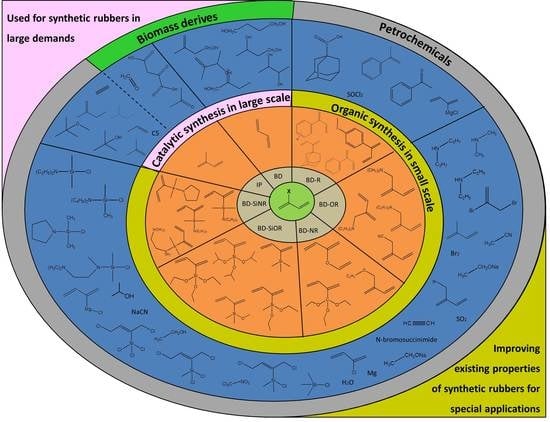
Synthesis of 1,3-Butadiene and Its 2-Substituted Monomers for Synthetic Rubbers
Abstract
:1. Introduction
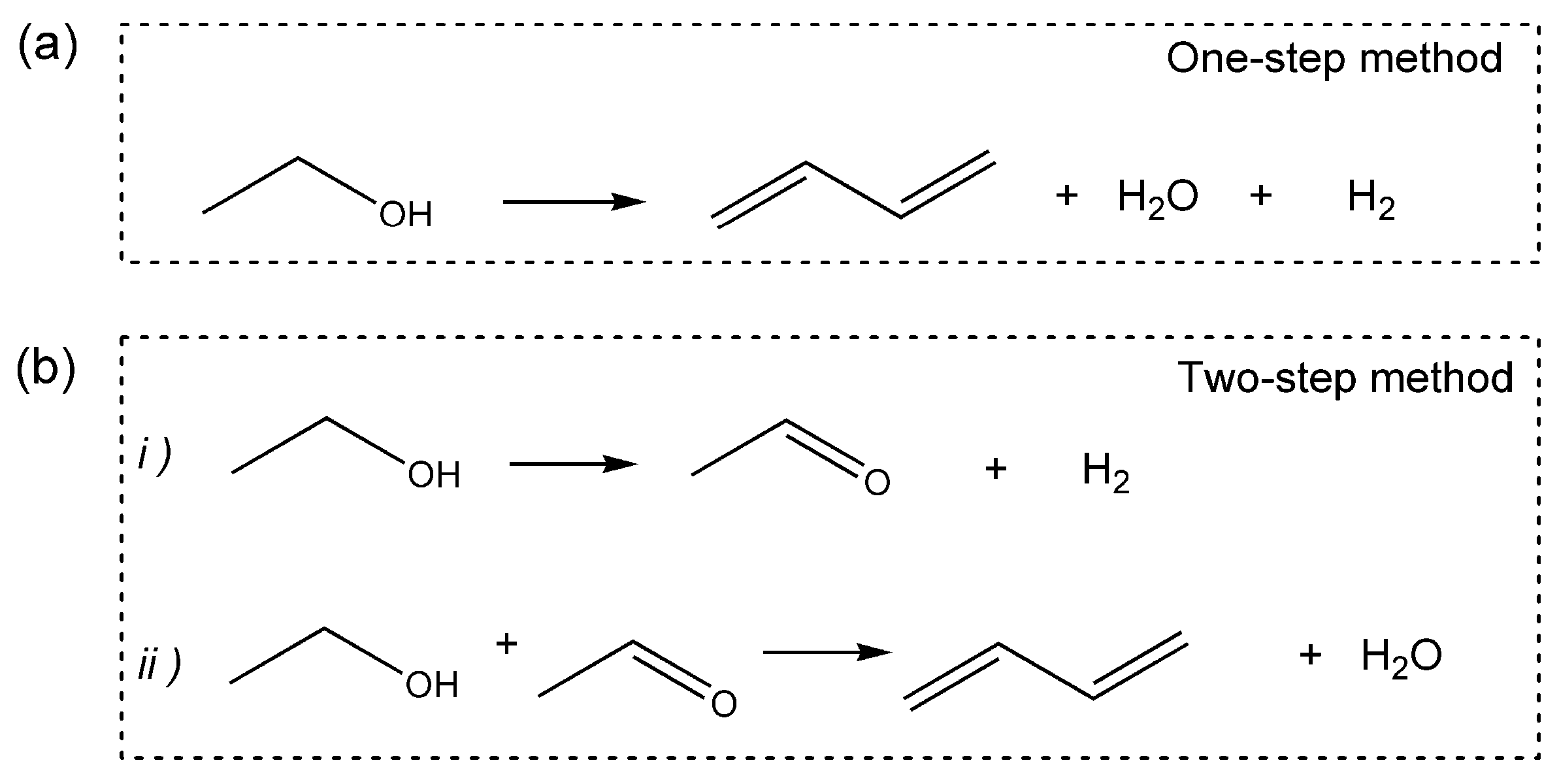
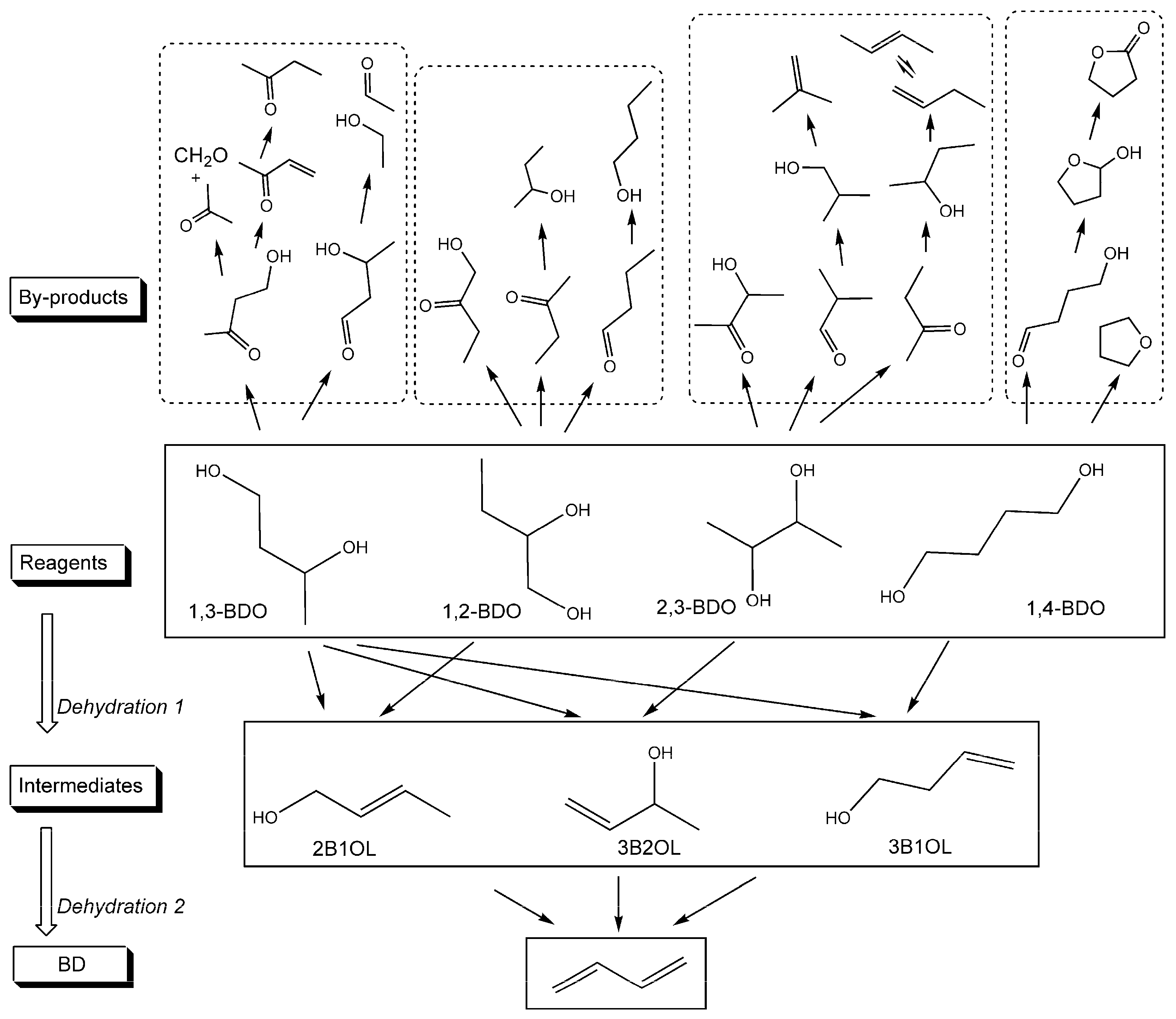
2. Synthesis of 1,3-butadiene
3. Synthesis of Isoprene
4. Rigid-Group Functionalized 1,3-butadienes
4.1. Synthesis of 2-(1-adamantyl)-1,3-butadiene
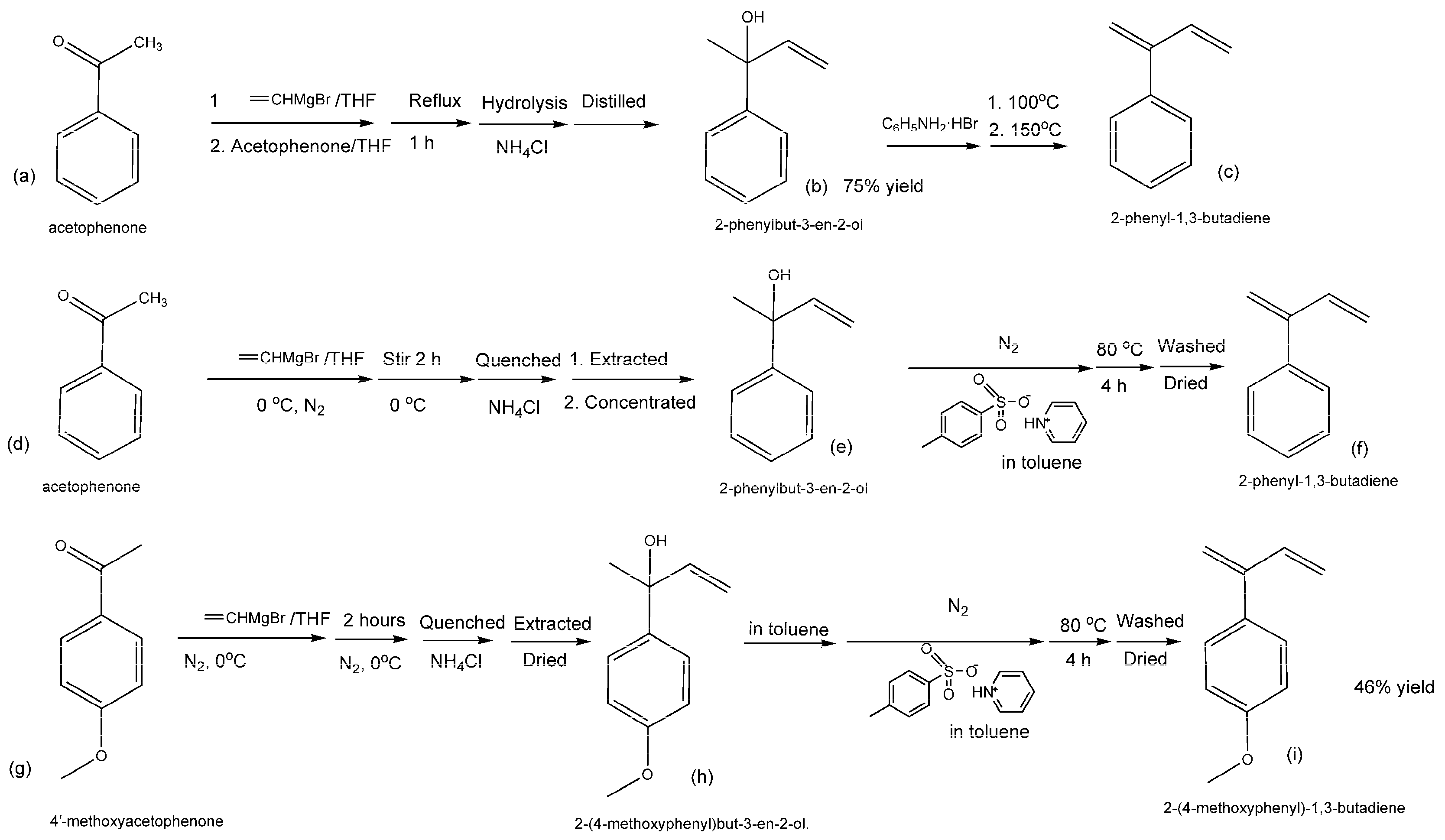
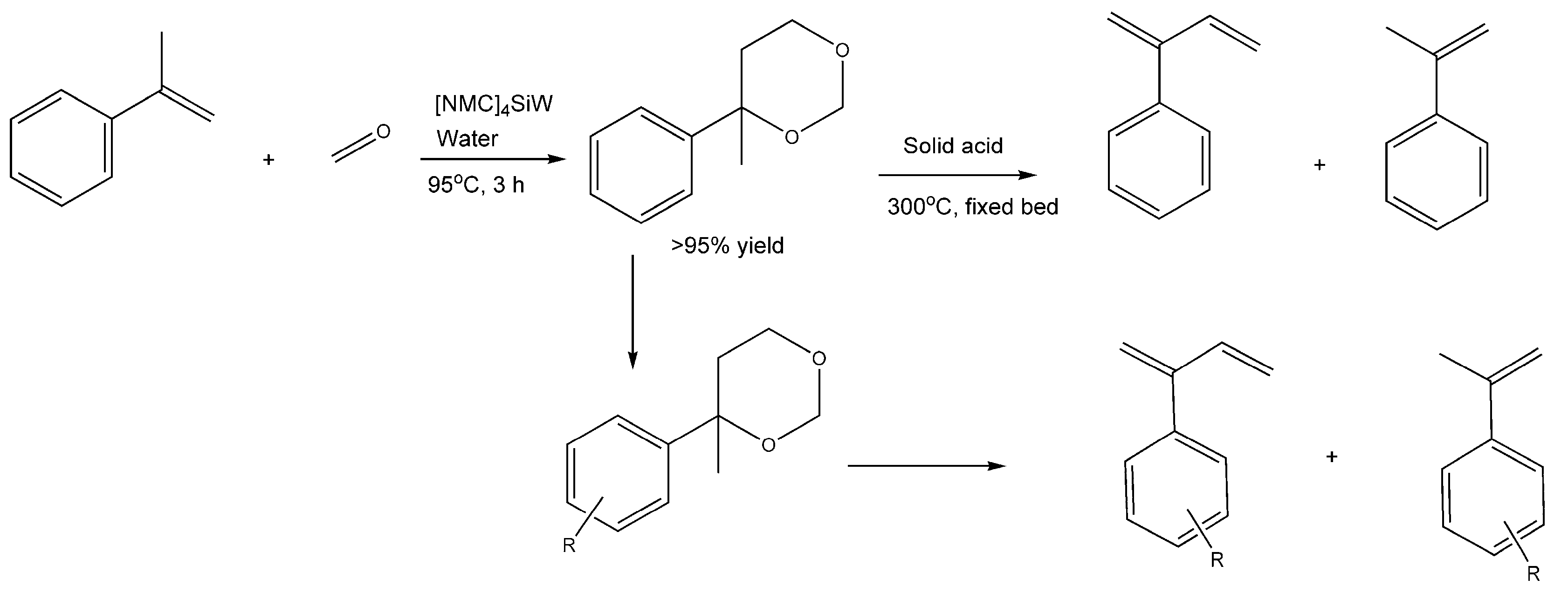
4.2. Synthesis of 2-phenyl-1,3-butadienes
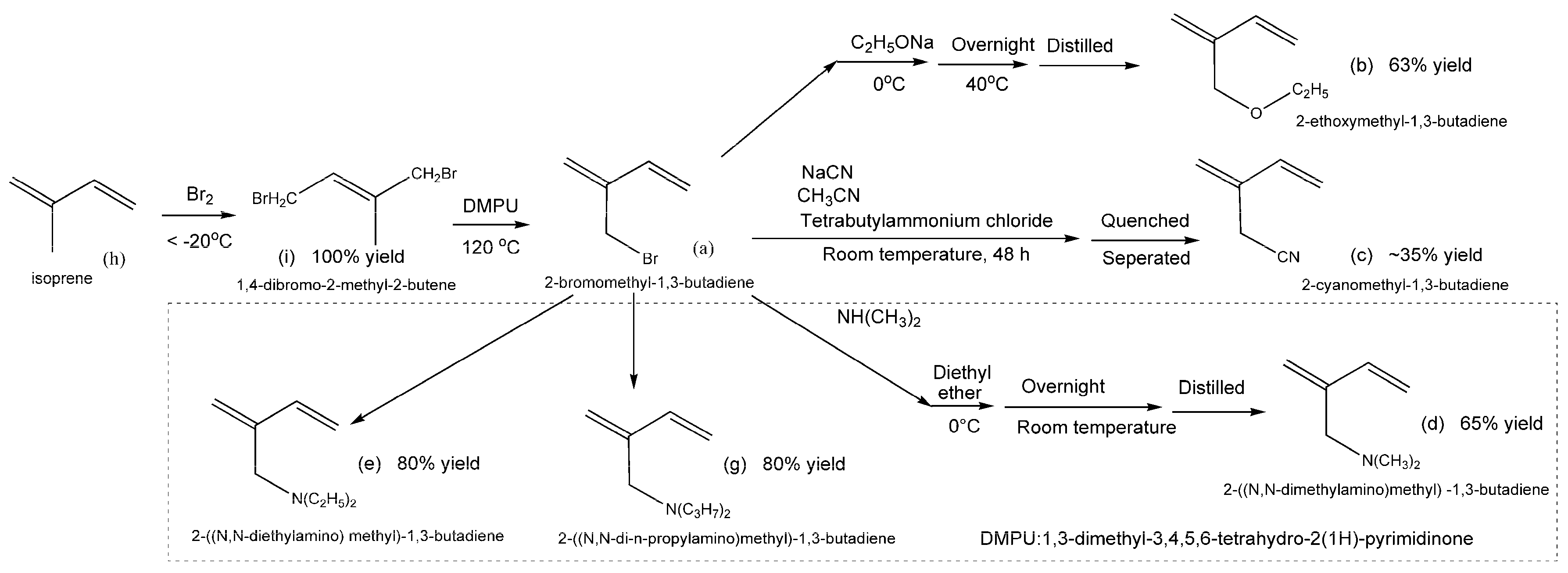
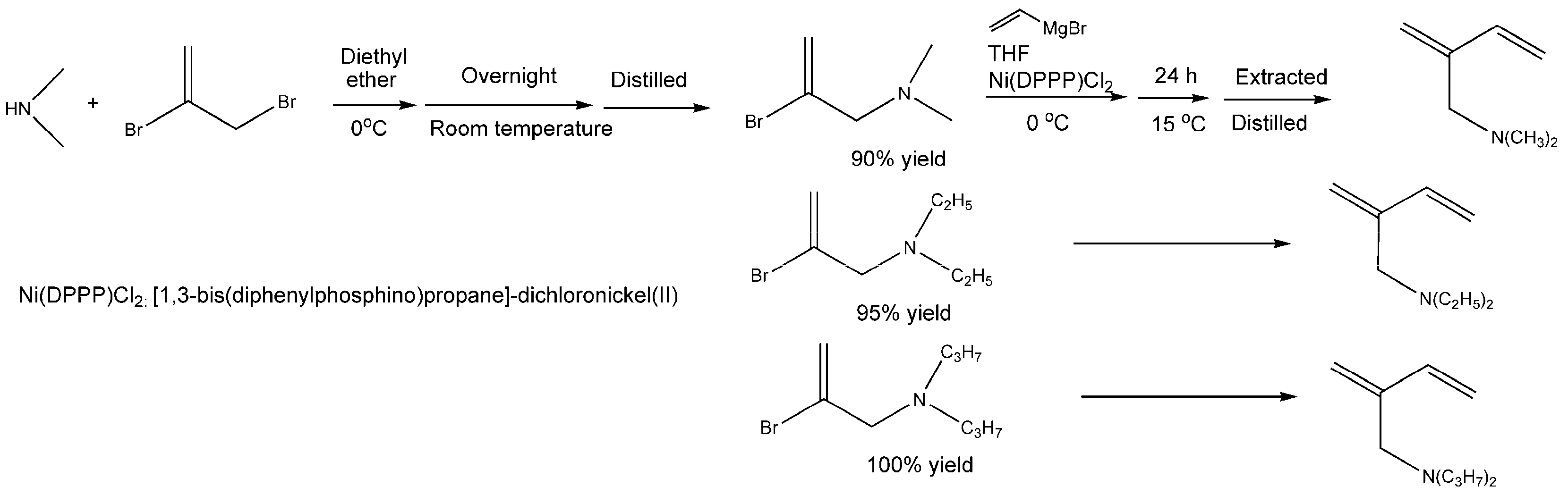
5. Polar-Group Functionalized 1,3-butadienes
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1,3-BDO | 1,3-Butanediol |
| 1,4-BDO | 1,4-Butanediol |
| 1-MPB | 1-(4-Methypenyl)-1,3-butadiene |
| 1-PB | 1-Phenyl-1,3-butadiene |
| 2,3-BDO | 2,3-Butanediol |
| 2B1OL | 2-Buten-1-ol |
| 2-MOPB | 2-(4-Methoxyphenyl)-1,3-butadiene |
| 2-MTHF | 2-Methyltetrahydrofuran |
| 2-PB | 2-Phenyl-1,3-butadiene |
| 3B1OL | 3-Buten-1-ol |
| 3B2OL | 3-Buten-2-ol |
| 3-MTHF | 3-Methyltetrahydrofuran |
| BD | 1,3-Butadiene |
| BDOs | Butanediols |
| BR | Butadiene rubber |
| CR | Chloroprene rubber |
| DMD | 4,4-Dimethyldioxane-1,3 |
| IP | Isoprene |
| IR | Isoprene rubber |
| MPD | 4-Methyl-4-phenyl-1,3-dioxane |
| MTBE | Methyl tertiarybutyl ether |
| MVK | Methyl ethyl ketone |
| NBR | Nitrile butadiene rubber |
| NB | Natural rubber |
| SBR | Styrene butadiene rubber |
| Tg | Glass transition temperature |
| THF | Tetrahydrofuran |
| UOLs | Unsaturated C4 alcohols |
References
- Sarkar, P.; Bhowmick, A.K. Sustainable rubbers and rubber additives. J. Appl. Polym. Sci. 2018, 135, 45701. [Google Scholar] [CrossRef]
- Zubov, A.; Pokorny, J.; Kosek, J. Styrene-butadiene rubber (SBR) production by emulsion polymerization: Dynamic modeling and intensification of the process. Chem. Eng. J. 2012, 207–208, 414–420. [Google Scholar] [CrossRef]
- Thiele, S.K.H.; Wilson, D.R. Alternate Transition Metal Complex Based Diene Polymerization. J. Macromol. Sci. Part C 2003, 43, 581–628. [Google Scholar] [CrossRef]
- Porri, L.; Giarrusso, A. Conjugated diene polymerization, Pergamon Press plc. Compr. Polym. Sci. 1989, 4, 53–108. [Google Scholar]
- Baugh, L.S.; Canich, J.A.M. Stereoselective Polymerization with Single-Site Catalysts; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Park, S.; Takeuchi, D.; Osakada, K. Pd Complex-Promoted Cyclopolymerization of Functionalized α,ω-Dienes and Copolymerization with Ethylene to Afford Polymers with Cyclic Repeating Units. J. Am. Chem. Soc. 2006, 128, 3510–3511. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhang, X.; Hu, Y.; He, J.; Shi, C.; Li, Y.; Bai, C. Regulation of the cis-1,4- and trans-1,4-Polybutadiene Multiblock Copolymers via Chain Shuttling Polymerization Using a Ternary Neodymium Organic Sulfonate Catalyst. Macromolecules 2017, 50, 7887–7894. [Google Scholar] [CrossRef]
- Wang, B.; Bi, J.; Zhang, C.; Dai, Q.; Bai, C.; Zhang, X.; Hu, Y.; Jiang, L. Highly active and trans-1,4 specific polymerization of 1,3-butadiene catalyzed by 2-pyrazolyl substituted 1,10-phenanthroline ligated iron (II) complexes. Polymer 2013, 54, 5174–5181. [Google Scholar] [CrossRef]
- Hirao, A.; Goseki, R.; Ishizone, T. Advances in Living Anionic Polymerization: From Functional Monomers, Polymerization Systems, to Macromolecular Architectures. Macromolecules 2014, 47, 1883–1905. [Google Scholar] [CrossRef]
- Marconi, W.; Mazzei, A.; Lugli, G.; Bruzzone, M. Slereospecific copolymers 1, 3-butadiene-2-phenylbutadiene. J. Polym. Sci. Part C Polym. Symp. 1967, 16, 805–819. [Google Scholar] [CrossRef]
- Buonerba, A.; Cuomo, C.; Speranza, V.; Grassi, A. Crystalline Syndiotactic Polystyrene as Reinforcing Agent of cis-1,4-Polybutadiene Rubber. Macromolecules 2010, 43, 367–374. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Ezinkwo, G.O.; Tretyakov, V.P.; Aliyu, A.; Ilolov, A.M. Fundamental Issues of Catalytic Conversion of Bio-Ethanol into Butadiene. ChemBioEng Rev. 2014, 1, 194–203. [Google Scholar] [CrossRef]
- Gallo, J.M.; Bueno, J.; Schuchardt, U. Catalytic transformations of ethanol for biorefineries. J. Braz. Chem. Soc. 2014, 25, 2229–2243. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Shale Gas Revolution: An Opportunity for the Production of Biobased Chemicals? Angew. Chem. Int. Ed. 2013, 52, 11980–11987. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, S.E.; Allen, D.T. Impact of Natural Gas and Natural Gas Liquids Supplies on the United States Chemical Manufacturing Industry: Production Cost Effects and Identification of Bottleneck Intermediates. ACS Sustain. Chem. Eng. 2015, 3, 451–459. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Sato, S. Future Prospect of the Production of 1,3-Butadiene from Butanediols. Chem. Lett. 2016, 45, 1036–1047. [Google Scholar] [CrossRef]
