Pt–Ni Seed-Core-Frame Hierarchical Nanostructures and Their Conversion to Nanoframes for Enhanced Methanol Electro-Oxidation
Abstract
:1. Introduction
2. Results and Discussion
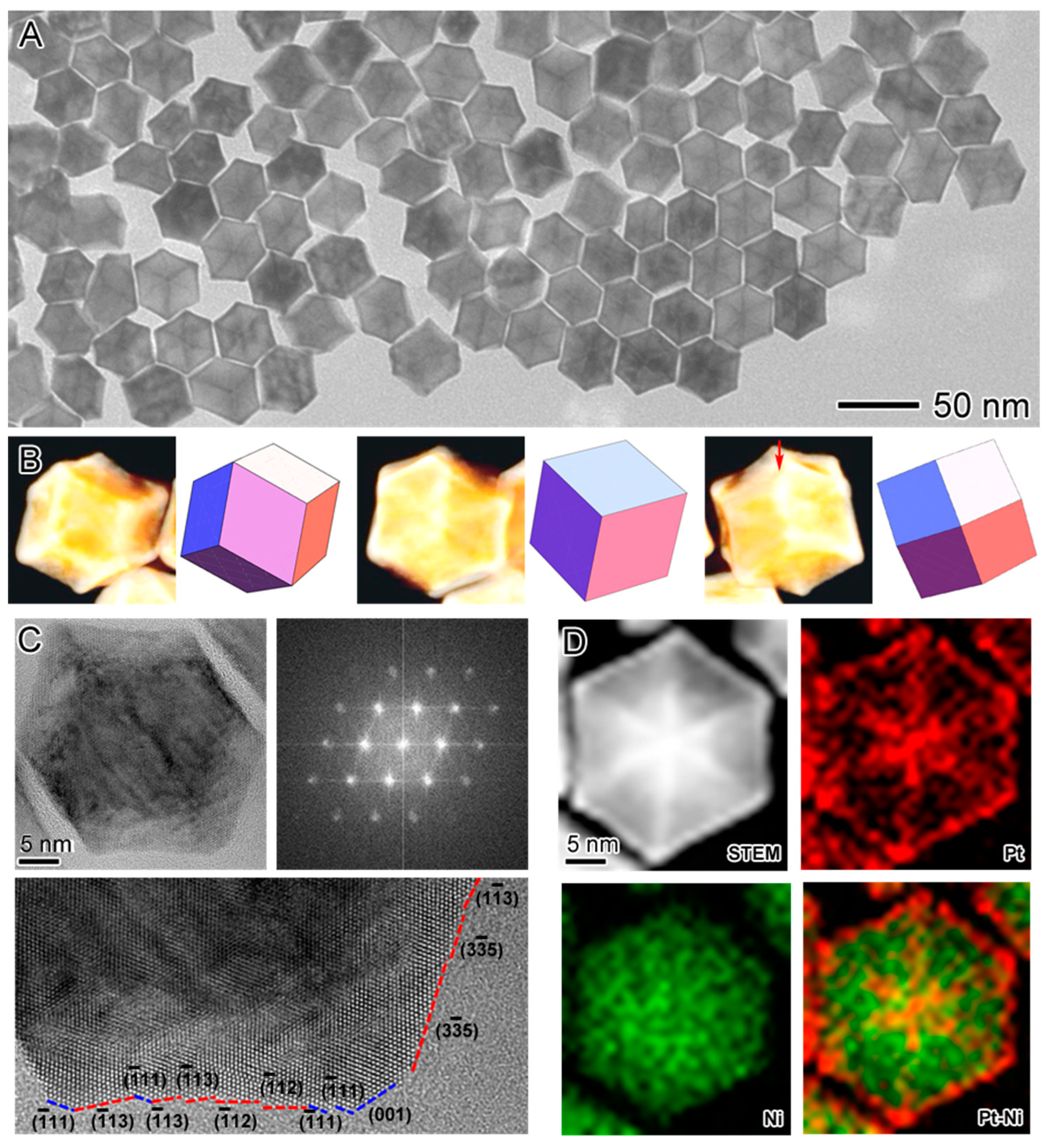
2.1. Synthesis of the Seed-Core-Frame Nanostructures
2.2. Conversion of the Seed-Core-Frame Hierarchical Nanostructures to Nanoframes
2.3. Electrocatalytic Performance of the Pt–Ni Nanostructures for Methanol Oxidation Reaction (MOR)
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Pt–Ni Seed-Core-Frame Nanostructures
3.3. Synthesis of Pt3Ni Nanoframes
3.4. Instrumentation
3.5. Electrocatalytic Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabis, A.; Rodriguez, P.; Schmidt, T.J. Electrocatalysis for Polymer Electrolyte Fuel Cells: Recent Achievements and Future Challenges. ACS Catal. 2012, 2, 864–890. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Bimetallic Nanocrystals: Liquid-Phase Synthesis and Catalytic Applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Peng, Z.; Yang, S.; Wagner, F.T.; Yang, H. Truncated Octahedral Pt3Ni Oxygen Reduction Reaction Electrocatalysts. J. Am. Chem. Soc. 2010, 132, 4984–4985. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, H. Synthesis and electrocatalytic oxygen reduction properties of truncated octahedral Pt3Ni nanoparticles. Nano Res. 2011, 4, 72–82. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Fang, J.; Zou, S. Synthesis and Oxygen Reduction Activity of Shape-Controlled Pt3Ni Nanopolyhedra. Nano Lett. 2010, 10, 638–644. [Google Scholar] [CrossRef]
- Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Atwan, M.H.; Tessema, M.M. Solvothermal Synthesis of Platinum Alloy Nanoparticles for Oxygen Reduction Electrocatalysis. J. Am. Chem. Soc. 2012, 134, 8535–8542. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Li, H.-H.; Yu, S.-H.; Heggen, M.; Strasser, P. Octahedral PtNi Nanoparticle Catalysts: Exceptional Oxygen Reduction Activity by Tuning the Alloy Particle Surface Composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef]
- Wang, C.; Chi, M.; Li, D.; Strmcnik, D.; van der Vliet, D.; Wang, G.; Komanicky, V.; Chang, K.-C.; Paulikas, A.P.; Tripkovic, D.; et al. Design and Synthesis of Bimetallic Electrocatalyst with Multilayered Pt-Skin Surfaces. J. Am. Chem. Soc. 2011, 133, 14396–14403. [Google Scholar] [CrossRef]
- Wang, C.; Chi, M.; Wang, G.; van der Vliet, D.; Li, D.; More, K.; Wang, H.-H.; Schlueter, J.A.; Markovic, N.M.; Stamenkovic, V.R. Correlation Between Surface Chemistry and Electrocatalytic Properties of Monodisperse PtxNi1−x Nanoparticles. Adv. Funct. Mater. 2011, 21, 147–152. [Google Scholar] [CrossRef]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Becknell, N.; Kang, Y.; Chen, C.; Resasco, J.; Kornienko, N.; Guo, J.; Markovic, N.M.; Somorjai, G.A.; Stamenkovic, V.R.; Yang, P. Atomic structure of Pt3Ni nanoframe electrocatalysts by in situ X-ray absorption spectroscopy. J. Am. Chem. Soc. 2015, 137, 15817–15824. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Becknell, N.; Yu, Y.; Kim, D.; Chen, C.; Kornienko, N.; Somorjai, G.A.; Yang, P. Anisotropic phase segregation and migration of Pt in nanocrystals en route to nanoframe catalysts. Nat. Mater. 