Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
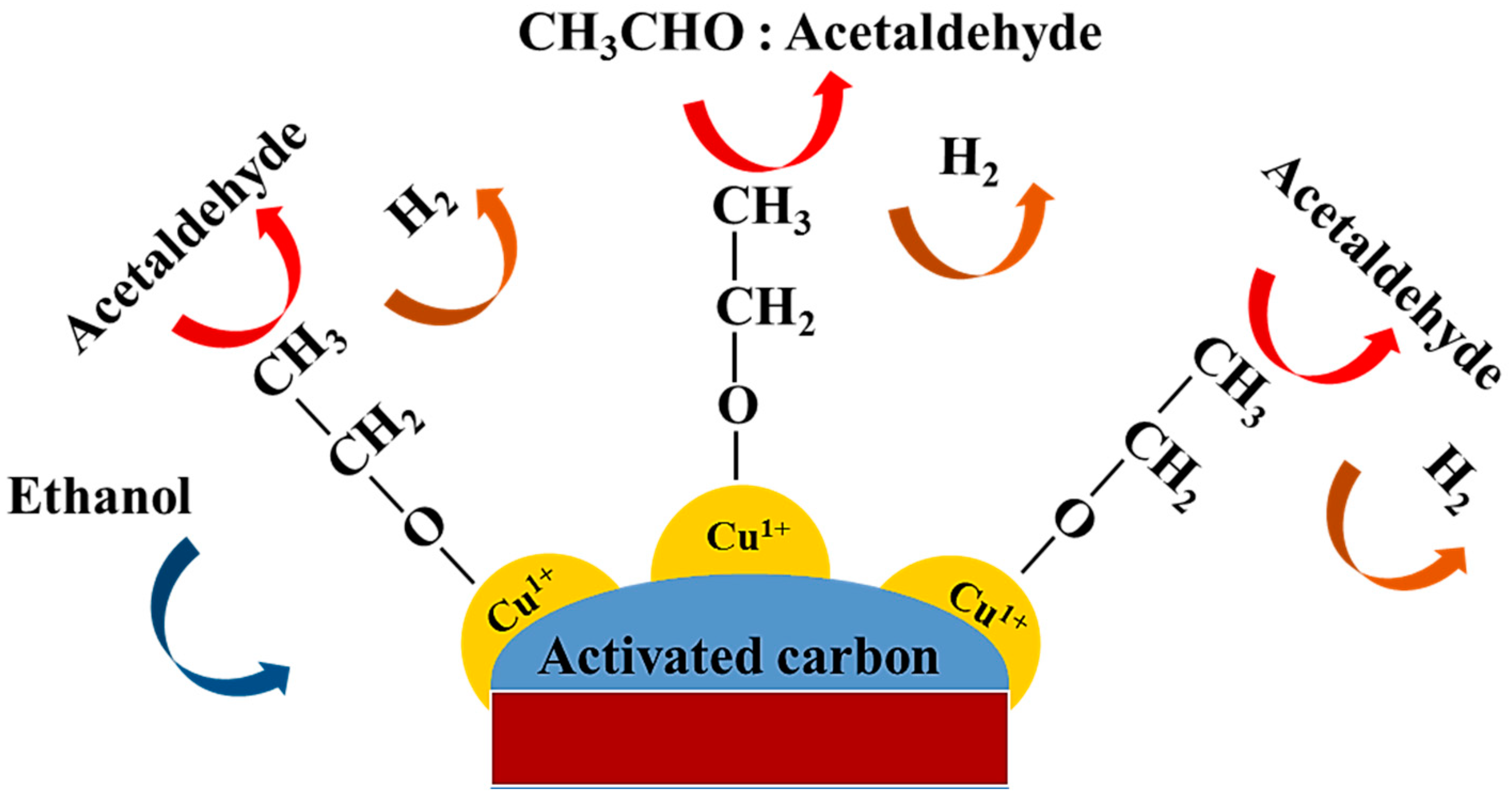
2.2. Catalyst Testing
3. Materials and Methods
3.1. Raw Materials and Chemicals
3.2. Preparation of Activated Carbons
3.3. Characterization of Activated Carbons
3.4. Catalytic Activity with Ethanol Dehydrogenation
3.4.1. Temperature-Programmed Reaction
3.4.2. Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- LookChem. Available online: https://www.lookchem.com/Chempedia/Chemical-Technology/Organic-Chemical-Technology/7521.html (accessed on 13 May 2018).