- Ipatieff, V. To the question of the decomposition of ethyl alcohol due to various catalysts. J. Russ. Phys. Chem. Soc. 1903, 35, 449–452. [Google Scholar]
- Gorin, Y.A. On The Catalytic Conversion of Alcohols Into Hydrocarbons of The Divinyl Series. 2. A Study of The Process of Formation of Divinyl From Ethyl Alcohol. Zhurnal Obshchei Khimii 1946, 16, 283–294. [Google Scholar]
- Goldstein, R.F.; Waddams, A.L. The Petroleum Chemicals Industry; Spon: Hamburg, Germany, 1967. [Google Scholar]
- Sushkevich, V.L.; Ivanova, I.I.; Taarning, E. Ethanol conversion into butadiene over Zr-containing molecular sieves doped with silver. Green Chem. 2015, 17, 2552–2559. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ivanova, I.I. With Open Arms: Open Sites of ZrBEA Zeolite Facilitate Selective Synthesis of Butadiene from Ethanol. ACS Catal. 2015, 5, 4833–4836. [Google Scholar] [CrossRef]
- Tret’yakov, V.F.; Talyshinskii, R.M.; Ilolov, A.M.; Maksimov, A.L.; Khadzhiev, S.N. Initiated conversion of ethanol to divinyl by the Lebedev reaction. Pet. Chem. 2014, 54, 195–206. [Google Scholar] [CrossRef]
- Gruver, V.; Sun, A.; Fripiat, J.J. Catalytic properties of aluminated sepiolite in ethanol conversion. Catal. Lett. 1995, 34, 359–364. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Ordóñez, S. Ethanol catalytic condensation over Mg–Al mixed oxides derived from hydrotalcites. Catal. Today 2011, 164, 436–442. [Google Scholar] [CrossRef]
- Larina, O.V.; Kyriienko, P.I.; Soloviev, S.O. Effect of the Addition of Zirconium Dioxide on the Catalytic Properties of ZnO/MgO-SiO2 Compositions in the Production of 1,3-Butadiene from Ethanol. Theor. Exp. Chem. 2015, 51, 252–258. [Google Scholar] [CrossRef]
- Ochoa, J.V.; Bandinelli, C.; Vozniuk, O.; Chieregato, A.; Malmusi, A.; Recchi, C.; Cavani, F. An analysis of the chemical, physical and reactivity features of MgO–SiO2 catalysts for butadiene synthesis with the Lebedev process. Green Chem. 2016, 18, 1653–1663. [Google Scholar] [CrossRef]
- Angelici, C.; Meirer, F.; van der Eerden, A.M.J.; Schaink, H.L.; Goryachev, A.; Hofmann, J.P.; Hensen, E.J.M.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Ex Situ and Operando Studies on the Role of Copper in Cu-Promoted SiO2–MgO Catalysts for the Lebedev Ethanol-to-Butadiene Process. ACS Catal. 2015, 5, 6005–6015. [Google Scholar] [CrossRef]
- Tsuchida, T.; Kubo, J.; Yoshioka, T.; Sakuma, S.; Takeguchi, T.; Ueda, W. Reaction of ethanol over hydroxyapatite affected by Ca/P ratio of catalyst. J. Catal. 2008, 259, 183–189. [Google Scholar] [CrossRef]
- Quattlebaum, W.; Toussaint, W.; Dunn, J. Deoxygenation of certain aldehydes and ketones: Preparation of butadiene and styrene1. J. Am. Chem. Soc. 1947, 69, 593–599. [Google Scholar] [CrossRef]
- Lebedev, S. Preparation of bivinyl directly from alcohol. I. Zh Obshch Khim 1933, 3, 698–717. [Google Scholar]
- Delacaillerie, J.B.D.; Gruver, V.; Fripiat, J.J. Modification of the Surface Properties of Natural Phyllosilicate Sepiolite by Secondary Isomorphic Substitution. J. Catal. 1995, 151, 420–430. [Google Scholar] [CrossRef]
- Arundale, E.; Mikeska, L. The Olefin-Aldehyde Condensation. The Prins Reaction. Chem. Rev. 1952, 51, 505–555. [Google Scholar] [CrossRef]
- Kvisle, S.; Aguero, A.; Sneeden, R.P.A. Transformation of ethanol into 1,3-butadiene over magnesium oxide/silica catalysts. Appl. Catal. 1988, 43, 117–131. [Google Scholar] [CrossRef]
- Bhattacharyya, S.K.; Avasthi, B.N. Catalytic conversion of ethanol to butadiene by two-step process in fluidised bed. J. Appl. Chem. 1966, 16, 239–244. [Google Scholar] [CrossRef]
- da Ros, S.; Jones, M.D.; Mattia, D.; Schwaab, M.; Noronha, F.B.; Pinto, J.C. Modelling the effects of reaction temperature and flow rate on the conversion of ethanol to 1, 3-butadiene. Appl. Catal. A Gen. 2017, 530, 37–47. [Google Scholar] [CrossRef]
- Baylon, R.A.; Sun, J.; Wang, Y. Conversion of ethanol to 1, 3-butadiene over Na doped ZnxZryOz mixed metal oxides. Catal. Today 2016, 259, 446–452. [Google Scholar] [CrossRef]
- Cheong, J.L.; Shao, Y.; Tan, S.J.R.; Li, X.; Zhang, Y.; Lee, S.S. Highly Active and Selective Zr/MCF Catalyst for Production of 1,3-Butadiene from Ethanol in a Dual Fixed Bed Reactor System. ACS Sustain. Chem. Eng. 2016, 4, 4887–4894. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, B.; Tan, T. Conversion of Ethanol and Acetaldehyde to Butadiene over MgO–SiO2 Catalysts: Effect of Reaction Parameters and Interaction between MgO and SiO2 on Catalytic Performance. ACS Sustain. Chem. Eng. 2017, 5, 722–733. [Google Scholar] [CrossRef]
