Synthesis of GO-SalenMn and Asymmetric Catalytic Olefin Epoxidation
Abstract
:1. Introduction
2. Results and Discussion
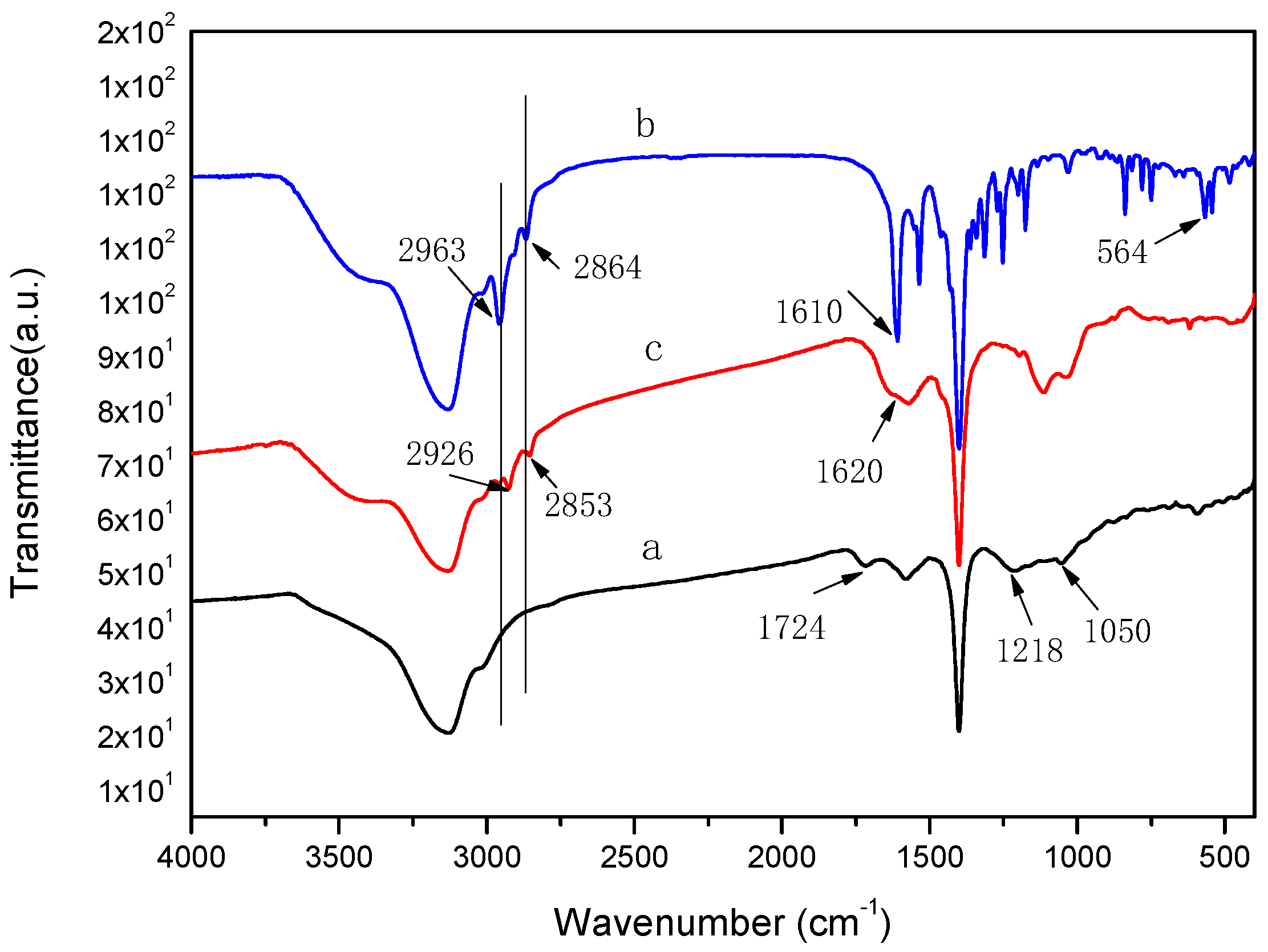
2.1. FT–IR Analysis
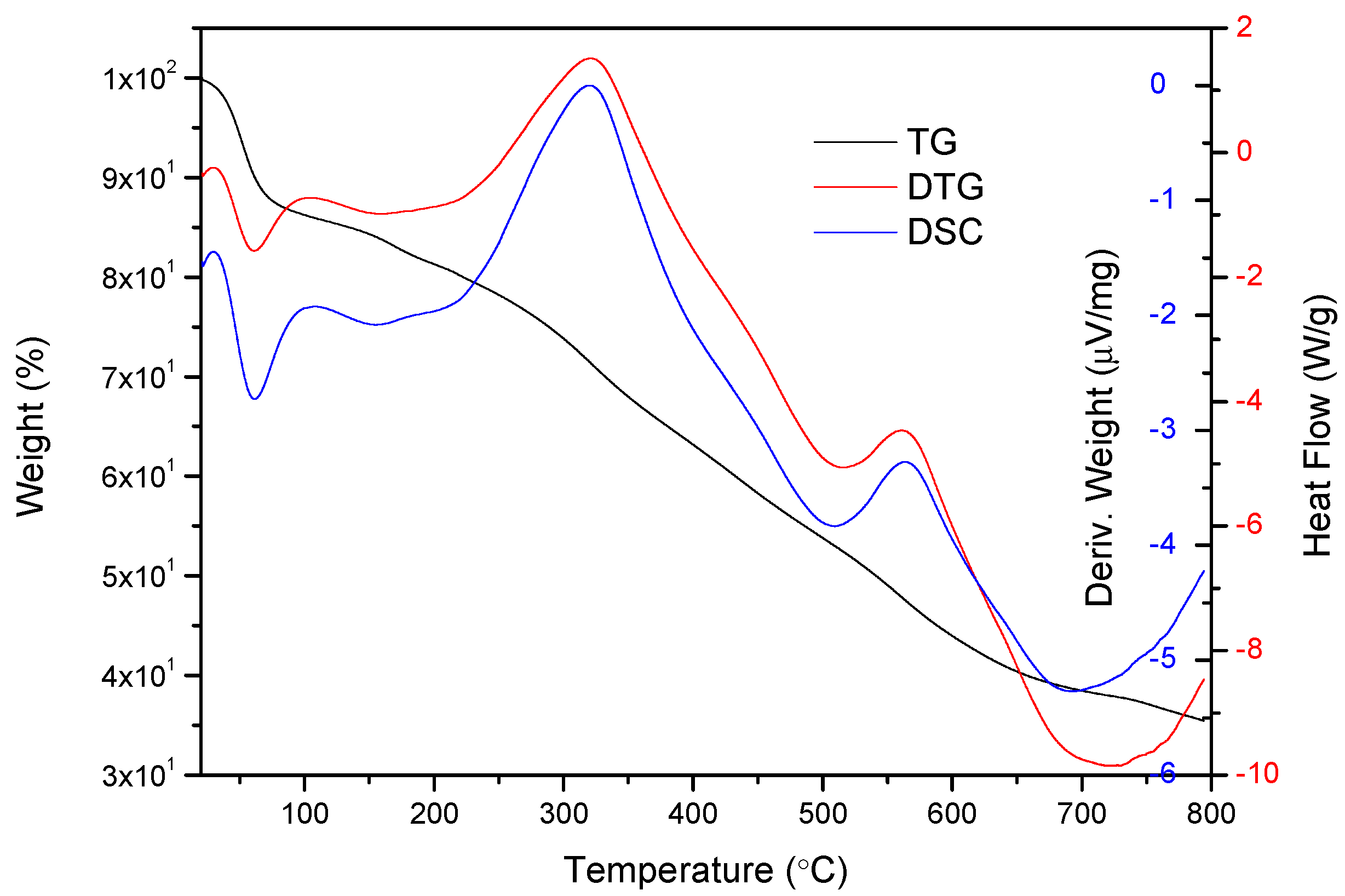
2.2. TG–DSC Analysis
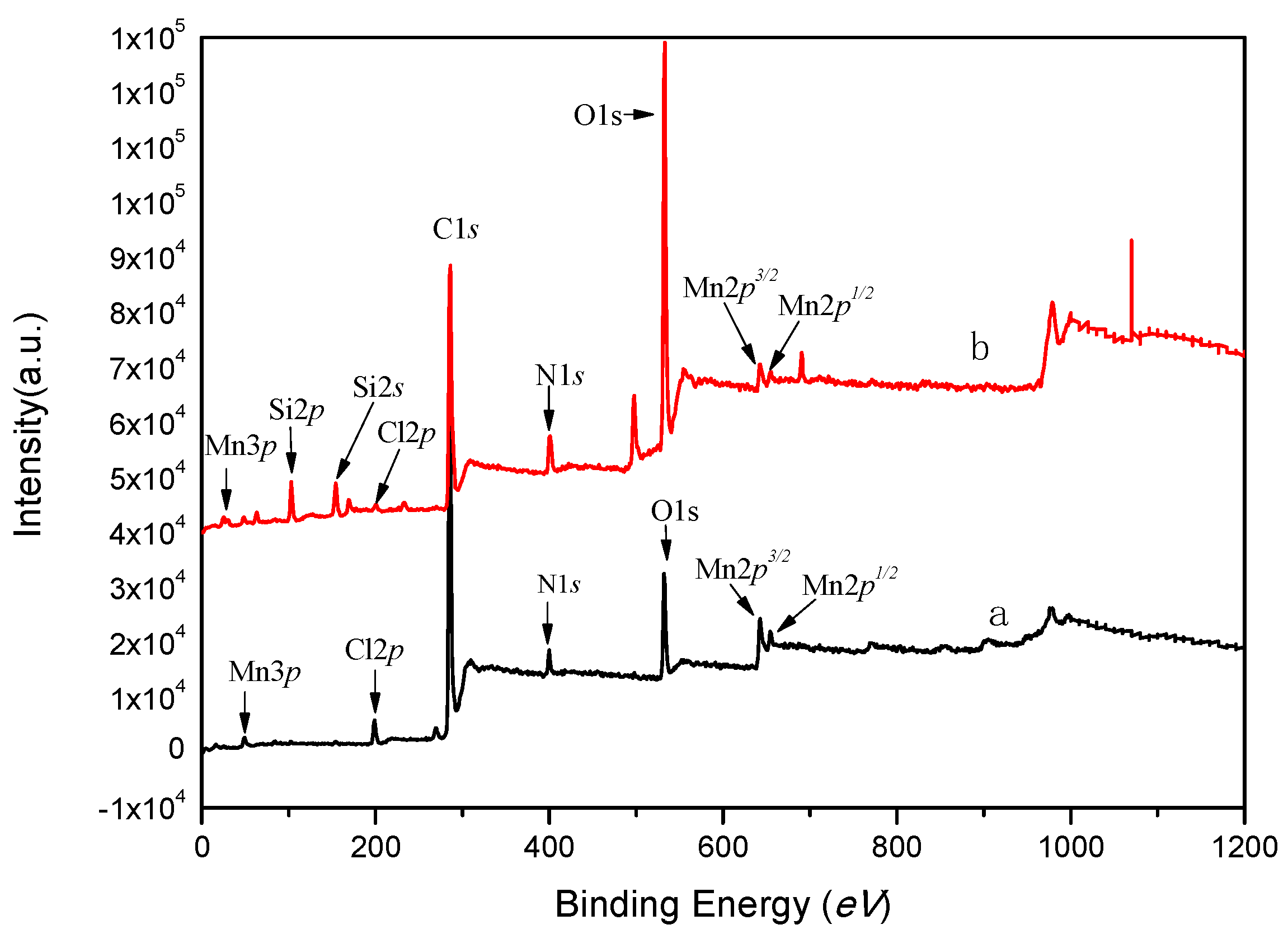
2.3. XPS Analysis
2.4. SEM and EDS Analysis
2.5. XRD Analysis
2.6. Asymmetric Epoxidation
2.7. Investigation of Reuse Performance of Supported Catalysts
3. Material and Methods
3.1. Materials
3.2. Methods
4. Preparation of Catalysts
4.1. Synthesis of GO and GO-NH2
4.2. Synthesis of GO-salenMn Catalyst
4.3. Asymmetric Epoxidation
4.4. Repeated Experiments of Supported Mn(Salen) Catalyst
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Luo, Y.F.; Zou, X.C.; Fu, X.K.; Jia, Z.Y.; Huang, X.M. The advance in asymmetric epoxidation of olefins catalyzed by chiral Mn(salen). Sci. China Chem. 2011, 41, 433–450. [Google Scholar]
- Zhang, H.D.; Zhang, Y.M.; Li, C. Asymmetric epoxidation of unfunctionalized olefins catalyzed by Mn(salen) axially immobilized onto insoluble polymers. Tetrahedron Asymmetry 2005, 16, 2417–2423. [Google Scholar] [CrossRef]
- Aneta, N.; Agnieszka, W.; Michael, W. Recent advances in the catalytic oxidation of alkene and alkane substrates using immobilized manganese complexes with nitrogen containing ligands. Coord. Chem. Rev. 2019, 382, 181–216. [Google Scholar]
- Reger, T.S.; Janda, K.D. Polymer-Supported (Salen)Mn Catalysts for Asymmetric Epoxidation: A Comparison Between Soluble and Insoluble Matrices. J. Am. Chem. Soc. 2000, 122, 6929–6934. [Google Scholar] [CrossRef]
- Peng, M.; Chen, Y.J.; Tan, R.; Zheng, W.G.; Yin, D.H. A highly efficient and recyclable catalyst-dendrimer supported chiral salen Mn(III) complexes for asymmetric epoxidation. RSC Adv. 2013, 3, 20684–20692. [Google Scholar] [CrossRef]
