Carbon Quantum Dots (CQDs) Decorated Bi2O3-x Hybrid Photocatalysts with Promising NIR-Light-Driven Photodegradation Activity for AO7
Abstract
1. Introduction
2. Results and Discussion
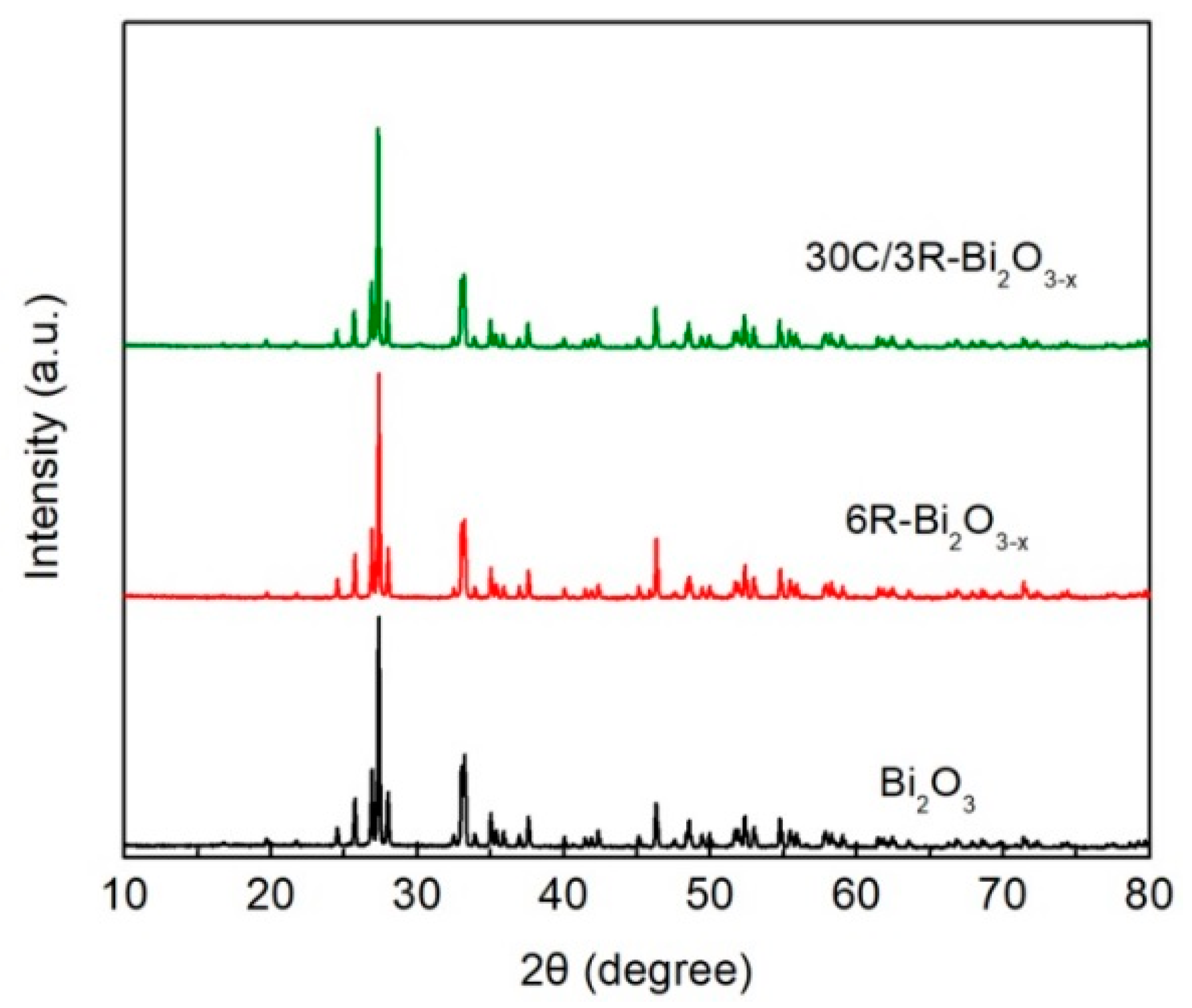
2.1. XRD and FTIR Analysis
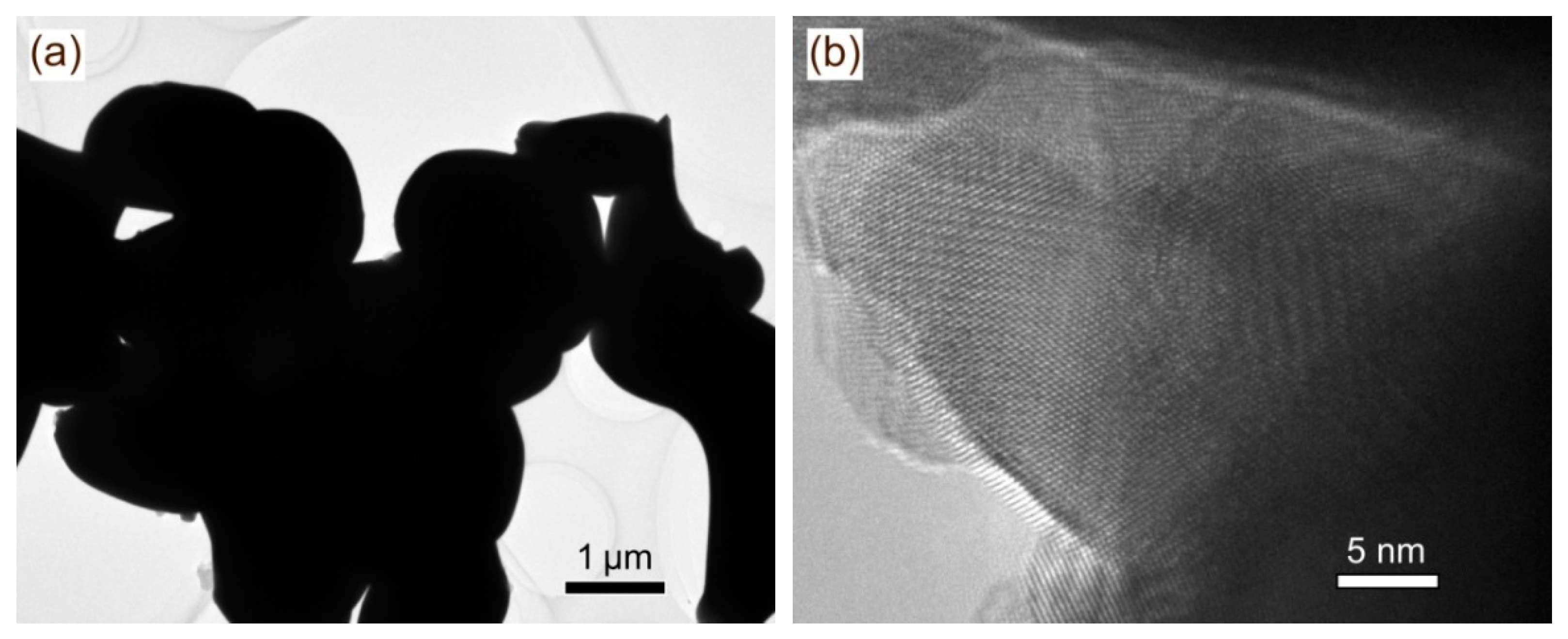
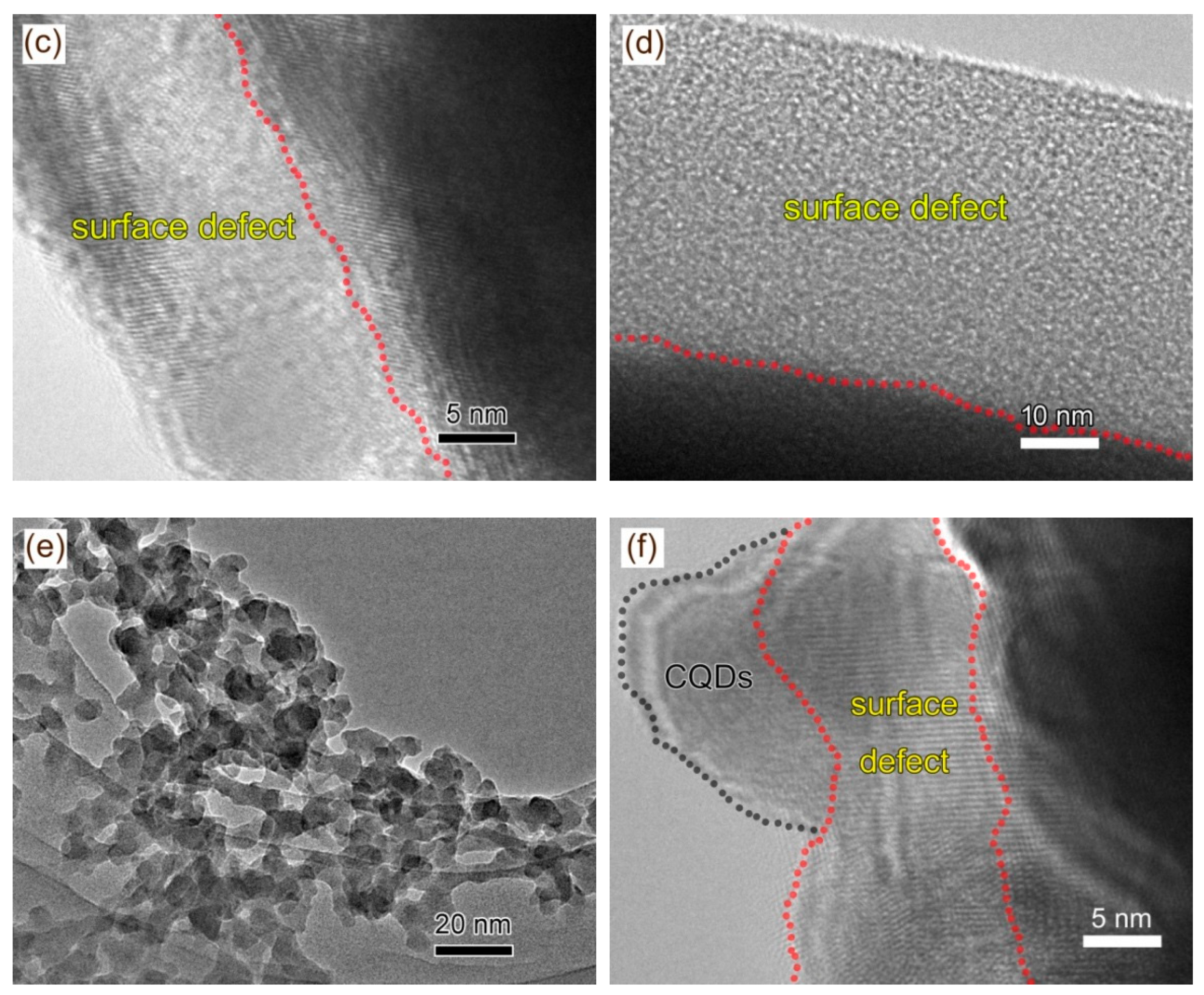
2.2. Morphology Observation
2.3. XPS Analysis
2.4. Optical Absorption Property
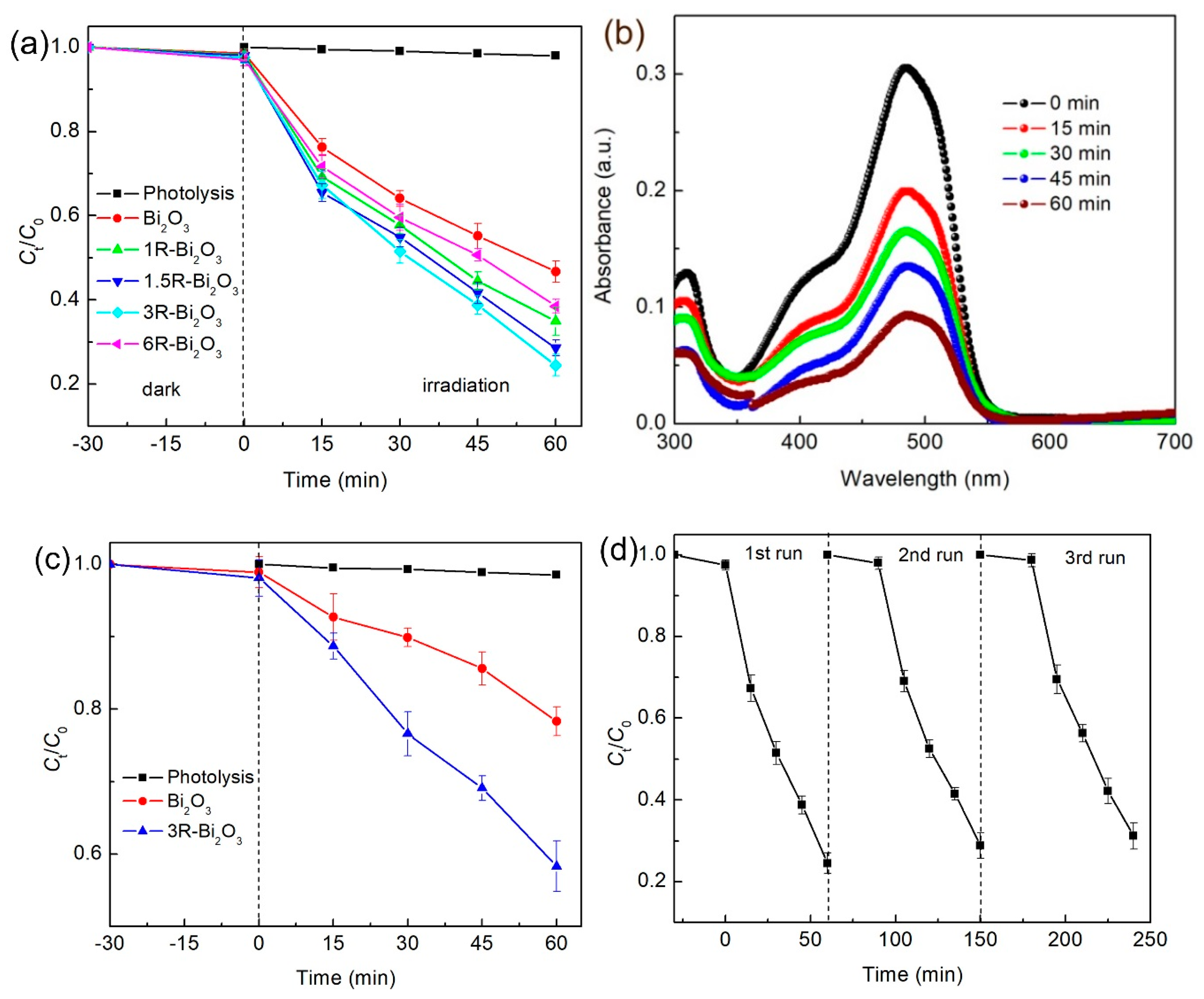
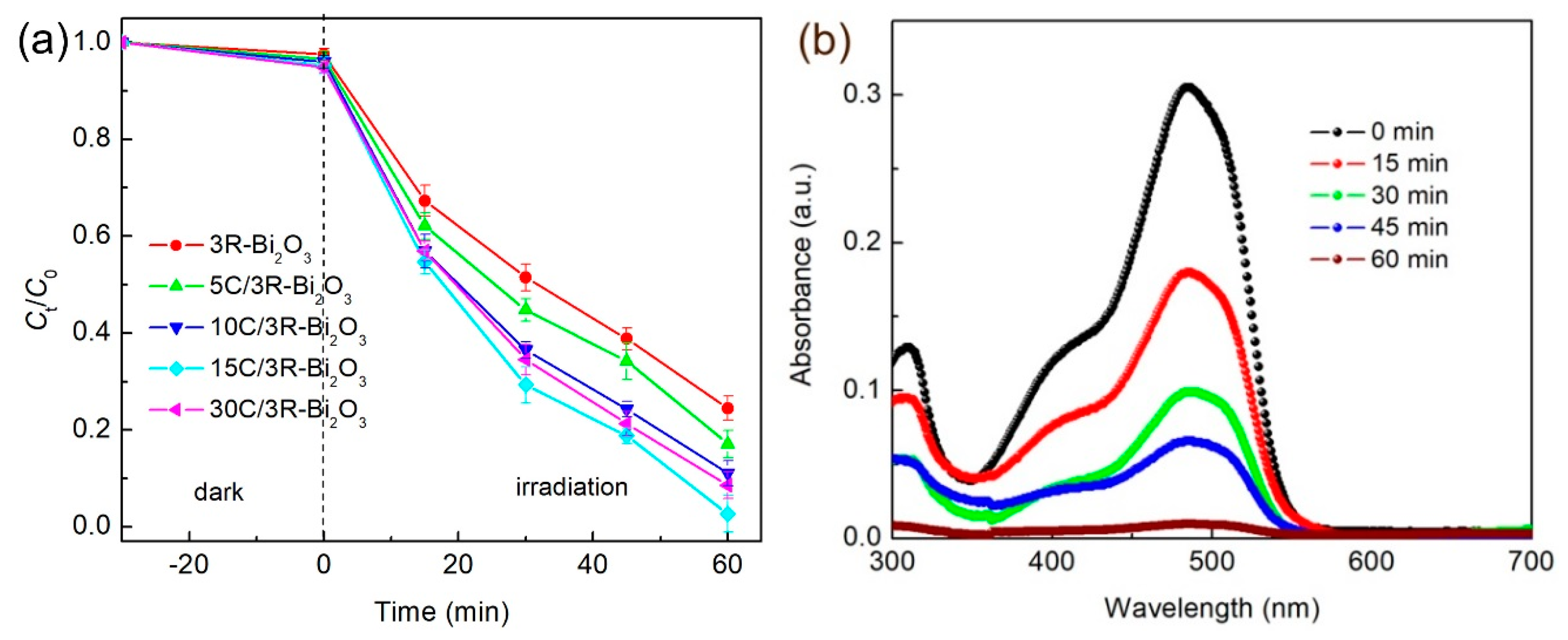
2.5. Photocatalytic Measurement
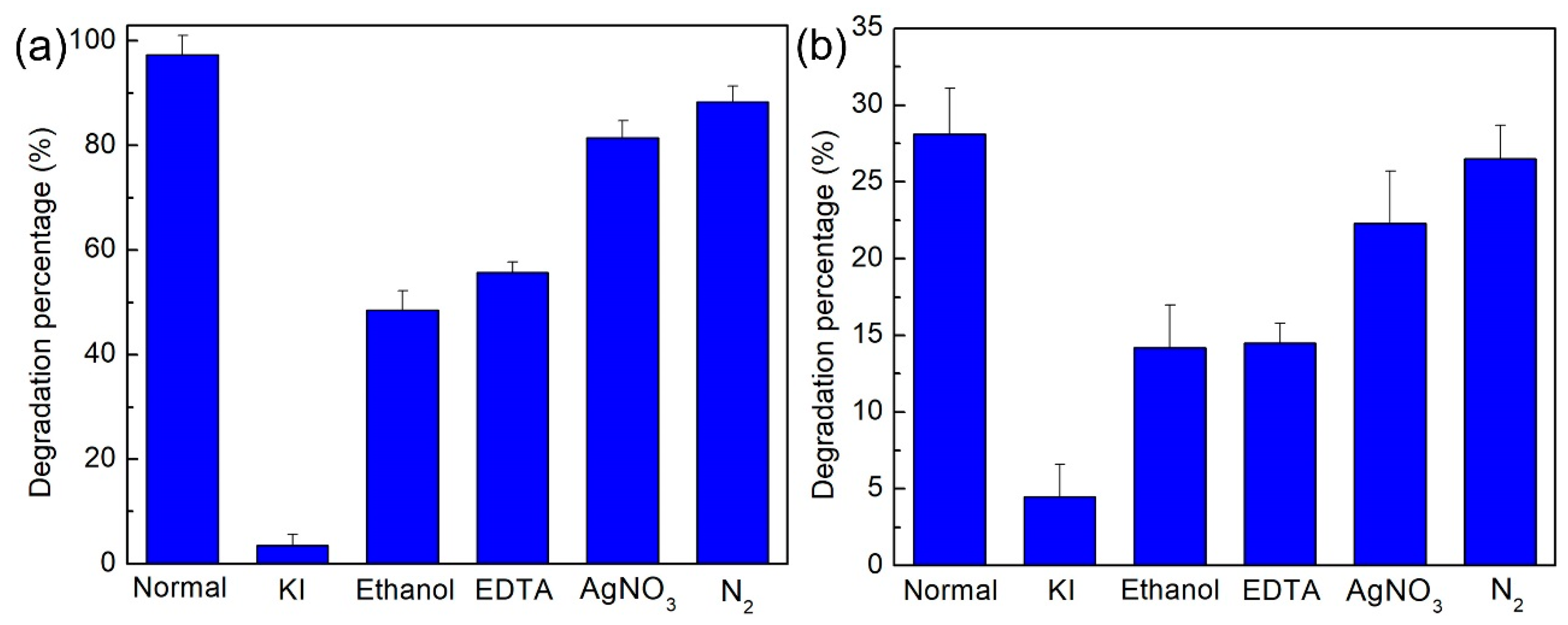
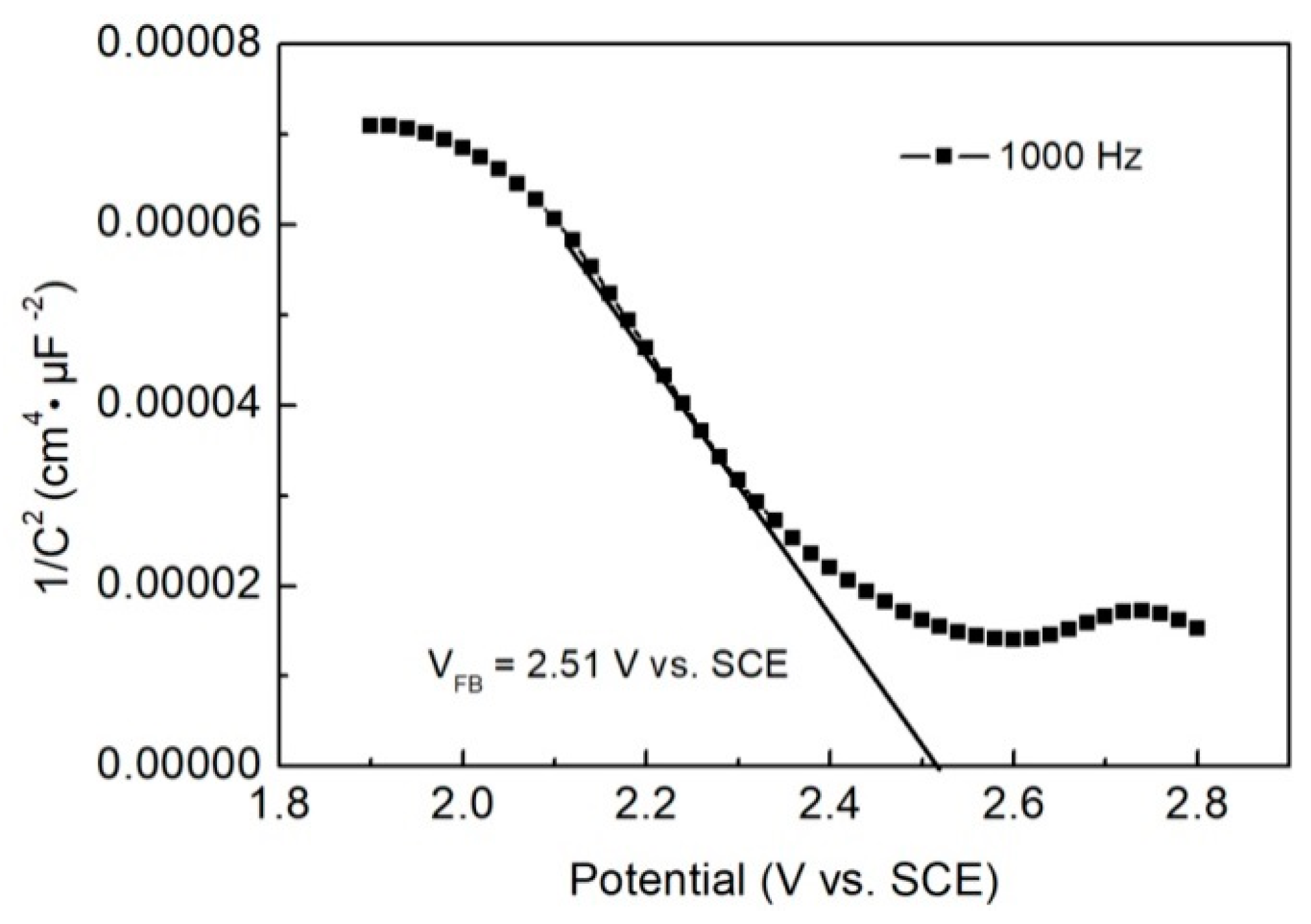
2.6. Photogenerated Charges Performance
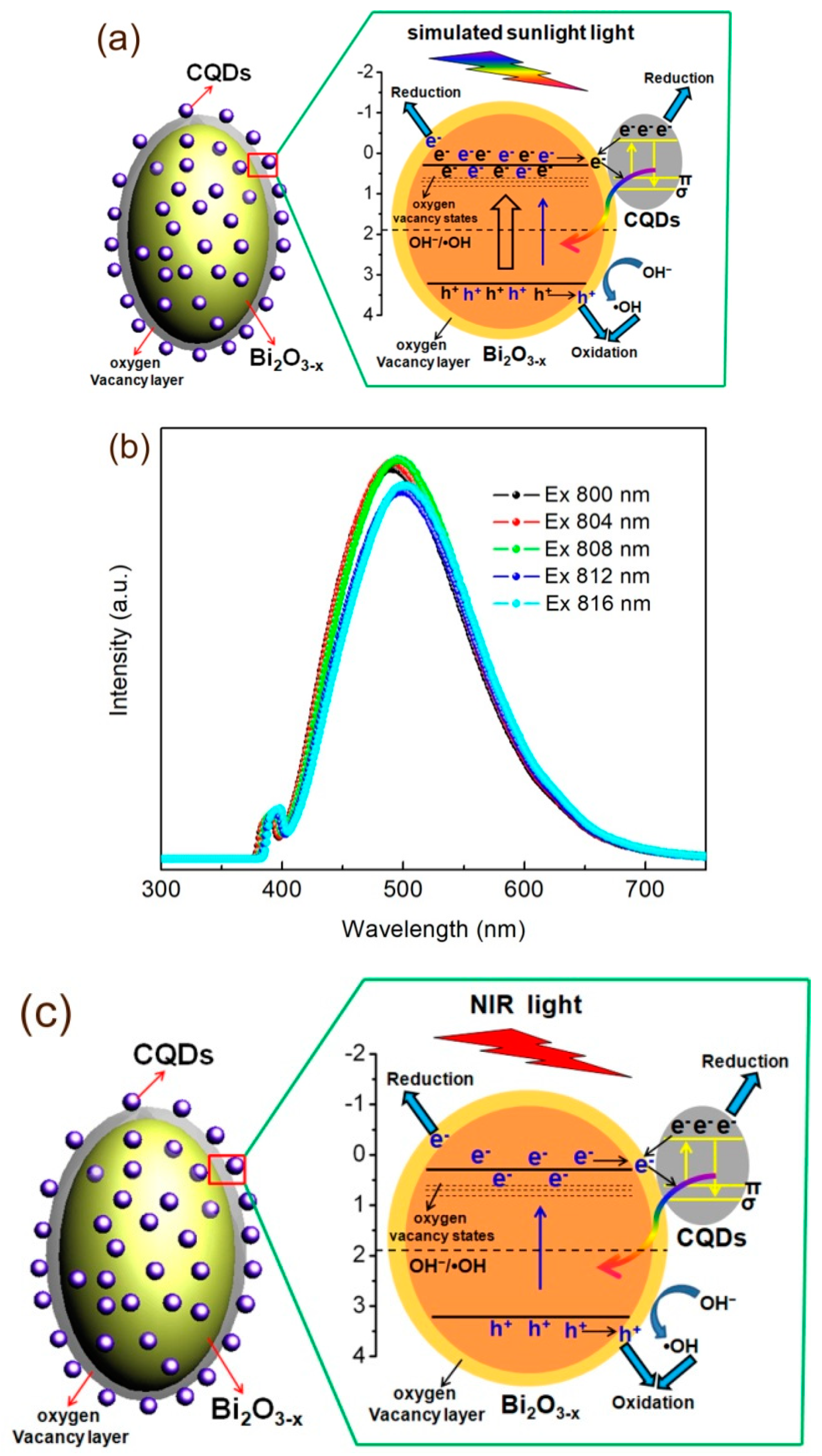
2.7. Photocatalytic Mechanism
3. Materials and Methods
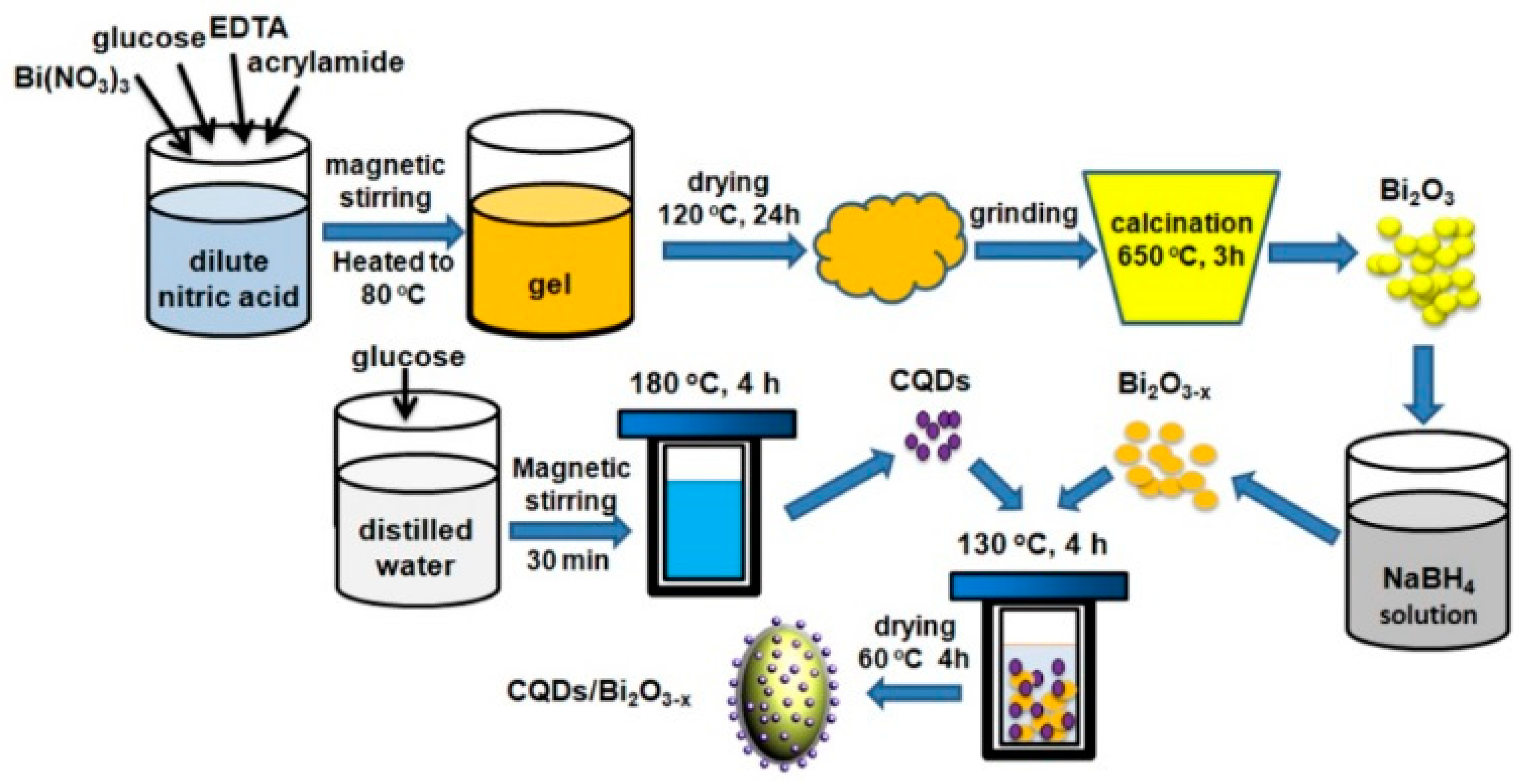
3.1. Fabrication of CQDs
3.2. Fabrication of CQDs/Bi2O3-x Composites
3.3. Photocatalytic Measurement
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chaneac, C.; Durupthy, O.; Ruaux, V.; Mariey, L.; Mauge, F.; Beaunier, P.; Jolivet, J.P. Bi2O3, BiVO4, and Bi2WO6: Impact of surface properties on photocatalytic activity under visible light. J. Phys. Chem. C 2011, 115, 5657–5666. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Liu, X.; Chen, X. Photocatalytic and photo-Fenton catalytic degradation activities of Z-scheme Ag2S/BiFeO3 heterojunction composites under visible-light irradiation. Nanomaterials 2019, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, M.; Cai, W.; Guo, J.; Xiao, C.; Zhang, G. The dominant {001} facet-dependent enhanced visible-light photoactivity of ultrathin BiOBr nanosheets. Phys. Chem. Chem. Phys. 2014, 16, 20909–20914. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Xian, T. NaBH4-reduction induced evolution of Bi nanoparticles from BiOCl nanoplates and construction of promising Bi@BiOCl hybrid photocatalysts. Catalysts 2019, 9, 795. [Google Scholar] [CrossRef]