- Hayashi, Y.; Akiyama, S.; Miyaji, A.; Sekiguchi, Y.; Sakamoto, Y.; Shiga, A.; Koyama, T.; Motokura, K.; Baba, T. Experimental and computational studies of the roles of MgO and Zn in talc for the selective formation of 1,3-butadiene in the conversion of ethanol. Phys. Chem. Chem. Phys. 2016, 18, 25191–25209. [Google Scholar] [CrossRef]
- Huang, X.; Men, Y.; Wang, J.; An, W.; Wang, Y. Highly active and selective binary MgO–SiO2 catalysts for the production of 1,3-butadiene from ethanol. Catal. Sci. Technol. 2017, 7, 168–180. [Google Scholar] [CrossRef]
- Shylesh, S.; Gokhale, A.A.; Scown, C.D.; Kim, D.; Ho, C.R.; Bell, A.T. From Sugars to Wheels: The Conversion of Ethanol to 1,3-Butadiene over Metal-Promoted Magnesia-Silicate Catalysts. ChemSusChem 2016, 9, 1462–1472. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I. Ag-Promoted ZrBEA Zeolites Obtained by Post-Synthetic Modification for Conversion of Ethanol to Butadiene. ChemSusChem 2016, 9, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Keisers, K.; Palkovits, R. Formation of 1,3-butadiene from ethanol in a two-step process using modified zeolite-β catalysts. Appl. Catal. A Gen. 2016, 514, 192–202. [Google Scholar] [CrossRef]
- Kyriienko, P.I.; Larina, O.V.; Soloviev, S.O.; Orlyk, S.M.; Dzwigaj, S. High selectivity of TaSiBEA zeolite catalysts in 1, 3-butadiene production from ethanol and acetaldehyde mixture. Catal. Commun. 2016, 77, 123–126. [Google Scholar] [CrossRef]
- Gao, M.; Jiang, H.; Zhang, M. The influence of calcination temperatures on the acid-based properties and catalytic activity for the 1, 3-butadiene synthesis from ethanol/acetaldehyde mixture. Appl. Surf. Sci. 2018, 439, 1072–1078. [Google Scholar] [CrossRef]
- Tripathi, A.; Faungnawakij, K.; Laobuthee, A.; Assabumrungrat, S.; Laosiripojna, N. Catalytic Activity of Bimetallic Cu-Ag/MgO-SiO2 Toward the Conversion of Ethanol to 1,3-Butadiene. Int. J. Chem. React. Eng. 2016, 14, 945–954. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S. Butadiene Production from Ethanol. J. Bioprocess Eng. Biorefinery 2012, 1, 33–43. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degrève, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef] [Green Version]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Chemocatalytic Conversion of Ethanol into Butadiene and Other Bulk Chemicals. ChemSusChem 2013, 6, 1595–1614. [Google Scholar] [CrossRef]
- Lanzafame, P.; Centi, G.; Perathoner, S. Catalysis for biomass and CO2 use through solar energy: Opening new scenarios for a sustainable and low-carbon chemical production. Chem. Soc. Rev. 2014, 43, 7562–7580. [Google Scholar] [CrossRef]
- Pomalaza, G.; Capron, M.; Ordomsky, V.; Dumeignil, F. Recent breakthroughs in the conversion of ethanol to butadiene. Catalysts 2016, 6, 203. [Google Scholar] [CrossRef]
- Miyaji, A.; Hiza, M.; Sekiguchi, Y.; Akiyama, S.; Shiga, A.; Baba, T. Catalysis by MgO and the Role of Zn2+ in Talc Catalysts for the Selective Production of 1,3-Butadiene from Ethanol. J. Jpn. Pet. Inst. 2018, 61, 171–181. [Google Scholar] [CrossRef]
- Ostromislenskiy, J. Production of butadiene. J. Russ. Phys. Chem. Soc. 1915, 47, 1472–1506. [Google Scholar]
- Jing, F.; Katryniok, B.; Araque, M.; Wojcieszak, R.; Capron, M.; Paul, S.; Daturi, M.; Clacens, J.M.; de Campo, F.; Liebens, A.; et al. Direct dehydration of 1,3-butanediol into butadiene over aluminosilicate catalysts. Catal. Sci. Technol. 2016, 6, 5830–5840. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.; Katryniok, B.; Paul, S.; Fang, L.; Liebens, A.; Shen, M.; Hu, B.; Dumeignil, F.; Pera-Titus, M. Al-doped SBA-15 Catalysts for Low-temperature Dehydration of 1,3-Butanediol into Butadiene. ChemCatChem 2017, 9, 258–262. [Google Scholar] [CrossRef]
- Zeng, F.; Tenn, W.J.; Aki, S.N.V.K.; Xu, J.; Liu, B.; Hohn, K.L. Influence of basicity on 1,3-butadiene formation from catalytic 2,3-butanediol dehydration over γ-alumina. J. Catal. 2016, 344, 77–89. [Google Scholar] [CrossRef]
- Fang, L.; Jing, F.; Lu, J.; Hu, B.; Pera-Titus, M. Nano-flowered Ce@MOR hybrids with modulated acid properties for the vapor-phase dehydration of 1,3-butanediol into butadiene. Green Chem. 2017, 19, 4610–4621. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Matei-Rutkovska, F.; Huchede, M.; Jaillardon, K.; Qingyi, G.; Michel, C.; Millet, J.M.M. Production of 1,3-butadiene in one step catalytic dehydration of 2,3-butanediol. Catal. Today 2019, 323, 62–68. [Google Scholar] [CrossRef]
