 2005, 59, 35–44. [Google Scholar] [CrossRef]
- Corbos, E.C.; Courtois, X.; Bion, N.; Marecot, P.; Duprez, D. Impact of the support oxide and Ba loading on the sulfur resistance and regeneration of Pt/Ba/support catalysts. Appl. Catal. B Environ. 2008, 80, 62–71. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J.H.; Wei, S.Y.; Chung, J.S.; Guo, Z.H. Sulfur Poisoning and Regeneration of NOx Storage-Reduction Cu/K2Ti2O5 Catalyst. Ind. Eng. Chem. Res. 2010, 49, 7330–7335. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Anderson, J.A. Influence of reductant on the regeneration of SO2-poisoned Pt/Ba/Al2O3 NOx storage and reduction catalyst. J. Catal. 2004, 228, 243–253. [Google Scholar] [CrossRef]
- Tanaka, T.; Amano, K.; Dohmae, K.; Takahashi, N.; Shinjoh, H. Studies on the regeneration of sulfur-poisoned NOx storage and reduction catalysts, including a Ba composite oxide. Appl. Catal. A Gen. 2013, 455, 16–24. [Google Scholar] [CrossRef]
- Le Phuc, N.; Corbos, E.C.; Courtois, X.; Can, F.; Marecot, P.; Duprez, D. NOx storage and reduction properties of Pt/CexZr1−xO2 mixed oxides: Sulfur resistance and regeneration, and ammonia formation. Appl. Catal. B Environ. 2009, 93, 12–21. [Google Scholar] [CrossRef]
- Doronkin, D.E.; Khan, T.S.; Bligaard, T.; Fogel, S.; Gabrielsson, P.; Dahl, S. Sulfur poisoning and regeneration of the Ag/γ-Al2O3 catalyst for H2-assisted SCR of NOx by ammonia. Appl. Catal. B Environ. 2012, 117, 49–58. [Google Scholar] [CrossRef]
- Chang, H.Z.; Li, J.H.; Yuan, J.; Chen, L.; Dai, Y.; Arandiyan, H.; Xu, J.Y.; Hao, J.M. Ge, Mn-doped CeO2–WO3 catalysts for NH3–SCR of NOx: Effects of SO2 and H2 regeneration. Catal. Today 2013, 201, 139–144. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Rodríguez, J.M.; Peral, A. Catalytic cracking of polyethylene over nanocrystalline HZSM-5: Catalyst deactivation and regeneration study. J. Anal. Appl. Pyrol. 