Synthesis of a Novel Catalyst MnO/CNTs for Microwave-Induced Degradation of Tetracycline
Abstract
:1. Introduction
2. Results and Discussion
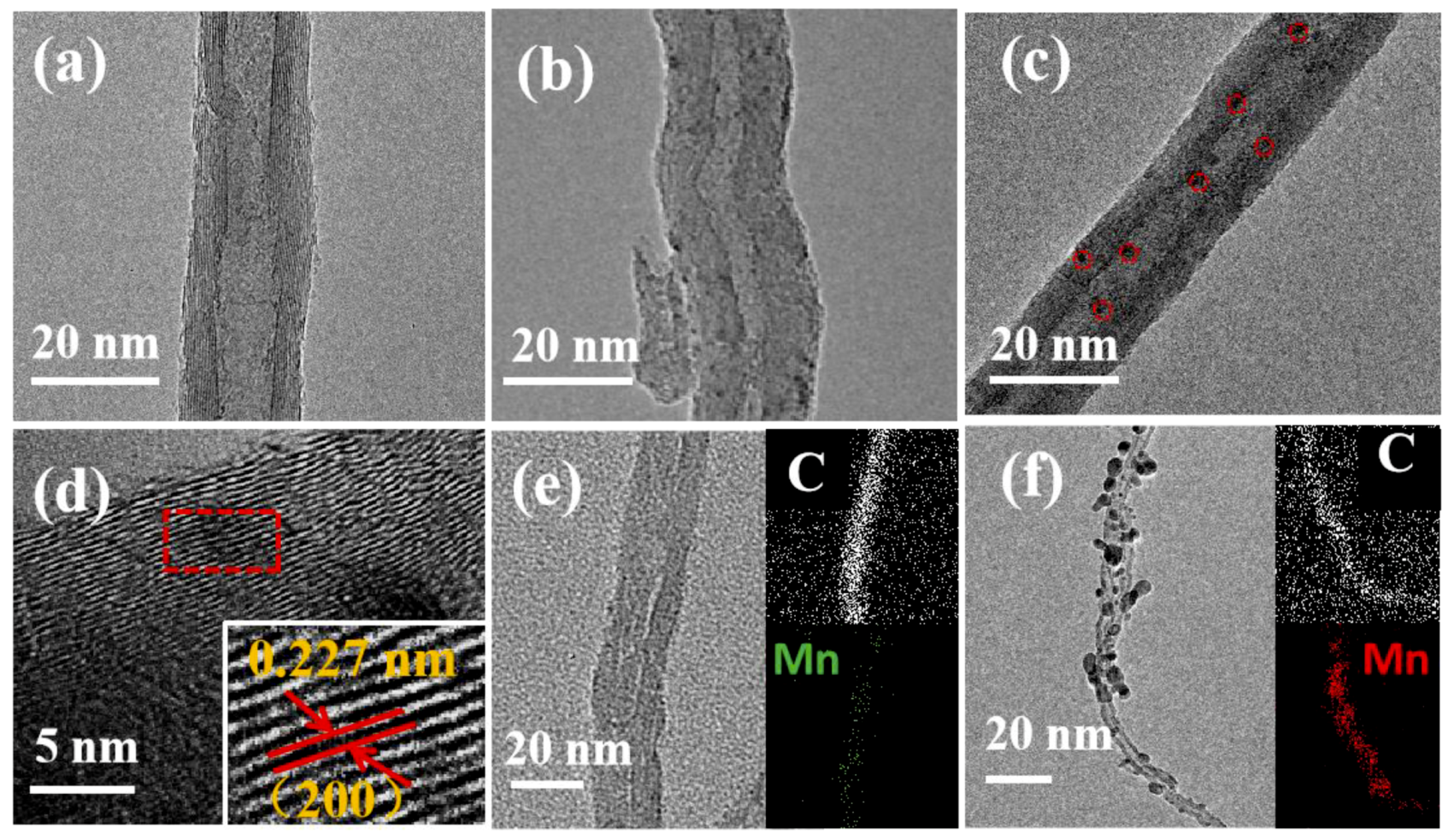
2.1. Characterization of MnO/CNTs
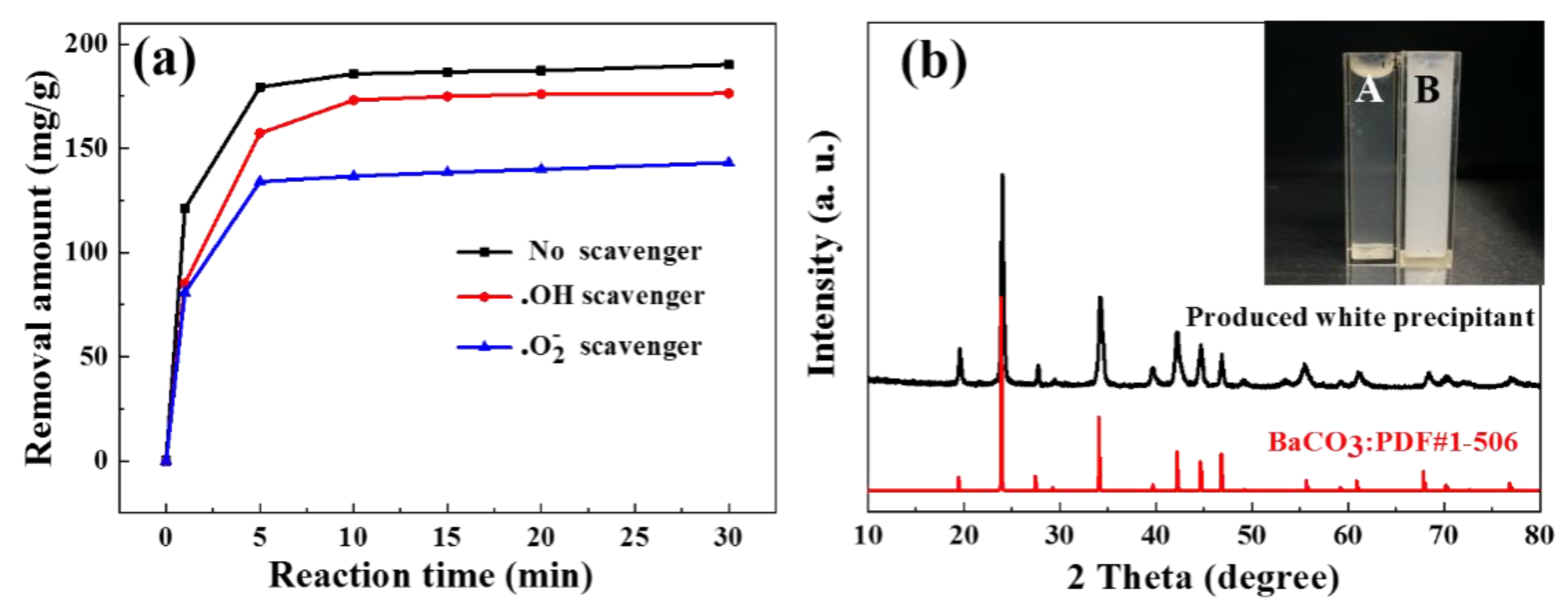
2.2. TC Removal by MnO/CNTs
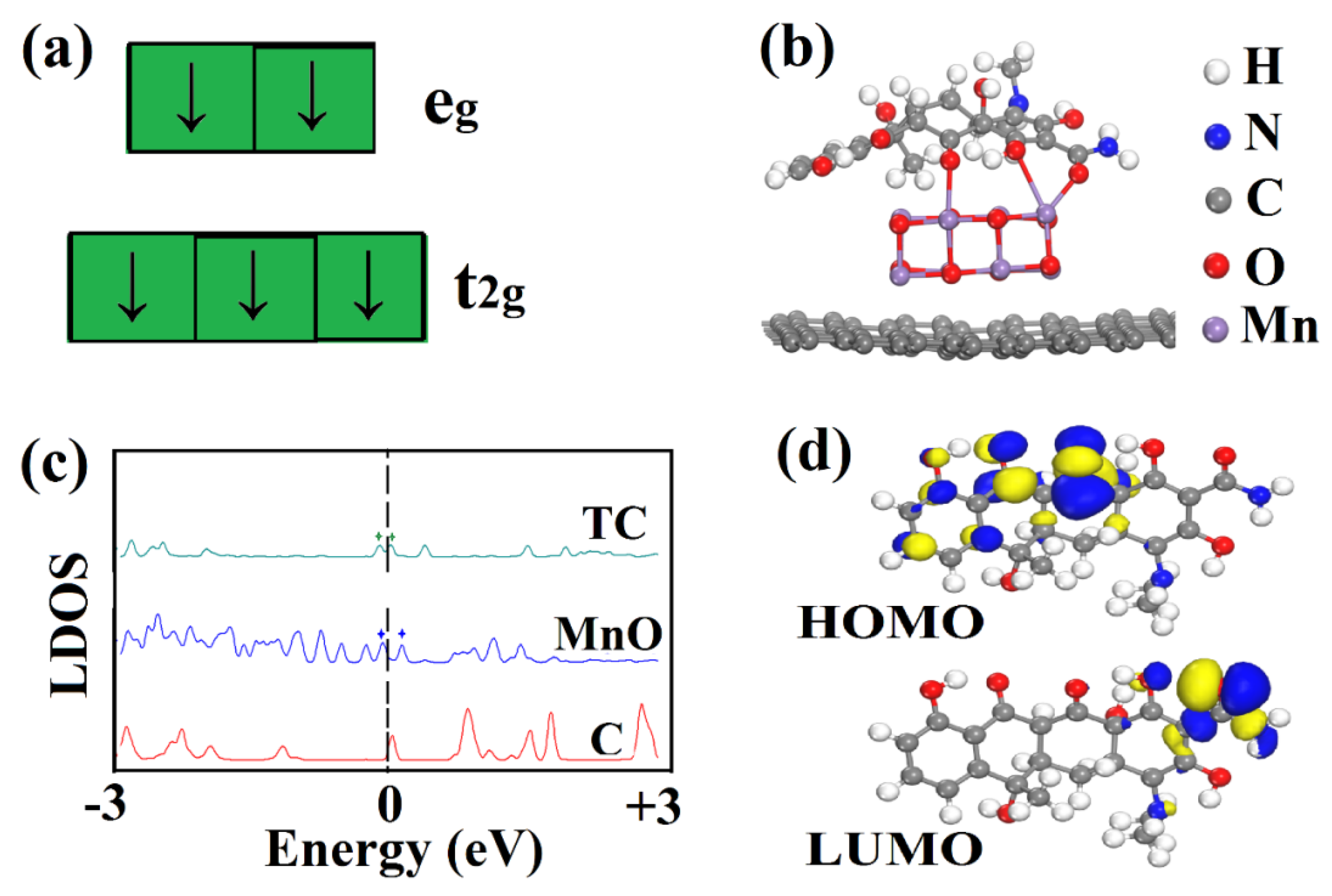
2.3. Possible Mechanism of Catalytic Degradation
2.4. Roles of CNTs and MnO Components
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of MnO/CNTs
3.3. Microwave Experiment
3.4. Computational Investigation
3.5. Methods of Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Yang, Y.; Kang, J.; Fan, M.; Qu, J. Removal of tetracycline from water by Fe-Mn binary oxide. J. Environ. Sci. 2012, 24, 242–247. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Delegan, N.; E1, M.A. Electrochemical degradation of chlortetracycline using N-Doped Ti/TiO2 photoanode under sunlight irradiations. Water Res. 2013, 47, 6801–6810. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Guo, F.; Yuan, S. In situ synthesis of Z-scheme Ag3PO4/CuBi2O4 photocatalysts and enhanced photocatalytic performance for the degradation of tetracycline under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 720–728. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Zhang, L. Fabrication of octahedral Cu@graphitic carbon cage complex porous structures and their microwave-driven catalytic activity. ACS Sustain. Chem. Eng. 2017, 9, 7800–7811. [Google Scholar] [CrossRef]
- Baquero, F.; Martinez, J.L.; Canton, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in stp effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Sumpter, J.P.; Johnson, A.C. Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environ. Sci. Technol. 2005, 39, 4321–4332. [Google Scholar] [CrossRef]
- Shi, Y.J.; Wang, X.H.; Qi, Z.; Diao, M.H.; Gao, M.M.; Xing, S.F.; Wang, S.G.; Zhao, X.C. Sorption and biodegradation of tetracycline by nitrifying granules and the toxicity of tetracycline on granules. J. Hazard. Mater. 2011, 191, 103–109. [Google Scholar] [CrossRef]
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl. Catal. B Environ. 2013, 140, 92–97. [Google Scholar] [CrossRef]