- Dutova, V.V.; Mamontova, G.V.; Soboleva, V.I.; Vodyankina, O.V. Silica-supported silver-containing OMS-2 catalysts for ethanol oxidative dehydrogenation. Catal. Today 2016, 278, 164–173. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Freitas, I.C.; Longo, E.; Bueno, J.M.C. Site-selective ethanol conversion over supported copper catalysts. Catal. Commun. 2012, 26, 122–126. [Google Scholar] [CrossRef]
- Freitasa, I.C.; Damyanovab, S.; Oliveirac, D.C.; Marquesd, C.M.P.; Buenoa, J.M.C. Effect of Cu content on the surface and catalytic properties of Cu/ZrO2 catalyst for ethanol dehydrogenation. J. Mol. Catal. A Chem. 2014, 381, 26–37. [Google Scholar] [CrossRef]
- DeWilde, J.F.; Czopinski, C.J.; Bhan, A. Ethanol Dehydration and Dehydrogenation on γ-Al2O3: Mechanism of Acetaldehyde Formation. ACS Catal. 2014, 4, 4425–4433. [Google Scholar] [CrossRef]
- Skinner, M.J.; Michor, E.L.; Fan, W.; Tsapatsis, M.; Bhan, A.; Schmidt, L.D. Ethanol Dehydration to Ethylene in a Stratified Autothermal Millisecond Reactor. ChemSusChem 2011, 4, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Autthanit, C.; Jongsomjit, B. Production of Ethylene through Ethanol Dehydration on SBA-15 Catalysts Synthesized by Sol-gel and One-step Hydrothermal Methods. J. Oleo Sci. 2018, 67, 235–243. [Google Scholar] [CrossRef] [PubMed]
- La-Salvia, N.; Lovon-Quintana, J.J.; Valenca, G.P. Vapor-phase catalytic conversion of ethanol into 1,3-butadiene on Cr-Ba/MCM-41 catalysts. Braz. J. Chem. Eng. 2015, 32, 489–500. [Google Scholar] [CrossRef]
- Kamsuwan, T.; Praserthdam, P.; Jongsomjit, B. Diethyl Ether Production during Catalytic Dehydration of Ethanol over Ru- and Pt- modified H-beta Zeolite Catalysts. J. Oleo Sci. 2017, 66, 199–207. [Google Scholar] [CrossRef]
- Almukhlifia, H.A.; Burns, R.C. Oxidative dehydrogenation of isobutane to isobutene by pyrovanadates, M2V2O7, where M(II) =Mn, Co, Ni, Cu and Zn, and Co2VO4 and ZnV2O4: The effect of gold nanoparticles. J. Mol. Catal. A Chem. 2015, 408, 26–40. [Google Scholar] [CrossRef]
- Guan, Y.; Hensen, E.J.M. Ethanol dehydrogenation by gold catalysts: The effect of the gold particle size and the presence of oxygen. Appl. Catal. A Gen. 2009, 361, 49–56. [Google Scholar] [CrossRef]
- Lippits, M.J.; Nieuwenhuys, B.E. Direct conversion of ethanol into ethylene oxide on gold-based catalysts Effect of CeOx and Li2O addition on the selectivity. J. Catal. 2010, 274, 142–149. [Google Scholar] [CrossRef]
- Ochoa-Hernándeza, C.; Yanga, Y.; Pizarroa, P.; O’Sheaa, V.A.P.; Coronadoa, J.M.; Serranoa, D.P. Hydrocarbons production through hydrotreating of methyl esters over Ni and Co supported on SBA-15 and Al-SBA-15. Catal. Today 2013, 210, 81–88. [Google Scholar] [CrossRef]
- Shan, J.; Janvelyan, N.; Li, H.; Liu, J.; Egle, T.M.; Ye, J.; Biener, M.M.; Biener, J.; Friend, C.M.; Flytzani-Stephanopoulos, M. Selective non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen on highly dilute NiCu alloys. Appl. Catal. B Environ. 2017, 205, 541–550. [Google Scholar] [CrossRef]
- Gao, D.; Feng, Y.; Yin, H.; Wang, A.; Jiang, T. Coupling reaction between ethanol dehydrogenation and maleic anhydride hydrogenation catalyzed by Cu/Al2O3, Cu/ZrO2, and Cu/ZnO catalysts. Chem. Eng. J. 2013, 233, 349–359. [Google Scholar] [CrossRef]
- Santacesaria, E.; Carotenuto, G.; Tesser, R.; Serio, M.D. Ethanol dehydrogenation to ethyl acetate by using copper and copper chromite catalysts. Chem. Eng. J. 2012, 179, 209–220. [Google Scholar] [CrossRef]