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.; Fan, Y.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609. [Google Scholar] [CrossRef]
- Yi, Z.; Li, X.; Wu, H.; Chen, X.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Fabrication of ZnO@Ag3PO4 core-shell nanocomposite arrays as photoanodes and their photoelectric properties. Nanomaterials 2019, 9, 1254. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, L.; Zhang, Y.; Chen, Y.; Wang, S.; Li, L.; Long, Y.; Jiang, F. Synthesis and characterization of α/β-Bi2O3 with enhanced photocatalytic activity for 17α-ethynylestradiol. Ceram. Int. 2017, 43, 7627–7635. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, X.; Liu, E.; Crittenden, J.C.; Ma, X.; Zhang, Y.; Cong, Y. Facile synthesis of AgI/BiOI-Bi2O3 multi-heterojunctions with high visible light activity for Cr (VI) reduction. J. Hazard. Mater. 2016, 317, 8–16. [Google Scholar] [CrossRef]
- Vignesh, K.; Priyanka, R.; Rajarajan, M.; Suganthi, A. Photoreduction of Cr (VI) in water using Bi2O3–ZrO2 nanocomposite under visible light irradiation. Mater. Sci. Eng. B 2013, 178, 149–157. [Google Scholar] [CrossRef]
- Muruganandham, M.; Amutha, R.; Lee, G.J.; Hsieh, S.H.; Wu, J.J.; Sillanpää, M. Facile fabrication of tunable Bi2O3 self-assembly and its visible light photocatalytic activity. J. Phys. Chem. C 2012, 116, 12906–12915. [Google Scholar] [CrossRef]
- Xiong, M.; Chen, L.; Yuan, Q.; He, J.; Luo, S.L.; Au, C.T.; Yin, S.F. Controlled synthesis of graphitic carbon nitride/beta bismuth oxide composite and its high visible-light photocatalytic activity. Carbon 2015, 86, 217–224. [Google Scholar] [CrossRef]
- Iyyapushpam, S.; Nishanthi, S.T.; Padiyan, D.P. Photocatalytic degradation of methyl orange using α-Bi2O3 prepared without surfactant. J. Alloys Compd. 2013, 563, 104–107. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, R.; Liu, C.; Xing, C.; Qian, C.; Zuo, X.; Nan, J.; Wang, L. Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl. Catal. B Environ. 2013, 140, 433–443. [Google Scholar] [CrossRef]