- Ahmadiab, M.; Motlaghc, H.R.; Jaafarzadehab, N.; Mostoufid, A.; Saeedie, R.; Barzegarc, G.; Jorfiab, S. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar]
- Pargoletti, E.; Mostoni, S.; Rassu, G.; Pifferi, V.; Meroni, D.; Falciola, L.; Davoli, E.; Marelli, M. Cappelletti, G. Zn- vs Bi-based oxides for o-toluidine photocatalytic treatment under solar light. Environ. Sci. Pollut. Res. 2017, 24, 8287–8296. [Google Scholar] [CrossRef] [PubMed]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Pérez-Moya, M.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today. 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Selvakumar, M.; Ganesh Babu, S.; Karuthapandian, S.; Chattopadhyay, S. CdO nanospheres: Facile synthesis and bandgap modification for the superior photocatalytic activity. Mater. Lett. 2005, 161, 45–48. [Google Scholar] [CrossRef]
- Tambosi, J.; Sena, R.; Favier, M.; Gebhardt, W.; José, H.; Schröder, H.F.; Moreira, R. Removal of pharmaceutical compounds in membrane bioreactors (MBR) applying submerged membranes. Desalination 2010, 261, 148–156. [Google Scholar] [CrossRef]
- Marcin, Z.; Magdalena, Z.; Marcin, D. Application of microwave radiation to biofilm heating during wastewater treatment in trickling filters. Bioresour. Technol. 2013, 127, 223–230. [Google Scholar]
- Remya, N.; Lin, J.G. Current status of microwave application in wastewater treatment-a review. Chem. Eng. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- He, S.; Wang, G.; Lu, C.; Liu, J.; Wen, B.; Liu, H.; Guo, L.; Cao, M. Enhanced wave absorption of nanocomposites based on the synthesized complex symmetrical CuS nanostructure and poly(vinylidene fluoride). J. Mater. Chem. A 2013, 15, 4685–4692. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Zhang, D.; Zhang, G.; Zhang, L. Superior performance of 3D Co-Ni bimetallic oxides for catalytic degradation of organic dye: Investigation on the effect of catalyst morphology and catalytic mechanism. Appl. Catal. B Environ. 2016, 186, 193–203. [Google Scholar] [CrossRef]
- Ai, Z.; Wang, Y.; Mi, X.; Zhang, L.; Qiu, J. Microwave-induced catalytic oxidation of RhB by a nanocomposite of Fe@Fe2O3 core−shell nanowires and carbon nanotubes. J. Phys. Chem. C 2008, 112, 9847–9853. [Google Scholar] [CrossRef]