- Kim, W.; Shin, W.; Lee, K.J.; Song, H.; Kim, H.S.; Seung, D.; Filimonov, I.N. 2,3-Butanediol dehydration catalyzed by silica-supported sodium phosphates. Appl. Catal. A Gen. 2016, 511, 156–167. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Sakami, S.; Ito, M.; Yamada, K.; Yonehara, T. Production of Bio-based 1,3-Butadiene by Highly Selective Dehydration of 2,3-Butanediol over SiO2-supported Cesium Dihydrogen Phosphate Catalyst. Chem. Lett. 2016, 45, 831–833. [Google Scholar] [CrossRef]
- Duan, H.; Yamada, Y.; Kubo, S.; Sato, S. Vapor-phase catalytic dehydration of 2,3-butanediol to 3-buten-2-ol over ZrO2 modified with alkaline earth metal oxides. Appl. Catal. A Gen. 2017, 530, 66–74. [Google Scholar] [CrossRef]
- Duan, H.; Hirota, T.; Ohtsuka, S.; Yamada, Y.; Sato, S. Vapor-phase catalytic dehydration of 1,4-butanediol to 3-buten-1-ol over modified ZrO2 catalysts. Appl. Catal. A Gen. 2017, 535, 9–16. [Google Scholar] [CrossRef]
- Sun, D.; Arai, S.; Duan, H.; Yamada, Y.; Sato, S. Vapor-phase dehydration of C4 unsaturated alcohols to 1,3-butadiene. Appl. Catal. A Gen. 2017, 531, 21–28. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Yamada, Y.; Sato, S. Selective production of 1,3-butadiene in the dehydration of 1,4-butanediol over rare earth oxides. Appl. Catal. A Gen. 2018, 562, 11–18. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.B.; Sandler, S.I.; Vlachos, D.G.; Lobo, R.F. Xylose Isomerization to Xylulose and its Dehydration to Furfural in Aqueous Media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Wang, S.; Vorotnikov, V.; Vlachos, D.G. Coverage-Induced Conformational Effects on Activity and Selectivity: Hydrogenation and Decarbonylation of Furfural on Pd(111). ACS Catal. 2015, 5, 104–112. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of Xylose to Furfural Using Lewis and Brønsted Acid Catalysts in Aqueous Media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Park, D.S.; Vinter, K.P.; Spanjers, C.S.; Ren, L.; Cho, H.J.; Vlachos, D.G.; Fan, W.; Tsapatsis, M.; Dauenhauer, P.J. Biomass-Derived Butadiene by Dehydra-Decyclization of Tetrahydrofuran. ACS Sustain. Chem. Eng. 2017, 5, 3732–3736. [Google Scholar] [CrossRef]
- Ezinkwo, G.; Tretjakov, V.; Talyshinky, R.; Ilolov, A.; Mutombo, T. Overview of the Catalytic Production of Isoprene from different raw materials; Prospects of Isoprene production from bio-ethanol. Catal. Sustain. Energy 2013, 1, 100–111. [Google Scholar] [CrossRef]
- Songsiri, N.; Rempel, G.L.; Prasassarakich, P. Liquid-phase synthesis of isoprene from MTBE and formalin using cesium salts of silicotungstic acid. Mol. Catal. 2017, 439, 41–49. [Google Scholar] [CrossRef]
- Songsiri, N.; Rempel, G.L.; Prasassarakich, P. Liquid-Phase Synthesis of Isoprene from Methyl tert-Butyl Ether and Formalin Using Keggin-Type Heteropolyacids. Ind. Eng. Chem. Res. 2016, 55, 8933–8940. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Park, D.S.; Vinter, K.P.; Spanjers, C.S.; Ren, L.; Cho, H.J.; Zhang, K.; Fan, W.; Tsapatsis, M.; Dauenhauer, P.J. Renewable Isoprene by Sequential Hydrogenation of Itaconic Acid and Dehydra-Decyclization of 3-Methyl-Tetrahydrofuran. ACS Catal. 2017, 7, 1428–1431. [Google Scholar] [CrossRef]
- Dumitriu, E.; On, D.T.; Kaliaguine, S. Isoprene by Prins Condensation over Acidic Molecular Sieves. J. Catal. 1997, 170, 150–160. [Google Scholar] [CrossRef]
- Dumitriu, E.; Hulea, V.; Fechete, I.; Catrinescu, C.; Auroux, A.; Lacaze, J.-F.; Guimon, C. Prins condensation of isobutylene and formaldehyde over Fe-silicates of MFI structure. Appl. Catal. A Gen. 1999, 181, 15–28. [Google Scholar] [CrossRef]
- An, L.-D.; Jiang, Z.-C.; Yin, Y.-G. Deactivation of AgxSbyOz/SiO2 Catalyst for The Condensation of Isobutene and Formaldehyde to Isopropene. Stud. Surf. Sci. Catal. 1987, 34, 159–171. [Google Scholar]
- Dang, Z.; Gu, J.; Yu, L. X-ray photoelectron spectroscopic study of CuSO4—MgO/SiO2 catalysts for isoprene synthesis. Appl. Catal. 1990, 63, 259–266. [Google Scholar] [CrossRef]
- Dang, Z.; Gu, J.; Yu, L.; Zhang, C. Vapor-phase synthesis of isoprene from formaldehyde and isobutylene over CuSO4−MOx/SiO2 catalysts. React. Kinet. Catal. Lett. 1991, 43, 495–500. [Google Scholar] [CrossRef]
- Krzywicki, A.; Wilanowicz, T.; Malinowski, S. Catalytic and physico-chemical properties of the Al2O3−H3PO4 system, I. Vapor phase condensation of isobutylene and formaldehyde—The Prins reaction. React. Kinet. Catal. Lett. 1979, 11, 399–403. [Google Scholar] [CrossRef]