- Afsaneh, F.; Hassan, H.M. Highly efficient asymmetric epoxidation of olefins with a chiral manganese-porphyrin covalently bound to mesoporous SBA-15: Support effect. J. Catal. 2017, 352, 229–238. [Google Scholar]
- Zou, X.C.; Shi, K.Y.; Li, J.; Wang, Y.; Deng, C.F.; Wang, C.; Ren, Y.R.; Tan, J. Research Progress on Epoxidation of Olefins Catalyzed by Mn(II, III, V) in Different Valence States. Chin. J. Org. Chem. 2016, 36, 1765–1778. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.W.; Fu, X.K.; Chen, J.X.; Li, Y.D.; Zou, X.C.; Tu, X.B.; Ding, P.P.; Ma, L.P. Synthesis of a new type of immobilized chiral salen Mn(III) complex as effective catalysts for asymmetric epoxidation of unfunctionalized olefins. J. Catal. 2009, 262, 9–17. [Google Scholar] [CrossRef]
- Zou, X.C.; Fu, X.K.; Li, Y.D.; Tu, X.B.; Fu, S.D.; Luo, Y.F.; Wu, X.J. Highly enantioselective epoxidation of unfunctionalized olefins catalyzed by chiral Jacobsen’s catalyst immobilized on phenoxyl modified Zirconium poly (styrene-phenylvinylphosphonate)-phosphate. Adv. Synth. Catal. 2010, 352, 163–170. [Google Scholar] [CrossRef]
- Zou, X.C.; Wang, C.; Wang, Y.; Shi, K.Y.; Wang, Z.M.; Li, D.W.; Fu, X.K. Chiral MnIII (Salen) Covalently Bonded on Modified ZPS-PVPA and ZPS-IPPA as Efficient Catalysts for Enantioselective Epoxidation of Unfunctionalized Olefins. Polymers 2017, 9, 108. [Google Scholar] [CrossRef]
- Zou, X.C.; Shi, K.Y.; Wang, C. Chiral MnIII(Salen) supported on tunable phenoxyl group modified zirconium poly (styrene-phenylvinylphosphonate)-phosphate as an efficient catalyst for epoxidation of unfunctionalized olefins. Chin. J. Catal. 2014, 35, 1446. [Google Scholar] [CrossRef]