2007, 79, 456–464. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Briones, L. Deactivation and regeneration of a Ni supported hierarchical Beta zeolite catalyst used in the hydroreforming of the oil produced by LDPE thermal cracking. Fuel 2013, 109, 679–686. [Google Scholar] [CrossRef]
- Madaan, N.; Gatla, S.; Kalevaru, V.N.; Radnik, J.; Lücke, B.; Brückner, A.; Martin, A. Deactivation and regeneration studies of a PdSb/TiO2 catalyst used in the gas-phase acetoxylation of toluene. J. Catal. 2011, 282, 103–111. [Google Scholar] [CrossRef]
- Villegas, J.I.; Kumar, N.; Heikkilä, T.; Lehto, V.P.; Salmi, T.; Murzin, D.Y. Isomerization of n-butane to isobutane over Pt-modified Beta and ZSM-5 zeolite catalysts: Catalyst deactivation and regeneration. Chem. Eng. J. 2006, 120, 83–89. [Google Scholar] [CrossRef]
- Mazzieri, V.A.; Pieck, C.L.; Vera, C.R.; Yori, J.C.; Grau, J.M. Analysis of coke deposition and study of the variables of regeneration and rejuvenation of naphtha reforming trimetallic catalysts. Catal. Today 2018, 133–135, 870–878. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Pan, H.; Li, W.; Shi, Y. SCR of NOx with C3H8 over Co/H-beta Modified by Zn at High GHSV. J. Chem. Eng. Chin. Univ. 2009, 23, 236–239. [Google Scholar]
- Khodayari, R. Regeneration of commercial TiO2-V2O5-WO3 SCR catalysts used in bio fuel plants. Appl. Catal. B Environ. 2001, 30, 87–99. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.F.; Yu, Y.K.; He, C.; Shen, Z.X.; Liu, H.X. Regeneration and sulfur poisoning behavior of In/H-BEA catalyst for NOx reduction by CH4. Appl. Surf. Sci. 2017, 401, 120–126. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Janas, J.; Machej, T.; Che, M. Selective catalytic reduction of NO by alcohols on Co- and Fe-Siβ catalysts. Catal. Today 2007, 119, 133–136. [Google Scholar] [CrossRef]