- Ai, M. The formation of isoprene by means of a vapor-phase prins reaction between formaldehyde and isobutene. J. Catal. 1987, 106, 280–286. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ordomsky, V.V.; Ivanova, I.I. Synthesis of isoprene from formaldehyde and isobutene over phosphate catalysts. Appl. Catal. A Gen. 2012, 441–442, 21–29. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ordomsky, V.V.; Ivanova, I.I. Isoprene synthesis from formaldehyde and isobutene over Keggin-type heteropolyacids supported on silica. Catal. Sci. Technol. 2016, 6, 6354–6364. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, W.; Zhai, S.; Bao, Q.; Cheng, D.; Xia, Y.; Wang, Z.; Zhang, W. Prins condensation for the synthesis of isoprene from isobutylene and formaldehyde over sillica-supported H3SiW12O40 catalysts. React. Kinet. Mech. Catal. 2016, 117, 761–771. [Google Scholar] [CrossRef]
- Ivanova, I.; Sushkevich, V.L.; Kolyagin, Y.G.; Ordomsky, V.V. Catalysis by Coke Deposits: Synthesis of Isoprene over Solid Catalysts. Angew. Chem. 2013, 125, 13199–13202. [Google Scholar] [CrossRef]
- Qi, Y.; Cui, L.; Dai, Q.; Li, Y.; Bai, C. Assembly line synthesis of isoprene from formaldehyde and isobutene over SiO2-supported MoP catalysts with active deposited carbon. RSC Adv. 2017, 7, 37392–37401. [Google Scholar] [CrossRef]
- Qi, Y.; Cui, L.; Li, Y.; Dai, Q.; Bai, C. Development a facile way to restore reactivity of deactivated phosphate catalysts for Prins reaction with the assistance of carbon deposition. Catal. Commun. 2018, 106, 11–15. [Google Scholar] [CrossRef]
- Canter, N. Manufacturing tires from renewable resources. Tribol. Lubr. Technol. 2017, 73, 20–22. [Google Scholar]
- Willke, T.; Vorlop, K.-D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Rozentsvet, V.A.; Kozlov, V.G.; Korovina, N.A.; Ivanova, V.P.; Kostjuk, S.V. Cationic polymerization of 1,3-pentadiene coinitiated by zinc halides. J. Appl. Polym. Sci. 2013, 128, 1771–1778. [Google Scholar] [CrossRef]
- Kostjuk, S.V. Recent progress in the Lewis acid co-initiated cationic polymerization of isobutylene and 1,3-dienes. RSC Adv. 2015, 5, 13125–13144. [Google Scholar] [CrossRef]
- Ren, L.; Liu, K.; He, Q.; Ou, E.; Lu, Y.; Xu, W. Anionic polymerization of 1,3-pentadiene in toluene: Homopolymer, alternating and block copolymers. RSC Adv. 2016, 6, 51533–51543. [Google Scholar] [CrossRef]
- Kumar, A.; Hackenberg, J.D.; Zhuo, G.; Steffens, A.M.; Mironov, O.; Saxton, R.J.; Goldman, A.S. High yields of piperylene in the transfer dehydrogenation of pentane catalyzed by pincer-ligated iridium complexes. J. Mol. Catal. A Chem. 2017, 426, 368–375. [Google Scholar] [CrossRef]
- Kundu, S.; Lyons, T.W.; Brookhart, M. Synthesis of Piperylene and Toluene via Transfer Dehydrogenation of Pentane and Pentene. ACS Catal. 2013, 3, 1768–1773. [Google Scholar] [CrossRef]
- Behr, A.; Neubert, P. Piperylene—A Versatile Basic Chemical in Catalysis. ChemCatChem 2014, 6, 412–428. [Google Scholar] [CrossRef]
- Burnette, L.W. The production of rubber from furfural. Rubber Chem. Technol. 1945, 18, 284–285. [Google Scholar] [CrossRef]
- Schniepp, L.; Geller, H. The Preparation of 1,3- and 1,4-Pentadienes from Furfural. J. Am. Chem. Soc. 1945, 67, 54–56. [Google Scholar] [CrossRef]
- Casas, E.; de Ancos, B.; Valderrama, M.J.; Cano, P.; Peinado, J.M. Pentadiene production from potassium sorbate by osmotolerant yeasts. Int. J. Food Microbiol. 2004, 94, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Bui, P.; Cecilia, J.A.; Oyama, S.T.; Takagaki, A.; Infantes-Molina, A.; Zhao, H.; Li, D.; Rodríguez-Castellón, E.; López, A.J. Studies of the synthesis of transition metal phosphides and their activity in the hydrodeoxygenation of a biofuel model compound. J. Catal. 2012, 294, 184–198. [Google Scholar] [CrossRef]
- Sun, R.; Zheng, M.; Li, X.; Pang, J.; Wang, A.; Wang, X.; Zhang, T. Production of renewable 1,3-pentadiene from xylitol via formic acid-mediated deoxydehydration and palladium-catalyzed deoxygenation reactions. Green Chem. 2017, 19, 638–642. [Google Scholar] [CrossRef]
- Kumbhalkar, M.D.; Buchanan, J.S.; Huber, G.W.; Dumesic, J.A. Ring Opening of Biomass-Derived Cyclic Ethers to Dienes over Silica/Alumina. ACS Catal. 2017, 7, 5248–5256. [Google Scholar] [CrossRef]
- Chern, Y.-T.; Shiue, H.-C. Low Dielectric Constants of Soluble Polyimides Based on Adamantane. Macromolecules 1997, 30, 4646–4651. [Google Scholar] [CrossRef]
- Fukukawa, K.-I.; Shibasaki, Y.; Ueda, M. A Photosensitive Semi-Alicyclic Poly(benzoxazole) with High Transparency and Low Dielectric Constant. Macromolecules 2004, 37, 8256–8261. [Google Scholar] [CrossRef]
- Feng, F.; Mitsuishi, M.; Miyashita, T.; Okura, I.; Asai, K.; Amao, Y. Preparation of Polymer Langmuir−Blodgett Films Containing Porphyrin Chromophore. Langmuir 1999, 15, 8673–8677. [Google Scholar] [CrossRef]