2016, 15, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Becknell, N.; Son, Y.; Kim, D.; Li, D.; Yu, Y.; Niu, Z.; Lei, T.; Sneed, B.T.; More, K.L.; Markovic, N.M. Control of architecture in rhombic dodecahedral Pt–Ni nanoframe electrocatalysts. J. Am. Chem. Soc. 2017, 139, 11678–11681. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Niu, Z.; Xie, C.; Gao, M.; Lai, M.; Li, M.; Yang, P. Effects of Catalyst Processing on the Activity and Stability of Pt–Ni Nanoframe Electrocatalysts. ACS Nano 2018, 12, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Bu, L.; Guo, S.; Zhao, Z.; Zhu, E.; Huang, Y.; Huang, X. Morphology and phase controlled construction of Pt–Ni nanostructures for efficient electrocatalysis. Nano Lett. 2016, 16, 2762–2767. [Google Scholar] [CrossRef]
- Gan, L.; Heggen, M.; Cui, C.; Strasser, P. Thermal facet healing of concave octahedral Pt–Ni nanoparticles imaged in situ at the atomic scale: Implications for the rational synthesis of durable high-performance ORR electrocatalysts. ACS Catal. 2015, 6, 692–695. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Kuhl, S.; Padgett, E.; Schmies, H.; Goerlin, M.; Erini, N.; Shviro, M.; Heggen, M.; Dunin-Borkowski, R.E. Tuning the Electrocatalytic Oxygen Reduction Reaction Activity and Stability of Shape-Controlled Pt–Ni Nanoparticles by Thermal Annealing—Elucidating the Surface Atomic Structural and Compositional Changes. J. Am. Chem. Soc. 2017, 139, 16536–16547. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Bastakoti, B.P.; Tang, J.; Jiang, B.; Kim, J.; Shahabuddin, M.; Bando, Y.; Kim, J.H.; Yamauchi, Y. Strategic synthesis of mesoporous Pt-on-Pd bimetallic spheres templated from a polymeric micelle assembly. J. Mater. Chem. A 2016, 4, 9169–9176. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yamauchi, Y.; Kaneti, Y.; Alsheri, S.; Ahamad, T.; Alhokbany, N.; Kim, J.; Ariga, K.; Wu, N. Micelle-Assisted Strategy for the Direct Synthesis of Large-Sized Mesoporous Platinum Catalysts by Vapor Infiltration of a Reducing Agent. Nanomaterials 2018, 8, 841. [Google Scholar] [CrossRef]
- Kani, K.; Zakaria, M.B.; Lin, J.; Alshehri, A.A.; Kim, J.; Bando, Y.; You, J.; Hossain, M.S.A.; Bo, J.; Yamauchi, Y. Synthesis and Characterization of Dendritic Pt Nanoparticles by Using Cationic Surfactant. Bull. Chem. Soc. Jpn. 2018, 91, 1333–1336. [Google Scholar] [CrossRef]
- Koenigsmann, C.; Santulli, A.C.; Gong, K.; Vukmirovic, M.B.; Zhou, W.-P.; Sutter, E.; Wong, S.S.; Adzic, R.R. Enhanced Electrocatalytic Performance of Processed, Ultrathin, Supported Pd–Pt Core–Shell Nanowire Catalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2011, 133, 9783–9795. [Google Scholar] [CrossRef]