- Huang, J.; Liu, S.R.; Ma, Y.; Cai, J.L. Chiral salen Mn (III) immobilized on ZnPS-PVPA through alkoxyl-triazole for superior performance catalyst in asymmetric epoxidation of unfunctionalized olefins. J. Organomet. Chem. 2019, 886, 27–33. [Google Scholar] [CrossRef]
- Huang, X.M.; Fu, X.K.; Jia, Z.Y.; Miao, Q.; Wang, G.M. Chiral salen Mn(III) complexes immobilized onto crystalline aluminium oligo-styrenyl phosphonate-hydrogen phosphate (AlSPP) for heterogeneous asymmetric epoxidation. Catal. Sci. Technol. 2013, 3, 415–424. [Google Scholar] [CrossRef]
- Huang, J.; Tang, M.; Li, X.; Zhong, G.Z.; Li, C.M. Novel layered crystalline organic polymer-inorganic hybrid material comprising calcium phosphate with unique architectures for superior performance catalyst support. Dalton Trans. 2014, 43, 17500–17508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Yang, C.L.; Cui, J.X.; Ma, Y.Y.; Kan, Q.B.; Guan, J.Q. Recent Advancements in Graphene-Based Supports of Metal Complexes/Oxides for Epoxidation of Alkenes. Chem. Asian J. 2018, 13, 3790–3799. [Google Scholar] [CrossRef]
- Siegfried, E.; Andreas, H. Chemistry with graphene and graphene oxide-challenges for synthetic chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar]
- Scheuermann, G.M.; Rumi, L.; Steurer, P.; Bannwarth, W.; Mülhaupt, R. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2009, 131, 8262–8270. [Google Scholar] [CrossRef]
- Shao, L.D.; Huang, X.; Teschner, D.; Zhang, W. Gold Supported on Graphene Oxide: An Active and Selective Catalyst for Phenylacetylene Hydrogenations at Low Temperatures. ACS Catal. 2014, 4, 2369–2373. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.G.; Wageh, S.; Ghamdi, A.A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Wang, H.L.; Casalongue, H.S.; Chen, Z.; Dai, H.J. TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef]