- Wang, C.; Fu, J.; Zhang, Y.; Zhao, H.; Wei, X.; Zhang, R. Microhydrangeas with a high ratio of low valence MnOx are capable of extremely fast degradation of organics. Chem. Commun. 2018, 54, 7330–7333. [Google Scholar] [CrossRef]
- Ding, S.; Wen, H.; Yang, S.; Mao, D.; Yuan, J.; Dai, Y. Degradation of Azo dye direct black BN based on adsorption and microwave-induced catalytic reaction. Environ. Sci. Eng. 2018, 12, 71–83. [Google Scholar] [CrossRef]
- He, H.; Yang, S.; Yu, K.; Ju, Y.; Sun, C.; Wang, L. Microwave induced catalytic degradation of crystal violet in nano-nickel dioxide suspensions. J. Hazard. Mater. 2010, 173, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, G.; Liao, L.; Wang, G. Manganese oxide-an excellent microwave absorbent for the oxidation of methylene blue. RSC Adv. 2015, 5, 55595–55601. [Google Scholar] [CrossRef]
- Gu, W.; Lv, G.; Liao, L.; Yang, C.; Liu, H.; Nebendahl, I.; Li, Z. Fabrication of Fe-doped birnessite with tunable electron spin magnetic moments for the degradation of tetracycline under microwave irradiation. J. Hazard. Mater. 2017, 338, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Fang, Z.; Liu, Z. Investigation on microwave absorption properties for multiwalled carbon nanotubes/Fe/Co/Ni nanopowders as lightweight absorbers. J. Phys. Chem. C 2011, 115, 14025–14030. [Google Scholar] [CrossRef]
- Lu, M.; Cao, M.; Chen, Y.; Cao, W.; Liu, J.; Shi, H.; Zhang, D.; Wang, W.; Yuan, J. Multiscale assembly of grape-like ferroferric oxide and carbon nanotubes: A smart absorber prototype varying temperature to tune intensities. ACS Appl. Mater. Interfaces 2015, 7, 19408–19415. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Wei, X.; Li, Y.; Nie, X.; Yu, R.; Shui, J. Magnetically aligned Co-C/MWCNTs composite derived from mwcnt-interconnected zeolitic imidazolate frameworks for a lightweight and highly efficient electromagnetic wave absorber. ACS Appl. Mater. Interfaces 2017, 9, 30850–30861. [Google Scholar] [CrossRef]