- Gurunathan, K. Photocatalytic hydrogen production using transition metal ions-doped γ-Bi2O3 semiconductor particles. Int. J. Hydrogen Energy 2004, 29, 933–940. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, L.; Zhao, Z.; Wang, T.; Liu, X.; Zhang, H.; Dong, F.; Zhang, Y. Mesoporous Ni-doped δ-Bi2O3 microspheres for enhanced solar-driven photocatalysis: A combined experimental and theoretical investigation. J. Phys. Chem. C 2017, 121, 9394–9401. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Immovilli, S.; Prudenziati, M. Powder X-ray diffraction data for the new polymorphic compound ω-Bi2O3. Powder Diffr. 1997, 12, 90–92. [Google Scholar] [CrossRef]
- Cornei, N.; Tancret, N.; Abraham, F.; Mentré, O. New ε-Bi2O3 metastable polymorph. Inorg. Chem. 2006, 45, 4886–4888. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ohta, H.; Nasu, H.; Ishihara, A. Preparation and photocatalytic activity of porous Bi2O3 polymorphisms. Int. J. Hydrogen Energy 2016, 41, 7388–7392. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Yang, J.; Chen, Z.; Zhang, W.; Zhou, L.; Liu, S. Sonochemical synthesis of nanocrystallite Bi2O3 as a visible-light-driven photocatalyst. Appl. Catal. A Gen. 2006, 308, 105–110. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Medina, J.C.; Bizarro, M.; Zanella, R.; Monroy, B.M.; Rodil, S.E. Photocatalytic activity of enlarged microrods of α-Bi2O3 produced using ethylenediamine-solvent. Ceram. Int. 2016, 42, 11866–11875. [Google Scholar] [CrossRef]
- Wang, C.; Shao, C.; Wang, L.; Zhang, L.; Li, X.; Liu, Y. Electrospinning preparation, characterization and photocatalytic properties of Bi2O3 nanofibers. J. Colloid Interface Sci. 2009, 333, 242–248. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Yu, Y. Enhanced photocatalytic activity of α-Bi2O3 with high electron-hole mobility by codoping approach: A first-principles study. Appl. Surf. Sci. 2015, 358, 449–456. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Cheng, K.; Lin, J. Crystalline metallic Au nanoparticle-loaded α-Bi2O3 microrods for improved photocatalysis. Phys. Chem. Chem. Phys. 2012, 14, 12114–12121. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Chen, X.; Yi, Z.; Tang, Y.; Yang, H.; Zhou, Z.; Duan, T.; Cheng, S.; Zhang, J.; Yi, Y. A numerical research of wideband solar absorber based on refractory metal from visible to near infrared. Opt. Mater. 2019, 97, 109400. [Google Scholar] [CrossRef]
- Liang, C.; Yi, Z.; Chen, X.; Tang, Y.; Yi, Y.; Zhou, Z.; Wu, X.; Huang, Z.; Yi, Y.; Zhang, G. Dual-band infrared perfect absorber based on a Ag-dielectric-Ag multilayer films with nanoring grooves arrays. Plasmonics 2019. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Sathishkumar, P.; Murugesan, S.; Anandan, S. Effective degradation of acid orange 10 by catalytic ozonation in the presence of Au-Bi2O3 nanoparticles. Chem. Eng. J. 2011, 168, 1227–1233. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, G.; Liu, P.; Luo, X.; Tan, C.; Jin, L.; Zhou, J. Synthesis and characterization of Fe-doped β-Bi2O3 porous microspheres with enhanced visible light photocatalytic activity. Superlattices Microstruct. 2014, 72, 272–282. [Google Scholar] [CrossRef]
- Liu, J.; Zou, S.; Wang, H.; Xiao, L.; Zhao, H.; Fan, J. Synergistic effect between Pt and Bi2O3−x for efficient room-temperature alcohol oxidation under base-free aqueous conditions. Catal. Sci. Technol. 2017, 7, 1203–1210. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Zhang, H.; Cui, Z.; Feng, W. Surface-disorder-engineering-induced enhancement in the photocatalytic activity of Bi4Ti3O12 nanosheets. Desalin. Water Treat. 2019, 145, 326–336. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, H.; Wang, X.; Feng, W. Surface disorder engineering of flake-like Bi2WO6 crystals for enhanced photocatalytic activity. J. Electron. Mater. 2019, 48, 2067–2076. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Labhsetwar, N.K.; Hishita, S.; Ohashi, N. Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 2005, 401, 579–584. [Google Scholar] [CrossRef]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Gao, H.; Zheng, C.; Yang, H.; Niu, X.; Wang, S. Construction of a CQDs/Ag3PO4/BiPO4 heterostructure photocatalyst with enhanced photocatalytic degradation of rhodamine B under simulated solar irradiation. Micromachines 2019, 10, 557. [Google Scholar] [CrossRef]
- Kulandaivalu, T.; Rashid, S.A.; Sabli, N.; Tan, T.L. Visible light assisted photocatalytic reduction of CO2 to ethane using CQDs/Cu2O nanocomposite photocatalyst. Diam. Relat. Mater. 2019, 91, 64–73. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Gao, H.; Wang, F.; Wang, S.; Wang, X.; Yi, Z.; Yang, H. Photocatalytic activity tuning in a novel Ag2S/CQDs/CuBi2O4 composite: Synthesis and photocatalytic mechanism. Mater. Res. Bull. 2019, 115, 140–149. [Google Scholar] [CrossRef]
- De, B.; Karak, N. Recent progress in carbon dot-metal based nanohybrids for photochemical and electrochemical applications. J. Mater. Chem. A 2017, 5, 1826–1859. [Google Scholar] [CrossRef]
- Sharma, S.; Mehta, S.K.; Ibahdon, A.O.; Kansal, S.K. Fabrication of novel Carbon Quantum Dots modified Bismuth Oxide (α-Bi2O3/C-dots): Material Properties and Catalytic Applications. J. Colloid Interface Sci. 2019, 533, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Liang, Q.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Luo, D.; Kang, Y. Controlled preparation of fiber-shaped 4-Br/Bi2O3 composite photocatalysts with excellent visible-light photocatalytic activity. J. Mater. Sci. 2019, 54, 1549–1565. [Google Scholar] [CrossRef]
- Faisal, M.; Ibrahim, A.A.; Bouzid, H.; Al-Sayari, S.A.; Al-Assiri, M.S.; Ismail, A.A. Hydrothermal synthesis of Sr-doped α-Bi2O3 nanosheets as highly efficient photocatalysts under visible light. J. Mol. Catal. A Chem. 2014, 387, 69–75. [Google Scholar] [CrossRef]
