Ru-Catalyzed Oxidative Cleavage of Guaiacyl Glycerol-β-Guaiacyl Ether-a Representative β-O-4 Lignin Model Compound
Abstract
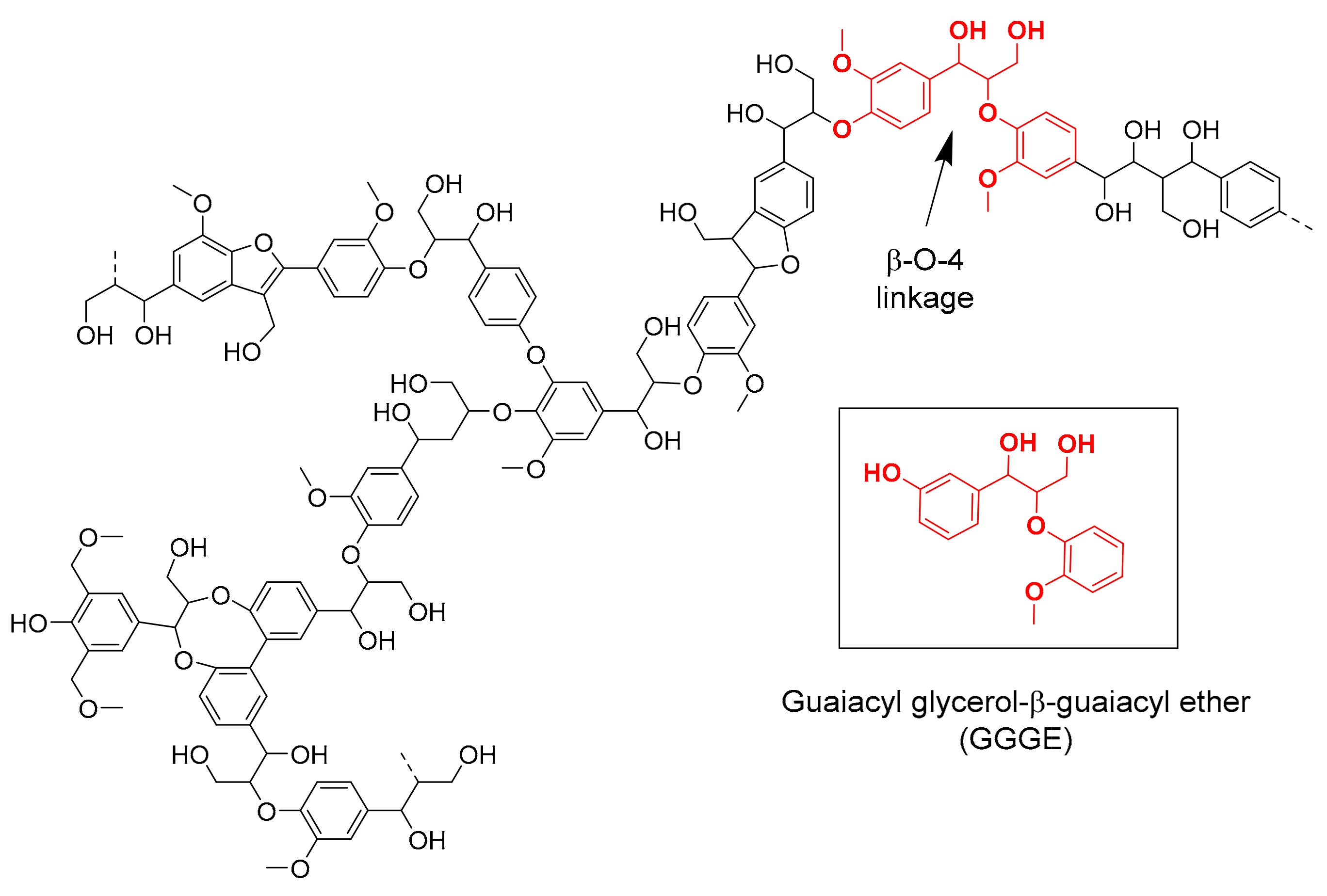
:1. Introduction
2. Results and Discussion
2.1. Catalyst Screening
2.2. Effect of Ru Precursor, Ru Loading and Catalyst Support
2.3. Effect of Reaction Time, Reaction Temperature and Oxygen Pressure
2.4. Catalyst Reuse
3. Materials and Methods
3.1. General
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Oxidation Reactions
3.5. Product Analysis
3.6. NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vlachos, D.G.; Caratzoulas, S. The roles of catalysis and reaction engineering in overcoming the energy and the environment crisis. Chem. Eng. Sci. 2010, 65, 18–29. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium (II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Functional nanoparticles obtained from cellulose: Engineering the shape and size of 6-carboxycellulose. Chem. Commun. 2013, 49, 8818–8820. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Haven, M.Ø.; Thirup, L. Inbicon makes lignocellulosic ethanol a commercial reality. Biomass Bioenergy 2012, 46, 36–45. [Google Scholar] [CrossRef]
- Chen, K.; Xu, L.J.; Yi, J. Bioconversion of Lignocellulose to Ethanol: A Review of Production Process. Adv. Mater. Res. 2011, 280, 246–249. [Google Scholar] [CrossRef]
- Lindedam, J.; Bruun, S.; Jørgensen, H.; Felby, C.; Magid, J. Cellulosic ethanol: Interactions between cultivar and enzyme loading in wheat straw processing. Biotechnol. Biofuels 2010, 3, 25. [Google Scholar] [CrossRef]
- Paniagua, M.; Saravanamurugan, S.; Melián-Rodríguez, M.; Melero, J.A.; Riisager, A. Xylose Isomerization with Zeolites in a Two-Step Alcohol-Water Process. ChemSusChem 2015, 8, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.C.; Dien, B.S.; Ting, K.C.; Singh, V. Influence of Feedstock Particle Size on Lignocellulose Conversion—A Review. Appl. Biochem. Biotechnol. 2011, 164, 1405–1421. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bonda, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Chamnankid, B.; Ratanatawanate, C.; Faungnawakij, K. Conversion of xylose to levulinic acid over modified acid functions of alkaline-treated zeolite Y in hot-compressed water. Chem. Eng. J. 2014, 258, 341–347. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Li, P.; Yi, H.; Yang, M.; Wang, G. Catalytic conversion of xylose to furfural over the solid acid SO42−-/ZrO2-Al2O3/SBA-15 catalysts. Carbohydr. Res. 2011, 346, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 188, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, M.B. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, H.; Wu, X.; Li, R.; Zhang, Q.; Wang, Y. Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles. Green Chem. 2015, 17, 5009–5018. [Google Scholar] [CrossRef]