- van Reenen, A.J.; Mathias, L.J.; Coetzee, L. Polymerization of olefins with bulky substituents. 1. Homo- and copolymerization of 3-(1-adamantyl)propene. Polymer 2004, 45, 799–804. [Google Scholar] [CrossRef]
- Acar, H.Y.; Jensen, J.J.; Thigpen, K.; McGowen, J.A.; Mathias, L.J. Evaluation of the Spacer Effect on Adamantane-Containing Vinyl Polymer Tg’s. Macromolecules 2000, 33, 3855–3859. [Google Scholar] [CrossRef]
- Ishizone, T.; Tajima, H.; Torimae, H.; Nakahama, S. Anionic Polymerizations of 1-Adamantyl Methacrylate and 3-Methacryloyloxy-1,1′-biadamantane. Macromol. Chem. Phys. 2002, 203, 2375–2384. [Google Scholar] [CrossRef]
- Hashimoto, T.; Makino, Y.; Urushisaki, M.; Sakaguchi, T. Living cationic polymerization of 2-adamantyl vinyl ether. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1629–1637. [Google Scholar] [CrossRef]
- Matsumoto, A.; Tanaka, S.; Otsu, T. Synthesis and characterization of poly (1-adamantyl methacrylate): Effects of the adamantyl group on radical polymerization kinetics and thermal properties of the polymer. Macromolecules 1991, 24, 4017–4024. [Google Scholar] [CrossRef]
- Kobayashi, S.; Matsuzawa, T.; Matsuoka, S.-I.; Tajima, H.; Ishizone, T. Living Anionic Polymerizations of 4-(1-Adamantyl)styrene and 3-(4-Vinylphenyl)-1,1‘-biadamantane. Macromolecules 2006, 39, 5979–5986. [Google Scholar] [CrossRef]
- Mathias, L.J.; Lewis, C.M.; Wiegel, K.N. Poly(ether ether ketone)s and Poly(ether sulfones) with Pendent Adamantyl Groups. Macromolecules 1997, 30, 5970–5975. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kataoka, H.; Ishizone, T.; Kato, T.; Ono, T.; Kobukata, S.; Arimoto, K.; Ogi, H. Synthesis of well-defined random and block copolymers of 2-(1-adamantyl)-1,3-butadiene with isoprene via anionic polymerization. React. Funct. Polym. 2009, 69, 409–415. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kataoka, H.; Ishizone, T. Synthesis of Well-Defined Poly(ethylene-alt-1-vinyladamantane) via Living Anionic Polymerization of 2-(1-Adamantyl)-1,3-butadiene, Followed by Hydrogenation. Macromolecules 2009, 42, 5017–5026. [Google Scholar] [CrossRef]
- Cai, Y.; Lu, J.; Zuo, D.; Li, S.; Cui, D.; Han, B.; Yang, W. Extremely High Glass Transition Temperature Hydrocarbon Polymers Prepared through Cationic Cyclization of Highly 3,4-Regulated Poly(Phenyl-1,3-Butadiene). Macromol. Rapid Commun. 2018, 39, 1800298. [Google Scholar] [CrossRef] [PubMed]
- Marvel, C.; Woolford, R. 2-Phenyl-1,3-butadiene and Related Compounds1. J. Org. Chem. 1958, 23, 1658–1660. [Google Scholar] [CrossRef]
- Pragliola, S.; Cipriano, M.; Boccia, A.C.; Longo, P. Polymerization of Phenyl-1,3-butadienes in the Presence of Ziegler-Natta Catalysts. Macromol. Rapid Commun. 2002, 23, 356–361. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsuji, Y.; Takegami, Y.; Harwood, H.J. Microstructure of poly (2-phenylbutadiene) prepared by anionic initiators. Macromolecules 1979, 12, 234–239. [Google Scholar] [CrossRef]
- Yao, C.; Xie, H.; Cui, D. Highly 3,4-selective living polymerization of 2-phenyl-1,3-butadiene with amidino N-heterocyclic carbene ligated rare-earth metal bis(alkyl) complexes. RSC Adv. 2015, 5, 93507–93512. [Google Scholar] [CrossRef]
- Yao, C.; Liu, N.; Long, S.; Wu, C.; Cui, D. Highly cis-1,4-selective coordination polymerization of polar 2-(4-methoxyphenyl)-1,3-butadiene and copolymerization with isoprene using a β-diketiminato yttrium bis(alkyl) complex. Polym. Chem. 2016, 7, 1264–1270. [Google Scholar] [CrossRef]
- Cai, Y.; Lu, J.; Jing, G.; Yang, W.; Han, B. High-Glass-Transition-Temperature Hydrocarbon Polymers Produced through Cationic Cyclization of Diene Polymers with Various Microstructures. Macromolecules 2017, 50, 7498–7508. [Google Scholar] [CrossRef]
- Yao, C.; Lin, F.; Wang, M.; Liu, D.; Liu, B.; Liu, N.; Wang, Z.; Long, S.; Wu, C.; Cui, D. Highly Syndioselective 3,4-Trans Polymerization of (E)-1-(4-Methylphenyl)-1,3-butadiene by Fluorenyl N-Heterocyclic Carbene Ligated Lutetium Bis(alkyl) Precursor. Macromolecules 2015, 48, 1999–2005. [Google Scholar] [CrossRef]
- Masuda, T.; Otsuki, M.; Higashimura, T. Structure and reactivity in cationic polymerization of butadiene derivatives. II. 1-phenylbutadiene. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 1385–1394. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsuji, Y.; Takegami, Y. Microstructure of poly (1-phenylbutadiene) prepared by anionic initiators. Macromolecules 1978, 11, 639–644. [Google Scholar] [CrossRef]