- Singh, V.K.; Yadav, P.K.; Chandra, S.; Bano, D.; Talat, M.; Hasan, S.H. Peroxidase mimetic activity of fluorescent NS-carbon quantum dots and their application in colorimetric detection of H2O2 and glutathione in human blood serum. J. Mater. Chem. B 2018, 6, 5256–5268. [Google Scholar] [CrossRef]
- Ren, H.; Ge, L.; Guo, Q.; Li, L.; Hu, G.; Li, J. The enhancement of photocatalytic performance of SrTiO3 nanoparticles via combining with carbon quantum dots. RSC Adv. 2018, 8, 20157–20165. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Wang, X.; Li, R.; Xian, T. Evolution of Bi nanowires from BiOBr nanoplates through a NaBH4 reduction method with enhanced photodegradation performance. Environ. Eng. Sci. 2019. [Google Scholar] [CrossRef]
- Imran, M.; Yousaf, A.B.; Farooq, M.; Kasak, P. Enhanced Z-scheme visible light photocatalytic hydrogen production over α-Bi2O3/CZS heterostructure. Int. J. Hydrogen Energy 2018, 43, 4256–4264. [Google Scholar] [CrossRef]
- Pooladi, M.; Shokrollahi, H.; Lavasani, S.A.N.H.; Yang, H. Investigation of the structural, magnetic and dielectric properties of Mn-doped Bi2Fe4O9 produced by reverse chemical co-precipitation. Mater. Chem. Phys. 2019, 229, 39–48. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, F.; Yi, Z.; Chen, X.; Zhou, Z.; Yang, H.; Liao, X.; Tang, Y.; Yao, W.; Yi, Y. Effect of slit width on surface plasmon resonance. Results Phys. 2019, 15, 102711. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Y.; Yi, Z.; Chen, X.; Zhou, Z.; Yang, H.; Yi, Y.; Tang, Y.; Yao, W.; Yi, Y. A broadband and polarization-independent metamaterial perfect absorber with monolayer Cr and Ti elliptical disks array. Results Phys. 2019, 15, 102635. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Zhang, G.; Liu, Z.; Qu, D.; Miao, X.; Feng, P.; Sun, Z. Effect of defects on photocatalytic activity of rutile TiO2 nanorods. Nano Res. 2015, 8, 4061–4071. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Chen, X. Facile synthesis and enhanced visible-light photocatalytic activity of novel p-Ag3PO4/n-BiFeO3 heterojunction composites for dye degradation. Nanoscale Res. Lett. 2018, 13, 257. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.; Lee, S.; Choi, W. Dye decolorization test for the activity assessment of visible light photocatalysts: Realities and limitations. Catal. Today 2014, 224, 21. [Google Scholar] [CrossRef]
- Barbero, N.; Vione, D. Why Dyes Should Not Be Used to Test the Photocatalytic Activity of Semiconductor Oxides. Environ. Sci. Technol. 2016, 50, 2130. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Cui, Z.; Yi, Z.; Yu, H. Synergistically enhanced photocatalytic performance of Bi4Ti3O12 nanosheets by Au and Ag nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 13785–13796. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Di, L.; Xian, T.; Sun, X.; Li, H.; Zhou, Y.; Ma, J.; Yang, H. Facile preparation of CNT/Ag2S nanocomposites with improved visible and NIR light photocatalytic degradation activity and their catalytic mechanism. Micromachines 2019, 10, 503. [Google Scholar] [CrossRef]
- Rivas, J.; Solis, R.R.; Gimeno, O.; Sagasti, J. Photocatalytic elimination of aqueous 2-methyl-4-chlorophenoxyacetic acid in the presence of commercial and nitrogen-doped TiO2. Int. J. Environ. Sci. Technol. 2015, 12, 513–526. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-band potential of a semiconductor: Using the Mott-Schottky equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Wang, S.; Yang, H.; Yi, Z.; Wang, X. Enhanced photocatalytic performance by hybridization of Bi2WO6 nanoparticles with honeycomb-like porous carbon skeleton. J. Environ. Manag. 2019, 248, 109341. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Cui, H.; Wang, C.; Zhang, G.; Song, Q.; Sun, W.; Song, X.; Sun, M.; Tian, J. Bi2O3 nanoparticles incorporated porous TiO2 films as an effective p-n junction with enhanced photocatalytic activity. J. Am. Ceram. Soc. 2017, 100, 1339–1349. [Google Scholar] [CrossRef]