- Wahyudiono, T.; Kanetake, M.; Sasaki, M.; Goto, M. Decomposition of a Lignin Model Compound under Hydrothermal Conditions. Chem. Eng. Technol. 2007, 30, 1113–1122. [Google Scholar] [CrossRef]
- Sedai, B.; Díaz-Urrutia, C.; Baker, R.T.; Wu, R.; Silks, L.P.; Hanson, S.K. Aerobic oxidation of β-1 lignin model compounds with copper and oxovanadium catalysts. ACS Catal. 2013, 3, 3111–3122. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Matsuura, B.S.; Stephenson, C.R.J. A photochemical strategy for lignin degradation at room temperature. J. Am. Chem. Soc. 2014, 136, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, W.; Xiao, L.-P.; Shi, J.; Sun, R.-C.; Song, G. Hydrogenolysis of biorefinery corncob lignin into aromatic phenols over activated carbon-supported nickel. Sustain. Energy Fuels 2019, 3, 401–408. [Google Scholar] [CrossRef]
- Sedai, B.; Baker, R.T. Copper Catalysts for Selective C-C Bond Cleavage of β-O-4 Lignin Model Compounds. Adv. Synth. Catal. 2014, 356, 3563–3574. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic Oxidation of Lignin in Solvent Systems for Production of Renewable Chemicals: A Review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef]
- Guadix-Montero, S.; Sankar, M. Review on Catalytic Cleavage of C–C Inter-unit Linkages in Lignin Model Compounds: Towards Lignin Depolymerisation. Top. Catal. 2018, 61, 183–198. [Google Scholar] [CrossRef]
- Son, S.; Toste, F.D. Non-Oxidative Vanadium-Catalyzed C-O Bond Cleavage: Application to Degradation of Lignin Model Compounds. Angew. Chem. Int. Ed. 2010, 49, 3791–3794. [Google Scholar] [CrossRef] [Green Version]
- Hanson, S.K.; Baker, R.T.; Gordon, J.C.; Scott, B.L.; Thorn, D.L. Aerobic oxidation of lignin models using a base metal vanadium catalyst. Inorg. Chem. 2010, 49, 5611–5618. [Google Scholar] [CrossRef]
- Hanson, S.K.; Wu, R.; Silks, L.A.P. C-C or C-O bond cleavage in a phenolic lignin model compound: Selectivity depends on vanadium catalyst. Angew. Chem. Int. Ed. 2012, 51, 3410–3413. [Google Scholar] [CrossRef]
- Zhang, G.; Scott, B.L.; Wu, R.; Silks, L.A.P.; Hanson, S.K. Aerobic oxidation reactions catalyzed by vanadium complexes of bis(phenolate) ligands. Inorg. Chem. 2012, 51, 7354–7361. [Google Scholar] [CrossRef]