- Yun, M.; Ahmed, M.S.; Jeon, S. Thiolated graphene oxide-supported palladium cobalt alloyed nanoparticles as high performance electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 293, 380–387. [Google Scholar] [CrossRef]
- Yuan, D.C.; Chen, B.B. Synthesis and Characterization of Graphene Oxide Supported Schiff Base Palladium Catalyst and Its Catalytic Performance to Suzuki Reaction. Chin. J. Org. Chem. 2014, 34, 1630–1638. [Google Scholar] [CrossRef]
- Tan, R.; Li, C.Y.; Luo, J.Q.; Kong, Y.; Zheng, W.G.; Yin, D.H. An effective heterogeneous L-proline catalyst for the direct asymmetric aldol reaction using graphene oxide as support. J. Catal. 2013, 298, 138–147. [Google Scholar] [CrossRef]
- Shi, H.; Yu, C.; He, J. On the Structure of Layered Double Hydroxides Intercalated with Titanium Tartrate Complex for Catalytic Asymmetric Sulfoxidation. J. Phys. Chem. C 2010, 114, 17819–17828. [Google Scholar] [CrossRef]
- Zheng, W.G.; Tan, R.; Yin, S.F.; Zhang, Y.Y.; Zhao, G.W.; Chen, Y.J.; Yin, D.H. Ionic liquid-functionalized graphene oxide as an efficient support for the chiral salen MnIII complex in asymmetric epoxidation of unfunctionalized olefins. Catal. Sci. Technol. 2015, 5, 2092–2102. [Google Scholar] [CrossRef]
- Domenech, A.; Formentin, P.; Garcia, H.; Sabater, M.J. Combined electrochemical and EPR studies of manganese Schiff base complexes encapsulated within the cavities of zeolite Y. Eur. J. Inorg. Chem. 2000, 2000, 1339–1344. [Google Scholar] [CrossRef]