- Aggrawal, S.; Chauhan, I.; Mohanty, P. Immobilization of Bi2O3 nanoparticles on the cellulose fibers of paper matrices and investigation of its antibacterial activity against E, coli in visible light. Mater. Express 2015, 5, 429–436. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Xian, T.; Wang, X. Direct Z-scheme CaTiO3@ BiOBr composite photocatalysts with enhanced photodegradation of dyes. Environ. Sci. Pollut. Res. 2019, 26, 29020–29031. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Chen, X. Construction of Z-scheme g-C3N4/CNT/Bi2Fe4O9 composites with improved simulated-sunlight photocatalytic activity for the dye degradation. Micromachines 2018, 9, 613. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 2013, 142, 1–7. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, T.; Sun, X.; Di, L.; Zhou, Y.; Ma, J.; Li, H.; Yang, H. Carbon Quantum Dots (CQDs) Decorated Bi2O3-x Hybrid Photocatalysts with Promising NIR-Light-Driven Photodegradation Activity for AO7. Catalysts 2019, 9, 1031. https://doi.org/10.3390/catal9121031
Xian T, Sun X, Di L, Zhou Y, Ma J, Li H, Yang H. Carbon Quantum Dots (CQDs) Decorated Bi2O3-x Hybrid Photocatalysts with Promising NIR-Light-Driven Photodegradation Activity for AO7. Catalysts. 2019; 9(12):1031. https://doi.org/10.3390/catal9121031
Chicago/Turabian StyleXian, Tao, Xiaofeng Sun, Lijing Di, Yongjie Zhou, Jun Ma, Hongqin Li, and Hua Yang. 2019. "Carbon Quantum Dots (CQDs) Decorated Bi2O3-x Hybrid Photocatalysts with Promising NIR-Light-Driven Photodegradation Activity for AO7" Catalysts 9, no. 12: 1031. https://doi.org/10.3390/catal9121031
APA StyleXian, T., Sun, X., Di, L., Zhou, Y., Ma, J., Li, H., & Yang, H. (2019). Carbon Quantum Dots (CQDs) Decorated Bi2O3-x Hybrid Photocatalysts with Promising NIR-Light-Driven Photodegradation Activity for AO7. Catalysts, 9(12), 1031. https://doi.org/10.3390/catal9121031