- Duan, Y.; Xiao, Z.; Yan, X.; Gao, Z.; Tang, Y.; Hou, L.; Li, Q.; Ning, G.; Li, Y. Enhanced electromagnetic microwave absorption property of peapod-like MnO@carbon nanowires. ACS Appl. Mater. Interfaces 2018, 10, 40078–40087. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Wei, X.; Yu, R.; Shui, J. Porous CNTs/Co composite derived from zeolitic imidazolate framework: A lightweight, ultrathin and highly efficient electromagnetic wave absorber. ACS Appl. Mater. Interfaces 2016, 8, 34686–34698. [Google Scholar] [CrossRef]
- Li, N.; Huang, G.; Li, Y.; Xiao, H.; Feng, Q.; Hu, N.; Fu, S. Enhanced microwave absorption performance of coated carbon nanotubes by optimizing the Fe3O4 nanocoating structure. ACS Appl. Mater. Interfaces 2017, 9, 2973–2983. [Google Scholar] [CrossRef]
- Shin, H.H.; Yeon, G.J.; Choi, H.K.; Park, S.M.; Lee, K.S.; Kim, Z.H. Frequency-Domain proof of the existence of atomic-scale SERS hot-spots. Nano Lett. 2018, 18, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mei, L.; Liang, X.; Liao, L.; Lv, G.; Ma, S.; Lu, S.; Abdel, A.; Xi, K. Anchoring Fe3O4 nanoparticles on carbon nanotube for microwave-induced catalytic degradation of antibiotics. ACS Appl. Mater. Interfaces 2018, 10, 29467–29475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Y.; Sun, L.; Liu, R. Direct conversion of metal-polyphenolic coordination assembly to MnOx-Carbon nanocomposites for catalytic degradation of methylene blue. Mater. Lett. 2018, 221, 97–100. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Wang, W.; Quan, H.; Luo, X.; Guo, L. rGO-stabilized MnO/N-doped carbon nanofibers for efficient removal of Pb(II) ion and catalytic degradation of methylene blue. J. Mater. Sci. 2017, 52, 5117–5132. [Google Scholar] [CrossRef]
- Mettela, G.; Bhogra, M.; Waghmare, U.V.; Kulkarni, G.U. Ambient stable tetragonal and orthorhombic phases in penta-twinned bipyramidal au microcrystals. J. Am. Chem. Soc. 2015, 137, 3024–3030. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Liu, P.; Li, Y.; Liu, X.; Li, G.; Wong, K.; An, W.; Zhao, H. Cross-linked ZnIn2S4/rGO composite photocatalyst for sunlight-driven photocatalytic degradation of 4-nitrophenol. Appl. Catal. B Environ. 2015, 168, 266–273. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Z.; An, T.; Li, G.; Zhao, H.; Wong, P.K. Enhanced visible light-driven photocatalytic bacterial inactivation by ultrathin carbon-coated magnetic cobalt ferrite nanoparticles. Environ. Sci. Technol. 