- Vom Stein, T.; den Hartog, T.; Buendia, J.; Stoychev, S.; Mottweiler, J.; Bolm, C.; Klankermayer, J.; Leitner, W. Ruthenium-Catalyzed C-C Bond Cleavage in Lignin Model Substrates. Angew. Chem. Int. Ed. 2015, 54, 5859–5863. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S.; Madison, A.R. Selective Aerobic Alcohol Oxidation Method For Conversion Of Lignin Into Simple Aromatic Compounds. U.S. Patent 8969534 B2, 3 March 2015. [Google Scholar]
- Yamaguchi, K.; Mizuno, N. Supported ruthenium catalyst for the heterogeneous oxidation of alcohols with molecular oxygen. Angew. Chem. Int. Ed. 2002, 41, 4538–4542. [Google Scholar] [CrossRef]
- Melián-Rodríguez, M.; Saravanamurugan, S.; Kegnæs, S.; Riisager, A. Aerobic Oxidation of Veratryl Alcohol to Veratraldehyde with Heterogeneous Ruthenium Catalysts. Top. Catal. 2015, 58, 1036–1042. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.F.; McCarty, J.G.; Wachsman, E.D. Effect of ruthenium-loading on the catalytic activity of Ru-NaZSM-5 zeolites for nitrous oxide decomposition. Appl. Catal. B Environ. 1995, 6, 21–33. [Google Scholar] [CrossRef]
- Harvey, T.G.; Pratt, K.C. The effect of ruthenium loading on Ru-zeolite/NiMo HDN catalysts. Appl. Catal. A Gen. 1996, 146, 317–321. [Google Scholar] [CrossRef]
- Betancourt, P.; Rives, A.; Hubaut, R.; Scott, C.E.; Goldwasser, J. A study of the ruthenium–alumina system. Appl. Catal. A Gen. 1998, 170, 307–314. [Google Scholar] [CrossRef]
- Gorbanev, Y.Y.; Kegnæs, S.; Riisager, A. Effect of support in heterogeneous ruthenium catalysts used for the selective aerobic oxidation of HMF in water. Top. Catal. 2011, 54, 1318–1324. [Google Scholar] [CrossRef]
- Laursen, A.B.; Gorbanev, Y.Y.; Cavalca, F.; Malacrida, P.; Kleiman-Shwarsctein, A.; Kegnæs, S.; Riisager, A.; Chorkendorff, I.; Dahl, S. Highly dispersed ruthenium oxide as an aerobic catalyst for acetic acid synthesis. Appl. Catal. A 2012, 433–434, 243–250. [Google Scholar] [CrossRef]
- Cruz, J.; Baglio, V. Preparation and Characterization of RuO2 Catalysts for Oxygen Evolution in a Solid Polymer Electrolyte. Int. J. Mol. Sci. 2011, 6, 6607–6619. [Google Scholar]
- Hwang, S.; Lee, C.-H.; Ahn, I.-S. Product identification of guaiacol oxidation catalyzed by manganese peroxidase. J. Ind. Chem. Eng. 2008, 14, 487–492. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Kegnæs, S.; Hanning, C.W.; Hansen, T.W.; Riisager, A. Acetic Acid Formation by Selective Aerobic Oxidation of Aqueous Ethanol over Heterogeneous Ruthenium Catalysts. ACS Catal. 2012, 2, 604–612. [Google Scholar] [CrossRef]