- Reddy, J.S.; Sayari, A. A simple method for the preparation of active Ti beta zeolite catalysts. Stud. Surf. Sci. Catal. 1995, 94, 309–316. [Google Scholar]
- Chung, K.S.; Massoth, F.E. Studies on molybdena-alumina catalysts: VII. Effect of cobalt on catalyst states and reducibility. J. Catal. 1980, 64, 320–331. [Google Scholar] [CrossRef]
- Stranick, M.S.; Houalla, M.; Hercules, D.M. Spectroscopic characterization of TiO2Al2O3 and CoAl2O3TiO2 catalysts. J. Catal. 1987, 106, 362–368. [Google Scholar] [CrossRef]
- Zsoldos, Z.; Guczi, L. Structure and Catalytic Activity of Alumina Supported Platinum Cobalt Bimetallic Catalysts. 3. Effect of Treatment on the Interface Layer. J. Phys. Chem. 1992, 96, 9393–9400. [Google Scholar] [CrossRef]
- Gomez-Garcia, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kung, M.C.; Sachtler, W.M.H.; Kung, H.H. Co/Al2O3 Lean NOx Reduction Catalyst. J. Catal. 1997, 172, 178–186. [Google Scholar] [CrossRef]
- Gong, T.; Qin, L.J.; Lu, J.; Feng, H. ZnO modified ZSM-5 and Y zeolites fabricated by atomic layer deposition for propane conversion. Phys. Chem. Chem. Phys. 2016, 18, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Kicir, N.; Tuken, T.; Erken, O.; Gumus, C.; Ufuktepe, Y. Nanostructured ZnO films in forms of rod, plate and flower: Electrodeposition mechanisms and characterization. Appl. Surf. Sci. 2016, 377, 191–199. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.Y.; Sachtler, W.M.H. Catalytic reduction of NOx by hydrocarbons over Co/ZSM-5 catalysts prepared with different methods. Appl. Catal. B Environ. 2000, 26, L227–L239. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.D. Charaterization of the phase and valency state of Co on Co/HZSM-5 catalysts. Chem. J. Chin. Univ. 2002, 23, 129–131. [Google Scholar]