- Li, H.-H.; Ma, S.-Y.; Fu, Q.-Q.; Liu, X.-J.; Wu, L.; Yu, S.-H. Scalable Bromide-Triggered Synthesis of Pd@Pt Core–Shell Ultrathin Nanowires with Enhanced Electrocatalytic Performance toward Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 7862–7868. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Su, D.; Sun, S. Seed-mediated synthesis of core/shell FePtM/FePt (M = Pd, Au) nanowires and their electrocatalysis for oxygen reduction reaction. J. Am. Chem. Soc. 2013, 135, 13879–13884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mo, Y.; Vukmirovic, M.B.; Klie, R.; Sasaki, K.; Adzic, R.R. Platinum Monolayer Electrocatalysts for O2 Reduction: Pt Monolayer on Pd(111) and on Carbon-Supported Pd Nanoparticles. J. Phys. Chem. B 2004, 108, 10955–10964. [Google Scholar] [CrossRef]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum Monolayer Fuel Cell Electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar] [CrossRef]
- Sasaki, K.; Wang, J.X.; Naohara, H.; Marinkovic, N.; More, K.; Inada, H.; Adzic, R.R. Recent advances in platinum monolayer electrocatalysts for oxygen reduction reaction: Scale-up synthesis, structure and activity of Pt shells on Pd cores. Electrochim. Acta 2010, 55, 2645–2652. [Google Scholar] [CrossRef]
- Adzic, R.R. Platinum Monolayer Electrocatalysts: Tunable Activity, Stability, and Self-Healing Properties. Electrocatalysis 2012, 3, 163–169. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuttiyiel, K.A.; Wang, J.X.; Vukmirovic, M.B.; Adzic, R.R. Recent Advances in Platinum Monolayer Electrocatalysts for the Oxygen Reduction Reaction. Electrocatal. Low Temp. Fuel Cells Fundam. Recent Trends 2017, 557–584. [Google Scholar] [CrossRef]
- Tian, X.L.; Xu, Y.Y.; Zhang, W.; Wu, T.; Xia, B.Y.; Wang, X. Unsupported Platinum-Based Electrocatalysts for Oxygen Reduction Reaction. ACS Energy Lett. 2017, 2, 2035–2043. [Google Scholar] [CrossRef]
- Li, C.; Tan, H.; Lin, J.; Luo, X.; Wang, S.; You, J.; Kang, Y.-M.; Bando, Y.; Yamauchi, Y.; Kim, J. Emerging Pt-based electrocatalysts with highly open nanoarchitectures for boosting oxygen reduction reaction. Nano Today 2018, 21, 91–105. [Google Scholar] [CrossRef]
- Park, K.-W.; Choi, J.-H.; Kwon, B.-K.; Lee, S.-A.; Sung, Y.-E.; Ha, H.-Y.; Hong, S.-A.; Kim, H.; Wieckowski, A. Chemical and Electronic Effects of Ni in Pt/Ni and Pt/Ru/Ni Alloy Nanoparticles in Methanol Electrooxidation. J. Phys. Chem. B 2002, 106, 1869–1877. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, L.; Hou, H.; Qi, J.; Wang, S.; Sun, G. Promoting Effect of Ni in PtNi Bimetallic Electrocatalysts for the Methanol Oxidation Reaction in Alkaline Media: Experimental and Density Functional Theory Studies. J. Phys. Chem. C 2010, 114, 19714–19722. [Google Scholar] [CrossRef]
- Sulaiman, J.E.; Zhu, S.; Xing, Z.; Chang, Q.; Shao, M. Pt–Ni octahedra as electrocatalysts for the ethanol electro-oxidation reaction. ACS Catal. 2017, 7, 5134–5141. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Zhou, G.; Yu, R.; Chen, C.; Li, Y. Sophisticated construction of Au islands on Pt–Ni: An ideal trimetallic nanoframe catalyst. J. Am. Chem. Soc. 2014, 136, 11594–11597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gan, Y.; Du, J.; Tian, D.; Zhang, R.; Yang, C.; Dai, Z. A review of hollow Pt-based nanocatalysts applied in proton exchange membrane fuel cells. J. Power Sources 2013, 232, 310–322. [Google Scholar] [CrossRef]