- Gorbanev, Y.Y.; Kegnæs, S.; Riisager, A. Selective aerobic oxidation of 5-hydroxymethylfurfural in water over solid ruthenium hydroxide catalysts with magnesium-based supports. Catal. Lett. 2011, 141, 1752–1760. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Xu, J. Hydrogenolysis of lignosulfonate into phenols over heterogeneous nickel catalysts. Chem. Commun. 2012, 48, 7019–7021. [Google Scholar] [CrossRef] [PubMed]






| Entry | Catalyst | BET Surface Area (m2/g) | GGGE conv. (%) | Product Yield (%) | ||
|---|---|---|---|---|---|---|
| Guaiacol | Vanillin | Vanillic Acid | ||||
| 1 | - | - | 70 | 11 | <1 | <1 |
| 2 | Al2O3 | 204 | 73 | 15 | <1 | <1 |
| 3 | 5 wt.% Fe/Al2O3 | 154 | 92 | 23 | 7 | 4 |
| 4 | 5 wt.% Mn/Al2O3 | 152 | >99 | 21 | 8 | 9 |
| 5 | 5 wt.% Cu/Al2O3 | 158 | >99 | 9 | 3 | <1 |
| 6 | 5 wt.% Ag/Al2O3 | 164 | >99 | 27 | 10 | 8 |
| 7 | 5 wt.% Ru/Al2O3 (1) b | 148 | >99 | 28 | 11 | 11 |
| 8 | 5 wt.% Ru/Al2O3 (1) b,c | 148 | - | 3 | 42 | 6 |
| 9 | 3 wt.% Ru/Al2O3 (1) b | 160 | >99 | 20 | 8 | 6 |
| 10 | 1 wt.% Ru/Al2O3 (1) b | 157 | >99 | 18 | 8 | 6 |
| 11 | 5 wt.% Ru/Al2O3 (2) d | 152 | >99 | 24 | 9 | 3 |
| 12 | 5 wt.% Ru/Al2O3 (3) e | 166 | >99 | 34 | 13 | 11 |
| Entry | Catalyst | BET Surface Area b (m2/g) | GGGEconv. (%) | Product Yield (%) | ||
|---|---|---|---|---|---|---|
| Guaiacol | Vanillin | Vanillic Acid | ||||
| 1 | 5 wt.% Ru/SiO2 | 278 | >99 | 22 | 7 | 10 |
| 2 | 5 wt.% Ru/spinel | 63 | >99 | 20 | 12 | 8 |
| 3 | 5 wt.% Ru/HY(6) c | 698 | >99 | 15 | 8 | 9 |
| 4 | 5 wt.% Ru/ZrO2 | 97 | >99 | 20 | 9 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melián-Rodríguez, M.; Saravanamurugan, S.; Meier, S.; Kegnæs, S.; Riisager, A. Ru-Catalyzed Oxidative Cleavage of Guaiacyl Glycerol-β-Guaiacyl Ether-a Representative β-O-4 Lignin Model Compound. Catalysts 2019, 9, 832. https://doi.org/10.3390/catal9100832
Melián-Rodríguez M, Saravanamurugan S, Meier S, Kegnæs S, Riisager A. Ru-Catalyzed Oxidative Cleavage of Guaiacyl Glycerol-β-Guaiacyl Ether-a Representative β-O-4 Lignin Model Compound. Catalysts. 2019; 9(10):832. https://doi.org/10.3390/catal9100832
Chicago/Turabian StyleMelián-Rodríguez, Mayra, Shunmugavel Saravanamurugan, Sebastian Meier, Søren Kegnæs, and Anders Riisager. 2019. "Ru-Catalyzed Oxidative Cleavage of Guaiacyl Glycerol-β-Guaiacyl Ether-a Representative β-O-4 Lignin Model Compound" Catalysts 9, no. 10: 832. https://doi.org/10.3390/catal9100832