- Hidalgoa, J.M.; Tislera, Z.; Kubicka, D.; Raabova, K.; Bulanek, R. (V)/Hydrotalcite, (V)/Al2O3, (V)/TiO2 and (V)/SBA-15 catalysts for the partial oxidation of ethanol to acetaldehyde. J. Mol. Catal. A: Chem. 2016, 420, 178–189. [Google Scholar] [CrossRef]
- Autthanit, C.; Praserthdam, P.; Jongsomjit, B. Oxidative and non-oxidative dehydrogenation of ethanol to acetaldehyde over different VOx/SBA-15 catalysts. J. Environ. Chem. Eng. 2018, 6, 6516–6529. [Google Scholar] [CrossRef]
- Lippits, M.J.; Nieuwenhuys, B.E. Direct conversion of ethanol into ethylene oxide on copper and silver nanoparticles Effect of addition of CeOx and Li2O. Catal. Today 2010, 154, 127–132. [Google Scholar] [CrossRef]
- Wachs, I.E.; Madix, R.J. The oxidation of methanol on a silver (110) catalyst. Surf. Sci. 1978, 76, 531–558. [Google Scholar] [CrossRef]
- Lim, S.P.; Pandikumar, A.; Huang, N.M.; Lim, H.N. Silver/titania nanocomposite-modified photoelectrodes for photoelectrocatalytic methanol oxidation. Int. J. Hydrogen Energy 2014, 39, 14720–14729. [Google Scholar] [CrossRef]
- Wu, X.; Fang, G.; Liang, Z.; Leng, W.; Xu, K.; Jiang, D.; Ni, J.; Li, X. Catalytic upgrading of ethanol to n-butanol over M-CeO2/AC (M = Cu, Fe, Co, Ni and Pd) catalysts. Catal. Commun. 2017, 100, 15–18. [Google Scholar] [CrossRef]
- Jasinska, J.; Krzyzynska, B.; Kozlowski, M. Influence of activated carbon modifications on their catalytic activity in methanol and ethanol conversion reactions. Cent. Eur. J. Chem. 2011, 9, 925–931. [Google Scholar] [CrossRef]
- Carrasco-Marin, F.; Mueden, A.; Moreno-Castilla, C. Surface-Treated Activated Carbons as Catalysts for the Dehydration and Dehydrogenation Reactions of Ethanol. J. Phys. Chem. B 1998, 102, 9239–9244. [Google Scholar] [CrossRef]
- Szymanski, G.S.; Rychlicki, G.; Terzyk, A.P. Catalytic conversion of ethanol on carbon catalysts. Carbon 1994, 32, 265–271. [Google Scholar] [CrossRef]
- Bekyarova, E.; Mehandjiev, D. Studies of Ni-Impregnated Active Carbon Part I. Effect of Thermal Treatment on the Texture of Active Carbon and the State of the Active Phase. J. Colloid Interface Sci. 1996, 176, 509–516. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. “Introduction”. In Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1982; pp. 3–5. [Google Scholar]
- Sun, G.; Li, X.; Qu, Y.; Wang, X.; Yan, H.; Zhang, Y. Preparation and characterization of graphite nanosheets from detonation technique. Mater. Lett. 2008, 62, 703–706. [Google Scholar] [CrossRef]
- Reina, T.R.; Ivanova, S.; Laguna, O.H.; Centeno, M.A.; Odriozola, J.A. WGS and CO-PrOx reactions using gold promoted copper-ceria catalysts: “Bulk CuO CeO2 vs. CuO CeO2/Al2O3 with low mixed oxide content”. Appl. Catal. B Environ. 2016, 197, 62–72. [Google Scholar] [CrossRef]
- Grzechowiak, J.R.; Szyszka, I.; Masalska, A. Effect of TiO2 content and method of titania–silica preparation on the nature of oxidic nickel phases and their activity in aromatic hydrogenation. Catal. Today 2008, 137, 433–438. [Google Scholar] [CrossRef]
- Bin, Q.; Wenhan, G.; Zibin, L.; Wei, X.; Song, G.; Qingfei, W.; Xiaofeng, Y.; Ruo, Z.; Ruqiang, Z. Fabrication of Co3O4 nanoparticles in thin porous carbon shells from metal–organic frameworks for enhanced electrochemical performance. RSC Adv. 2017, 7, 13340–13346. [Google Scholar] [CrossRef]