- Zou, X.C.; Wang, Y.; Wang, C.; Shi, K.Y.; Ren, Y.R.; Zhao, X. Chiral MnIII (Salen) Immobilized on Organic Polymer/Inorganic Zirconium Hydrogen Phosphate Functionalized with 3-Aminopropyltrimethoxysilane as an Efficient and Recyclable Catalyst for Enantioselective Epoxidation of Styrene. Polymers 2019, 11, 212. [Google Scholar] [CrossRef]
- Caplan, N.A.; Hancock, F.E.; Bulman, P.P.C.; Hutchings, G.J. Heterogeneous Enantioselective Catalyzed Carbonyl- and Imino-Ene Reactions using Copper Bis(Oxazoline) Zeolite, Y. Angew. Chem. Int. Ed. 2004, 43, 1685–1688. [Google Scholar] [CrossRef]
- Venkataramanana, N.S.; Rajagopal, S. Effect of added donor ligands on the selective oxygenation of organic sulfides by oxo(salen)chromium(V) complexes. Tetrahedron 2006, 62, 5645–5651. [Google Scholar] [CrossRef]
- Ma, X.B.; Wang, Y.H.; Wang, W.; Cao, J. Synthesis and characterization of mesoporous zirconium phosphonates: A novel supported cinchona alkaloid catalysts in asymmetric catalysis. Catal. Commun. 2010, 11, 401–407. [Google Scholar] [CrossRef]
- Jia, H.P.; Dreyer, D.R.; Bielawski, C.W. Graphite Oxide as an Auto-Tandem Oxidation–Hydration–Aldol Coupling Catalyst. Adv. Synth. Catal. 2011, 53, 528–532. [Google Scholar] [CrossRef]
- Zou, Z.G.; Yu, H.J.; Long, F.; Fan, Y.H. Preparation of Graphene Oxide by Ultrasound-Assisted Hummers Method. Chin. J. Inorg. Chem. 2011, 27, 1753–1757. [Google Scholar]
- Zhang, F.; Jiang, H.Y.; Li, X.Y.; Wu, X.T.; Li, H.X. Amine-Functionalized GO as an Active and Reusable Acid–Base Bifunctional Catalyst for One-Pot Cascade Reactions. ACS Catal. 2014, 4, 394−401. [Google Scholar] [CrossRef]






| Entry | Substrate | Catalyst | Oxidant | Time (h) | Conv (%) b | Sele (%) b | ee (%) b | TOFe × 10−4 (s−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 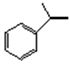 | salenMnCl | m-CPBA/NMO (seen in Section 3.1) | 1 | >99 | >99 | 52.0 c | 27.2 |