- Asami, R.; Hasegawa, K.-I.; Onoe, T. Cationic Polymerization of Phenylbutadienes. I. Cationic Polymerization of trans-1-Phenyl-1,3-butadieiie. Polym. J. 1976, 8, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Ukai, J.; Ikeda, N.; Yamamoto, H. Stereoselective synthesis of (z)- and (e)-1,3-alkadienes from aldehydes using organotitanium and lithium reagents. Tetrahedron 1987, 43, 723–730. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Cui, D. Statistically Syndioselective Coordination (Co)polymerization of 4-Methylthiostyrene. Macromolecules 2016, 49, 781–787. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Wang, Z.; Cui, D. Development of Group 3 Catalysts for Alternating Copolymerization of Ethylene and Styrene Derivatives. ACS Catal. 2018, 8, 6086–6093. [Google Scholar] [CrossRef]
- Liu, D.; Wang, M.; Wang, Z.; Wu, C.; Pan, Y.; Cui, D. Stereoselective Copolymerization of Unprotected Polar and Nonpolar Styrenes by an Yttrium Precursor: Control of Polar-Group Distribution and Mechanism. Angew. Chem. Int. Ed. 2017, 56, 2714–2719. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cui, F.; He, J.; Cui, L.; Li, Y.; Dai, Q.; Bai, C. Insight into performance of lactam-based Brønsted-acidic catalysts for Prins condensation and their self-separation in water. Mol. Catal. 2018, 445, 80–86. [Google Scholar] [CrossRef]
- Trofimov, B.A. Acetylene and its Derivatives in Reactions with Nucleophiles: Recent Advances and Current Trends. Curr. Org. Chem. 2002, 6, 1121–1162. [Google Scholar] [CrossRef]
- Boris, A.T.; Nina, K.G. Acetylene: New prospects of classical reactions. Russ. Chem. Rev. 2007, 76, 507–527. [Google Scholar]
- Trofimov, B. New Intermediates for Organic Synthesis based on Acetylene. Zeitschrift für Chemie 1986, 26, 41–49. [Google Scholar] [CrossRef]
- Vitkovskaya, N.M.; Larionova, E.Y.; Skitnevskaya, A.D.; Kobychev, V.B.; Trofimov, B.A. Nucleophilic addition of methanol and methanethiol to acetylene in the superbasic system KOH-DMSO: A quantum chemical model. Russ. Chem. Bull. 2013, 62, 26–32. [Google Scholar] [CrossRef]
- Vitkovskaya, N.M.; Larionova, E.Y.; Skitnevskaya, A.D.; Trofimov, B.A. Hydrative trimerization of acetylene into 2-vinyloxy-1,3-butadiene in the KOH/DMSO system: A quantum chemical insight. Tetrahedron Lett. 2015, 56, 1063–1066. [Google Scholar] [CrossRef]
- Hirao, A.; Hiraishi, Y.; Nakahama, S.; Takenaka, K. Polymerization of Monomers Containing Functional Silyl Groups. 13. Anionic Polymerization of 2-[(N,N-Dialkylamino)dimethylsilyl]-1,3-butadiene Derivatives. Macromolecules 1998, 31, 281–287. [Google Scholar] [CrossRef]
- Takenaka, K.; Akagawa, Y.; Takeshita, H.; Miya, M.; Shiomi, T. Polymerization of 1, 3-Dienes Containing Functional Groups 6: Unexpected Collapse of Monomer Structure in the Anionic Polymerization of 2-Ethoxymethyl-l, 3-butadiene. Polym. J. 2009, 41, 106–107. [Google Scholar] [CrossRef]
- Petzhold, C.; Morschhaeuser, R.; Kolshorn, H.; Stadler, R. On the Anionic Polymerization of (Dialkylamino) isoprenes. 2. Influence of the Tertiary Amino Group on the Polymer Microstructure. Macromolecules 1994, 27, 3707–3713. [Google Scholar] [CrossRef]
- Jing, Y.; Sheares, V.V. Polar Functionalized Diene-Based Materials. 1. Bulk, Solution, and Emulsion Free Radical Polymerization of 2-Cyanomethyl-1,3-butadiene. Macromolecules 2000, 33, 6255–6261. [Google Scholar] [CrossRef]
- Jing, Y.; Sheares, V.V. Polar, Functionalized Diene-Based Materials. 2. Free Radical Copolymerization Studies of 2-Cyanomethyl-1,3-butadiene with Styrene and Acrylonitrile. Macromolecules 2000, 33, 6262–6268. [Google Scholar] [CrossRef]
- Yang, Y.; Sheares, V.V. Synthesis of disubstituted amine-functionalized diene-based polymers. Polymer 2007, 48, 105–109. [Google Scholar] [CrossRef]
- Wu, L.; Sheares, V.V. Polar, functionalized diene-based materials. V. Free-radical polymerization of 2-[(N-benzyl-n-methylamino)methyl]-1,3-butadiene and copolymerization with styrene. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3227–3238. [Google Scholar] [CrossRef]
- Mannebach, G.; Morschhäuser, R.; Stadler, R.; Petzhold, C. On the anionic polymerization of dialkylaminoisoprenes, 4. Experimental observation of the unusual polymerization kinetics. Macromol. Chem. Phys. 1998, 199, 909–912. [Google Scholar] [CrossRef]
- Sheares, V.; Li, Y.; Emmick, T.; Martin, C.; Jing, Y.; Beery, M. Polar functionalized materials via free radical polymerization of substituted dienes. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 1999; Volume 80, p. 79. [Google Scholar]
- Sheares, V.V.; Wu, L.; Li, Y.; Emmick, T.K. Polar, functionalized diene-based material. III. Free-radical polymerization of 2-[(N,N-dialkylamino)methyl]-1,3-butadienes. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4070–4080. [Google Scholar] [CrossRef]