2018, 52, 4774–4784. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; El Khakani, M.A. Photoelectrocatalytic oxidation of chlortetracycline using Ti/TiO2 photo-anode with simultaneous H2O2 production. Electrochim. Acta 2013, 87, 18–31. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Porous carbon-modified MnO disks prepared by a microwave-polyol process and their superior lithium-ion storage properties. J. Mater. Chem. 2012, 22, 19190–19195. [Google Scholar] [CrossRef]
- Xiao, Y.; Xua, C.; Wang, P.; Fang, H.; Sun, X.; Ma, F.; Pei, Y.; Zhen, L. Encapsulating MnO nanoparticles within foam-like carbon nanosheet matrix for fast and durable lithium storage. Nano Energy 2018, 50, 675–684. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Guo, X.; Su, M.; Xu, T.; Song, X. Investigation on the degradation of brilliant green induced oxidation by NiFe2O4 under microwave irradiation. Chem. Eng. J. 2011, 173, 734–742. [Google Scholar] [CrossRef]
- Chen, J.; Xue, S.; Song, Y.T.; Shen, M.L.; Zhang, Z.H.; Yuan, T.X.; Tian, F.Y. Dionysiou, D.D. Microwave-induced carbon nanotubes catalytic degradation of organic pollutants in aqueous solution. J. Hazard. Mater. 2016, 310, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.C.; Xing, X.B.; Liao, L.B.; An, P.F.; Yin, H.; Mei, L.F.; Li, Z. Synthesis of birnessite with adjustable electron spin magnetic moments for the degradation of tetracycline under microwave induction. Chem. Eng. J. 2017, 326, 329–338. [Google Scholar] [CrossRef]
- Colaizzi, J.L.; Klink, P.R. pH-Partition behavior of tetracyclines. J. Pharm. Sci. 1969, 58, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Sassman, S.A.; Lee, L.S. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef]
- Liu, M.; Lv, G.; Mei, L.; Wang, X.; Xing, X.; Liao, L. Degradation of tetracycline by birnessite under microwave irradiation. Adv. Mater. Sci. Eng. 2015, 2014, 1–5. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- An, T.; An, J.; Gao, Y.; Li, G.; Fang, H.; Song, W. Photocatalytic degradation and mineralization mechanism and toxicity assessment of antivirus drug acyclovir: Experimental and theoretical studies. Appl. Catal. B Environ. 2015, 164, 279–287. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Li, R.; Sun, H.; Zhao, X.; Liu, S. The degradation of tetracycline in a photo-electro-fenton system. Chem. Eng. J. 2013, 231, 441–448. [Google Scholar]