| 2 | GO-salenMn | m-CPBA/NMO | 2 | >99 | 97.9 | 83.2 c | 13.5 | |
| 3 | GO-salenMn | m-CPBA | 2 | 65.9 | 76.5 | 8.6 c | 7.0 | |
| 4 | 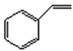 | salenMnCl | m-CPBA/NMO | 1 | >99 | 98.7 | 46.2 c | 27.1 |
| 5 | GO-salenMn | m-CPBA/NMO | 2 | 92.5 | 93.1 | 67.8 c | 12.0 | |
| 6 | GO-salenMn | m-CPBA | 2 | 73.2 | 72.9 | 5.9 c | 7.4 | |
| 7 | 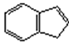 | salenMnCl | m-CPBA/NMO | 1 | >99 | >99 | 65.0 d | 27.2 |
| 8 | GO-salenMn | m-CPBA/NMO | 2 | >99 | >99 | 83.2 d | 13.6 | |
| 9 | GO-salenMn | m-CPBA | 2 | 77.9 | 81.6 | 13.1 d | 8.8 |
| Run | Conv (%) b | Sele (%) b | ee (%) b,c | TOF d × 10−4 (s−1) |
|---|---|---|---|---|
| 1 | >99 | 97.9 | 83.2 | 13.5 |
| 2 | >99 | 97.0 | 83.0 | 13.3 |
| 3 | >99 | 96.3 | 82.4 | 13.3 |
| 4 | 98.2 | 95.2 | 82.2 | 13.0 |
| 5 | 96.9 | 94.3 | 81.7 | 12.7 |
| 6 | 95.3 | 92.2 | 81.0 | 12.2 |
| 7 | 92.1 | 86.9 | 80.3 | 11.1 |
| 8 | 89.3 | 79.2 | 78.7 | 9.8 |
| 9 | 86.2 | 73.2 | 76.2 | 8.8 |
| 10 | 80.6 | 69.0 | 71.9 | 7.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Huang, T.; Rao, S.; Chen, Q.; Huang, C.; Tan, Z.; Ding, X.; Zou, X. Synthesis of GO-SalenMn and Asymmetric Catalytic Olefin Epoxidation. Catalysts 2019, 9, 824. https://doi.org/10.3390/catal9100824
Wang F, Huang T, Rao S, Chen Q, Huang C, Tan Z, Ding X, Zou X. Synthesis of GO-SalenMn and Asymmetric Catalytic Olefin Epoxidation. Catalysts. 2019; 9(10):824. https://doi.org/10.3390/catal9100824
Chicago/Turabian StyleWang, Fengqin, Tiankui Huang, Shurong Rao, Qian Chen, Cheng Huang, Zhiwen Tan, Xiyue Ding, and Xiaochuan Zou. 2019. "Synthesis of GO-SalenMn and Asymmetric Catalytic Olefin Epoxidation" Catalysts 9, no. 10: 824. https://doi.org/10.3390/catal9100824