- Blackley, D.C. Monomers for Synthetic Rubber Production, Synthetic Rubbers: Their Chemistry and Technology; Springer: Dordrecht, The Netherlands, 1983; pp. 32–58. [Google Scholar]
- Blackley, D.C. Synthetic Rubbers: Their Chemistry and Technology; Applied Science Publishers: Barking, UK, 1983; pp. 126–141. [Google Scholar]
- Sato, F.; Uchiyama, H.; Samaddar, A.K. 1984. Available online: https://pubs.acs.org/servlet/linkout?suffix=ol070089eb00011/ol070089eb00011_1&dbid=32&doi=10.1021%2Fol070089e&key=1%3ACAS%3A528%3ADyaL2MXisVOjtw%253D%253D (accessed on 17 November 2018).
- Takenaka, K.; Kawamoto, S.; Miya, M.; Takeshita, H.; Shiomi, T. Polymerization of 1,3-dienes containing functional groups: 8. Free-radical polymerization of 2-triethoxysilyl1,3-butadiene. Polym. Int. 2010, 59, 891–895. [Google Scholar] [CrossRef]
- Takenaka, K.; Hattori, T.; Hirao, A.; Nakahama, S. Polymerization of monomers containing functional silyl groups. 6. Anionic polymerization of 2-(trialkoxysilyl)-1,3-butadiene. Macromolecules 1989, 22, 1563–1567. [Google Scholar] [CrossRef]
- Takenaka, K.; Hanada, K.; Shiomi, T. Polymerization of 1,3-Dienes with Functional Groups. 1. Free-Radical Polymerization of 2-Triethoxymethyl-1,3-butadiene. Macromolecules 1999, 32, 3875–3877. [Google Scholar] [CrossRef]
- Nunomoto, S.; Yamashita, Y. Reaction of 2-(1,3-butadienyl) magnesium chloride with carbonyl compounds and epoxides. A regioselectivity study. J. Org. Chem. 1979, 44, 4788–4791. [Google Scholar] [CrossRef]
- Leicht, H.; Göttker-Schnetmann, I.; Mecking, S. Stereoselective Copolymerization of Butadiene and Functionalized 1,3-Dienes. ACS Macro Lett. 2016, 5, 777–780. [Google Scholar] [CrossRef]
- Beery, M.D.; Rath, M.K.; Sheares, V.V. Polar, Functionalized Diene-Based Materials. 4. Polymerization Studies of 2,3-Bis(4-ethoxy-4-oxobutyl)-1,3-butadiene and Copolymerization with Styrene. Macromolecules 2001, 34, 2469–2475. [Google Scholar] [CrossRef]
- Marshall, J.D.; Toffel, M.W. Framing the Elusive Concept of Sustainability: A Sustainability Hierarchy. Environ. Sci. Technol. 2005, 39, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Sikdar, S.K. Sustainability Perspective and Chemistry-Based Technologies. Ind. Eng. Chem. Res. 2007, 46, 4727–4733. [Google Scholar] [CrossRef]
- Hillmyer, M.A. The promise of plastics from plants. Science 2017, 358, 868–870. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I.; Ordomsky, V.V.; Taarning, E. Design of a Metal-Promoted Oxide Catalyst for the Selective Synthesis of Butadiene from Ethanol. ChemSusChem 2014, 7, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals: Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-S.; Kim, B.; Shin, J.H.; Choi, Y.J.; Choi, S.; Song, C.W.; Lee, J.; Park, H.G.; Lee, S.Y. Bio-based production of C2–C6 platform chemicals. Biotechnol. Bioeng. 2012, 109, 2437–2459. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]




| Catalyst | Reaction Temperature °C | Conversion% | BD Selectivity % | Ref. | BD Formation rate/mmol (gcat.h)−1 |
|---|---|---|---|---|---|
| ZnO–MgO/H–β280 | 350 | 43.6 | 63.4 | [36] | 0.92 |
| NaZn1Zr10Oz–H | 350 | 54.4 | 28 | [37] | 9.07 |
| Cu/MCF–Zr/MCF | 235, 400 | 96 | 37 | [38] | 25.92 |
| MgO–SiO2–500 a | 500 | Total 29.7 | 80.7 | [39] | 0.96 |
| Talc/Zn | 400 | 48.4 | 61.0 | [40] | 8.53 |
| MgO–SiO2 (65:35) | 450 | 95 | 77 | [41] | 25 |
| 3%Au/MgO–SiO2 | 300 | >45 | ~60 | [42] | 2.4 |
| Ag/Zr(3.3)BEA(38) | 320 | 15 | ~60 | [43] | 10.3 |
| Cu/SiO2 MgO/H–β280 | 100, 300 | - | 33% yield | [44] | - |
| Ta3.0SiBEA–EtOH/AA=3.2 | 350 | 58.9 | 73.1 | [45] | - |
| 2%ZrO2/NanoSiO2–500 a | 320 | 58.52 | 93.18 | [46] | - |
| 5 % Ag/MgO–SiO2 | 275 | >50 | >28 yield | [47] | - |
| 2.5% Cu-2.5%wt Ag/MgO–SiO2 | 300 | 60 | >40 yield | [47] | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Liu, Z.; Liu, S.; Cui, L.; Dai, Q.; He, J.; Dong, W.; Bai, C. Synthesis of 1,3-Butadiene and Its 2-Substituted Monomers for Synthetic Rubbers. Catalysts 2019, 9, 97. https://doi.org/10.3390/catal9010097
Qi Y, Liu Z, Liu S, Cui L, Dai Q, He J, Dong W, Bai C. Synthesis of 1,3-Butadiene and Its 2-Substituted Monomers for Synthetic Rubbers. Catalysts. 2019; 9(1):97. https://doi.org/10.3390/catal9010097
Chicago/Turabian StyleQi, Yanlong, Zaizhi Liu, Shijun Liu, Long Cui, Quanquan Dai, Jianyun He, Wei Dong, and Chenxi Bai. 2019. "Synthesis of 1,3-Butadiene and Its 2-Substituted Monomers for Synthetic Rubbers" Catalysts 9, no. 1: 97. https://doi.org/10.3390/catal9010097
APA StyleQi, Y., Liu, Z., Liu, S., Cui, L., Dai, Q., He, J., Dong, W., & Bai, C. (2019). Synthesis of 1,3-Butadiene and Its 2-Substituted Monomers for Synthetic Rubbers. Catalysts, 9(1), 97. https://doi.org/10.3390/catal9010097