- Shi, Y.; Su, Q.F.; Chen, J.; Wei, J.W.; Yang, J.T.; Pan, H. Combination non-thermal plasma and low temperature-C3H8-SCR over Co-In/H-beta catalyst for NOx abatement. Environ. Eng. Sci. 2009, 26, 1107–1113. [Google Scholar] [CrossRef]
- Pan, H.; Guo, Y.H.; Bi, H.T. NOx adsorption and reduction with C3H6 over Fe/zeolite catalysts: Effect of catalyst support. Chem. Eng. J. 2015, 280, 66–73. [Google Scholar] [CrossRef]


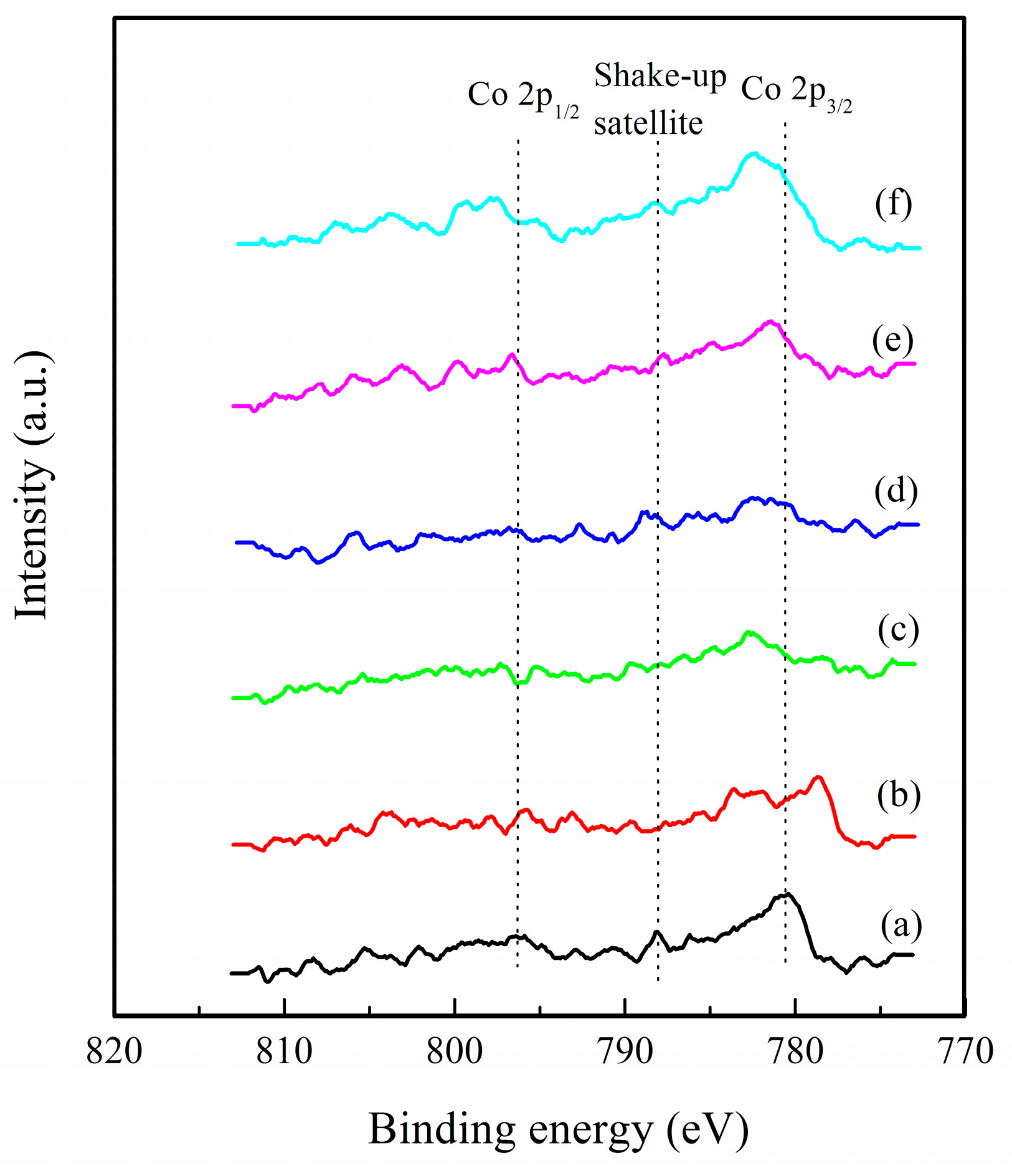
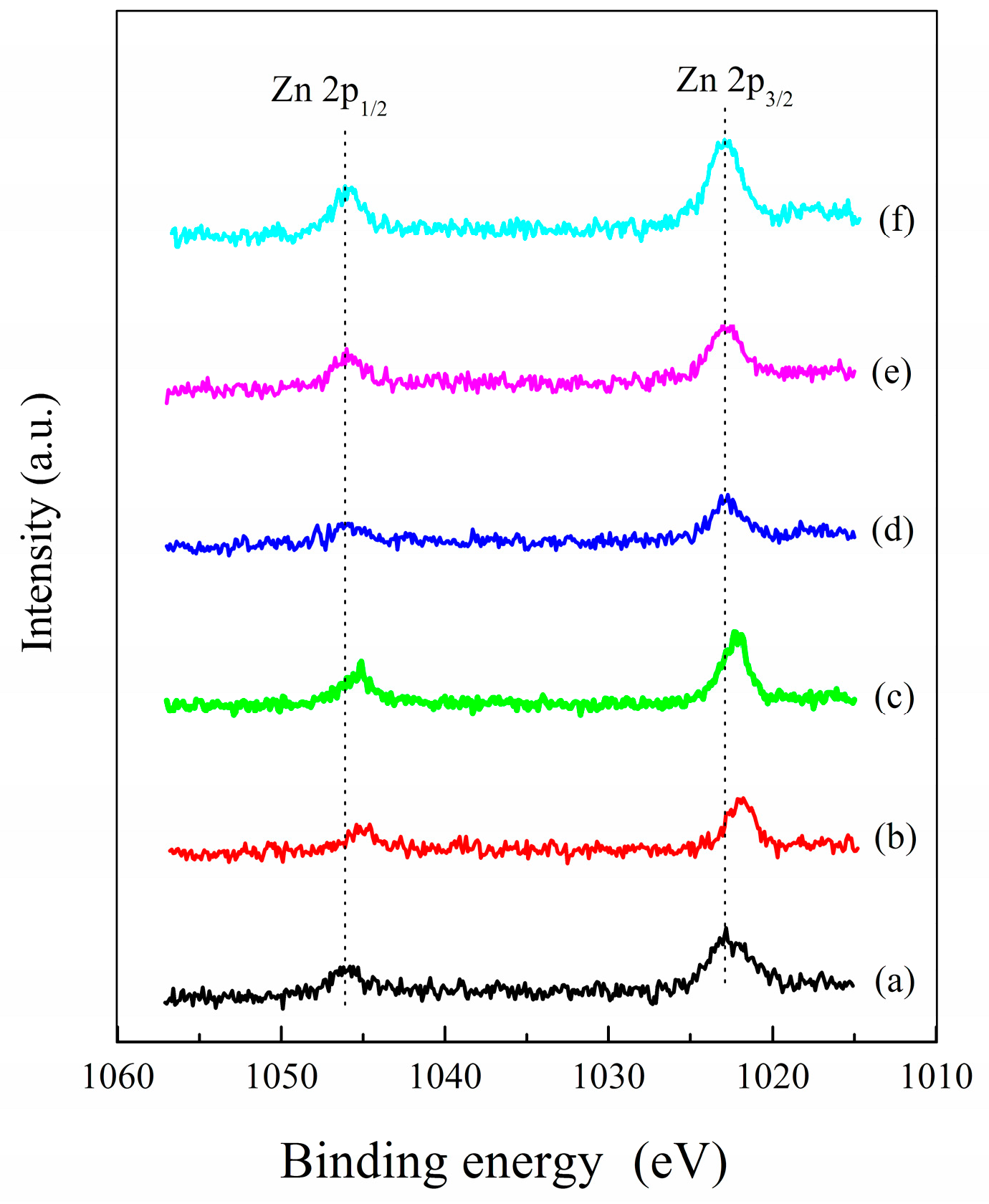
| Sample | SBETa (m2 g−1) | Smicrob (m2 g−1) | Vtotala (cm3 g−1) | Binding Energy of Co2p3/2 (eV) | Interval between Co2p3/2 and Co2p1/2 (eV) |
|---|---|---|---|---|---|
| Co-Zn/H-Beta-F | 428.42 | 338.88 | 0.17 | 780.5 | 15.8 |
| Co-Zn/H-Beta-D | 333.65 | 264.14 | 0.13 | 778.6 | 17.2 |
| Co-Zn/H-Beta-R (O2, 550 °C) | 384.56 | 298.22 | 0.15 | / | / |
| Co-Zn/H-Beta-R (H2, 550 °C) | 360.12 | 282.36 | 0.14 | / | / |
| Co-Zn/H-Beta-R (H2 + O2, 450 °C) | 388.50 | 305.97 | 0.15 | 782.7 | 14.6 |
| Co-Zn/H-Beta-R (O2 + H2, 450 °C) | 418.49 | 323.97 | 0.16 | 781.5 | 15.2 |
| Co-Zn/H-Beta-R (O2 + H2, 500 °C) | 419.91 | 336.47 | 0.17 | 781.4 | 15.1 |
| Co-Zn/H-Beta-R (O2 + H2, 550 °C) | 426.54 | 337.37 | 0.17 | 782.5 | 15.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Xu, D.; He, C.; Shen, C. In Situ Regeneration and Deactivation of Co-Zn/H-Beta Catalysts in Catalytic Reduction of NOx with Propane. Catalysts 2019, 9, 23. https://doi.org/10.3390/catal9010023
Pan H, Xu D, He C, Shen C. In Situ Regeneration and Deactivation of Co-Zn/H-Beta Catalysts in Catalytic Reduction of NOx with Propane. Catalysts. 2019; 9(1):23. https://doi.org/10.3390/catal9010023
Chicago/Turabian StylePan, Hua, Dongmei Xu, Chi He, and Chao Shen. 2019. "In Situ Regeneration and Deactivation of Co-Zn/H-Beta Catalysts in Catalytic Reduction of NOx with Propane" Catalysts 9, no. 1: 23. https://doi.org/10.3390/catal9010023