- Goodarznia, S.; Smith, K.J. The effect of Cu loading on the formation of methyl formate and C2-oxygenates from CH3OH and CO over K- or Cs-promoted Cu-MgO catalysts. J. Mol. Catal. A Chem. 2012, 353–354, 58–66. [Google Scholar] [CrossRef]
- Church, J.M.; Joshp, H.K. Acetaldehyde by Dehydrogenation of Ethyl Alcohol. Ind. Eng. Chem. 1951, 43, 1804–1811. [Google Scholar] [CrossRef]
- Kassim, M.A. The transport process in the catalytic dehydrogenation of ethyl alcohol. Adv. Sci. Technol. Res. 2015, 2, 19–41. [Google Scholar]
- Volanti, D.P.; Sato, A.G.; Orlandi, M.O.; Bueno, J.M.C.; Longo, E.; Andres, J. Insight into Copper-Based Catalysts: Microwave-Assisted Morphosynthesis, In Situ Reduction Studies, and Dehydrogenation of Ethanol. ChemCatChem 2011, 3, 839–843. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Meira, D.M.; Damyanova, S.; Longo, E.; Bueno, J.M.C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Chatchawanrat, S. Dehydrogenation of Ethanol to Acetaldehyde over Activated Carbon Catalysts. Master’s Thesis, Engineering Program in Chemical Engineering, Chulalongkorn University, Bangkok, Thailand, 2013. [Google Scholar]
- El-Molla, S.A.; El-Shobaky, G.A.; Sayed Ahmed, S.A. Catalytic Promotion of Activated carbon by Treatment with Some Transition Metal Cations. Chin. J. Catal. 2007, 28, 611–616. [Google Scholar] [CrossRef]
- Wu, X.; Fang, G.; Tong, Y.; Jiang, D.; Zhe, L.; Leng, W.; Liu, L.; Tu, P.; Wang, H.; Ni, J.; et al. Catalytic Upgrading of Ethanol to n-Butanol: Progress in Catalyst Development. ChemSusChem 2018, 11, 71–85. [Google Scholar] [CrossRef]









| Catalysts | ACC | Ce/ACC | Co/ACC | Cu/ACC | Ni/ACC |
|---|---|---|---|---|---|
| SBET (m2/g) | 852 | 837 | 744 | 823 | 699 |
| Smicropore(m2/g) | 310.4 | 805.5 | 723.0 | 779.1 | 626.8 |
| Sexternal (m2/g) | 541.7 | 31.8 | 20.9 | 43.6 | 71.7 |
| Vtotal (cm3/g) | 0.86 | 0.45 | 0.38 | 0.44 | 0.40 |
| Vmic (cm3/g) | 0.16 | 0.38 | 0.34 | 0.37 | 0.31 |
| Dp (nm) | 1.4 | 3.7 | 3.8 | 3.9 | 3.7 |
| Activated Carbons | wt% of Metal |
|---|---|
| Ce/ACC | 8.8 |
| Co/ACC | 8.0 |
| Cu/ACC | 7.7 |
| Ni/ACC | 11.4 |
| Activated Carbons | Total Acidity a (µmol/g) | Total Basicity b (µmol/g) |
|---|---|---|
| ACC | 133 | 56 |
| Ce/ACC | 164 | 174 |
| Co/ACC | 358 | 319 |
| Cu/ACC | 549 | 90 |
| Ni/ACC | 199 | 158 |
| Catalysts | Reaction Temperature (°C) | Ethanol Conversion (%) | Acetaldehyde Yield (%) | LHSV [mL/(h·gcat)] | Refs |
|---|---|---|---|---|---|
| Cu-CeO2/ACC | 250 | 46 | 3.3 | 4 | [39] |
| Co-CeO2/ACC | 250 | 34 | 2.5 | 4 | [39] |
| Ni-CeO2/ACC | 250 | 32 | 1.5 | 4 | [39] |
| CeO2/ACC | 250 | 3 | 0.4 | 4 | [39] |
| 4Cu1Ce/ACC | 250 | 46 | 3.6 | 4 | [39] |
| 5%Ni/ACC | 250 | 17 | 16.1 | 290 | [37] |
| Cu/ACC | 250 | 15 | 15.1 | 290 | [this work] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ob-eye, J.; Praserthdam, P.; Jongsomjit, B. Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts. Catalysts 2019, 9, 66. https://doi.org/10.3390/catal9010066
Ob-eye J, Praserthdam P, Jongsomjit B. Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts. Catalysts. 2019; 9(1):66. https://doi.org/10.3390/catal9010066
Chicago/Turabian StyleOb-eye, Jeerati, Piyasan Praserthdam, and Bunjerd Jongsomjit. 2019. "Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts" Catalysts 9, no. 1: 66. https://doi.org/10.3390/catal9010066
APA StyleOb-eye, J., Praserthdam, P., & Jongsomjit, B. (2019). Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts. Catalysts, 9(1), 66. https://doi.org/10.3390/catal9010066