- Taylor, E.; Chen, S.; Tao, J.; Wu, L.; Zhu, Y.; Chen, J. Synthesis of Pt–Cu Nanodendrites through Controlled Reduction Kinetics for Enhanced Methanol Electro-Oxidation. ChemSusChem 2013, 6, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, L.E.; Benamara, M.; Tao, J.; Zhu, Y.; Chen, J. Tailoring the Surface Structures of CuPt and CuPtRu 1D Nanostructures by Coupling Coreduction with Galvanic Replacement. Part. Part. Syst. Charact. 2018, 35, 1800053. [Google Scholar] [CrossRef]
- Mathurin, L.E.; Tao, J.; Xin, H.; Li, J.; Zhu, Y.; Chen, J. Dendritic Core-Frame and Frame Multimetallic Rhombic Dodecahedra: A Comparison Study of Composition and Structure Effects on Electrocatalysis of Methanol Oxidation. ChemNanoMat 2018, 4, 76–87. [Google Scholar] [CrossRef]
- Chen, S.; Jenkins, S.V.; Tao, J.; Zhu, Y.; Chen, J. Anisotropic Seeded Growth of Cu–M (M = Au, Pt, or Pd) Bimetallic Nanorods with Tunable Optical and Catalytic Properties. J. Phys. Chem. C 2013, 117, 8924–8932. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Göttinger Nachrichten Math. Phys. Rev. A 1918, 2, 98–100. [Google Scholar]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the’Debye-Scherrer equation. Nat. Nanotech. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Smigelskas, A.; Kirkendall, E. Zinc diffusion in alpha brass. Trans. Aime 1947, 171, 130–142. [Google Scholar]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Biegler, T.; Rand, D.; Woods, R. Limiting oxygen coverage on platinized platinum; relevance to determination of real platinum area by hydrogen adsorption. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29, 269–277. [Google Scholar] [CrossRef]
- Markovic, N.; Grgur, B.; Ross, P. Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions. J. Phys. Chem. B 1997, 101, 5405–5413. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Ferrin, P.; Tritsaris, G.A.; Nilekar, A.U.; Koh, S.; Bae, S.E.; Brankovic, S.R.; Strasser, P.; Mavrikakis, M. Bifunctional anode catalysts for direct methanol fuel cells. Energy Environ. Sci. 2012, 5, 8335–8342. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wu, H.; Tao, J.; Xin, H.; Zhu, Y.; Chen, J. Pt–Ni Seed-Core-Frame Hierarchical Nanostructures and Their Conversion to Nanoframes for Enhanced Methanol Electro-Oxidation. Catalysts 2019, 9, 39. https://doi.org/10.3390/catal9010039
Chen S, Wu H, Tao J, Xin H, Zhu Y, Chen J. Pt–Ni Seed-Core-Frame Hierarchical Nanostructures and Their Conversion to Nanoframes for Enhanced Methanol Electro-Oxidation. Catalysts. 2019; 9(1):39. https://doi.org/10.3390/catal9010039
Chicago/Turabian StyleChen, Shutang, Haibin Wu, Jing Tao, Huolin Xin, Yimei Zhu, and Jingyi Chen. 2019. "Pt–Ni Seed-Core-Frame Hierarchical Nanostructures and Their Conversion to Nanoframes for Enhanced Methanol Electro-Oxidation" Catalysts 9, no. 1: 39. https://doi.org/10.3390/catal9010039
APA StyleChen, S., Wu, H., Tao, J., Xin, H., Zhu, Y., & Chen, J. (2019). Pt–Ni Seed-Core-Frame Hierarchical Nanostructures and Their Conversion to Nanoframes for Enhanced Methanol Electro-Oxidation. Catalysts, 9(1), 39. https://doi.org/10.3390/catal9010039