- Zhu, Z.; Yu, Y.; Huang, H.; Yao, X.; Dong, H.; Liu, Z.; Yan, Y.; Li, C.; Huo, P. Microwave-hydrothermal synthesis of a novel, recyclable and stable photocatalytic nanoreactor for recognition and degradation of tetracycline. Catal. Sci. Technol. 2017, 7, 4092–4104. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Ye, Z.; Wang, H.; Ma, C.; Huo, P.; Yan, Y. Enhanced photocatalytic activity of g-C3N4-ZnO/HNTs composite heterostructure photocatalysts for degradation of tetracycline under visible light irradiation. RSC Adv. 2015, 5, 91177–91189. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, G.; Li, J.; Li, Y.; Wu, X. 0D/2D Z Scheme heterojunctions of bismuth tantalate quantum dots/ultrathin g-C3N4 nanosheets for highly efficient visible light photocatalytic degradation of antibiotics. ACS Appl. Mater. Interfaces 2017, 9, 43704–43715. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-Consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised perdew-burke-ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Goerigk, L.; Grimme, S. A general database for main group thermochemistry, kinetics, and noncovalent interactions-assessment of common and reparameterized (meta-)gga density functionals. J. Chem. Theory Comput. 2010, 6, 107–126. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Yuan, G.; Lv, G.; Li, Y.; Liao, L.; Qiu, S.; Sun, C. Synthesis of a Novel Catalyst MnO/CNTs for Microwave-Induced Degradation of Tetracycline. Catalysts 2019, 9, 911. https://doi.org/10.3390/catal9110911
Liu T, Yuan G, Lv G, Li Y, Liao L, Qiu S, Sun C. Synthesis of a Novel Catalyst MnO/CNTs for Microwave-Induced Degradation of Tetracycline. Catalysts. 2019; 9(11):911. https://doi.org/10.3390/catal9110911
Chicago/Turabian StyleLiu, Tianming, Guobao Yuan, Guocheng Lv, Yuxin Li, Libing Liao, Siyao Qiu, and Chenghua Sun. 2019. "Synthesis of a Novel Catalyst MnO/CNTs for Microwave-Induced Degradation of Tetracycline" Catalysts 9, no. 11: 911. https://doi.org/10.3390/catal9110911




