Pd Nanoparticles Supported on Amine-Functionalized MgAl Layered Double Hydroxides for Solvent-Free Aerobic Oxidation of Benzyl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
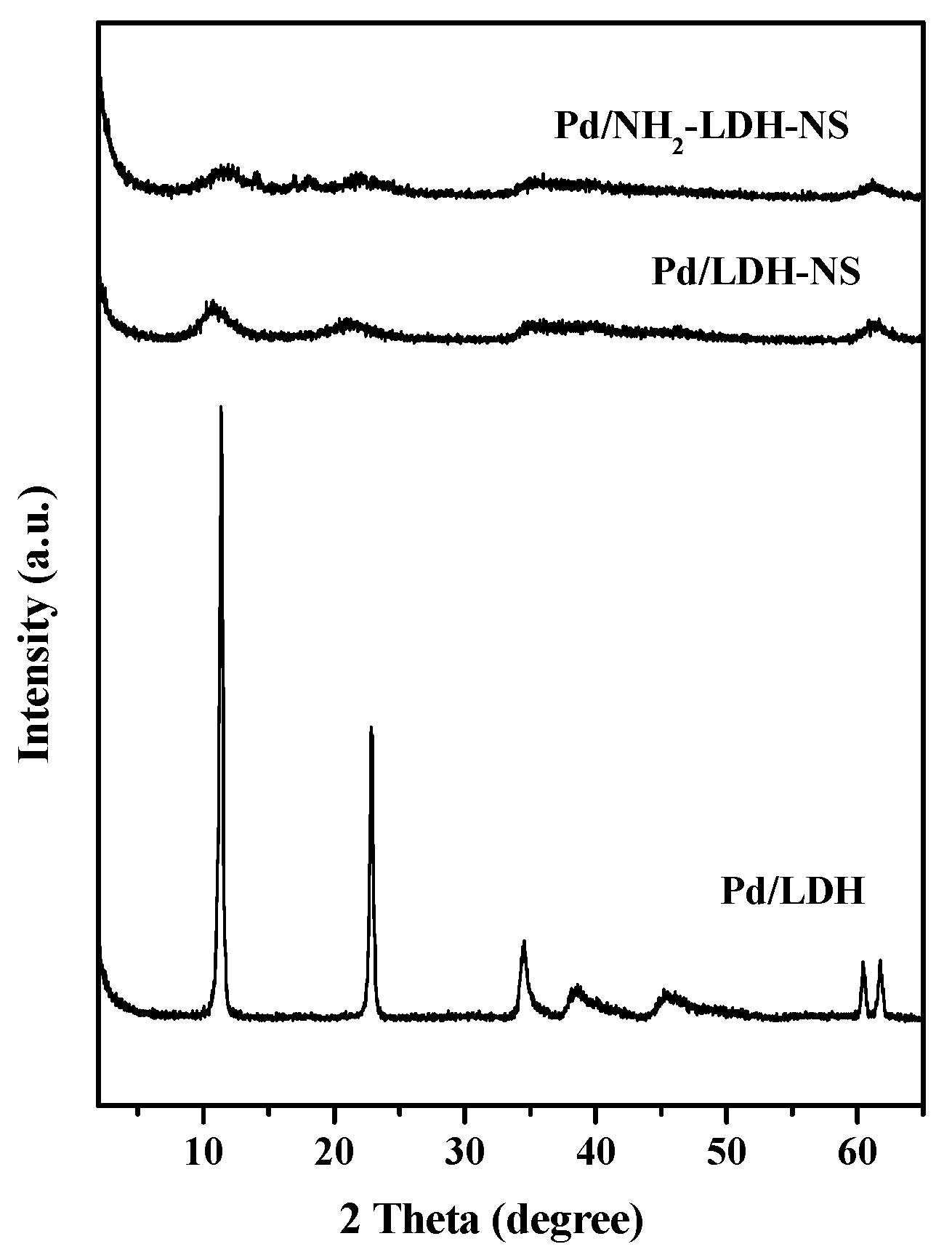
2.1. Characterization of LDH-NS and NH2-LDH-NS
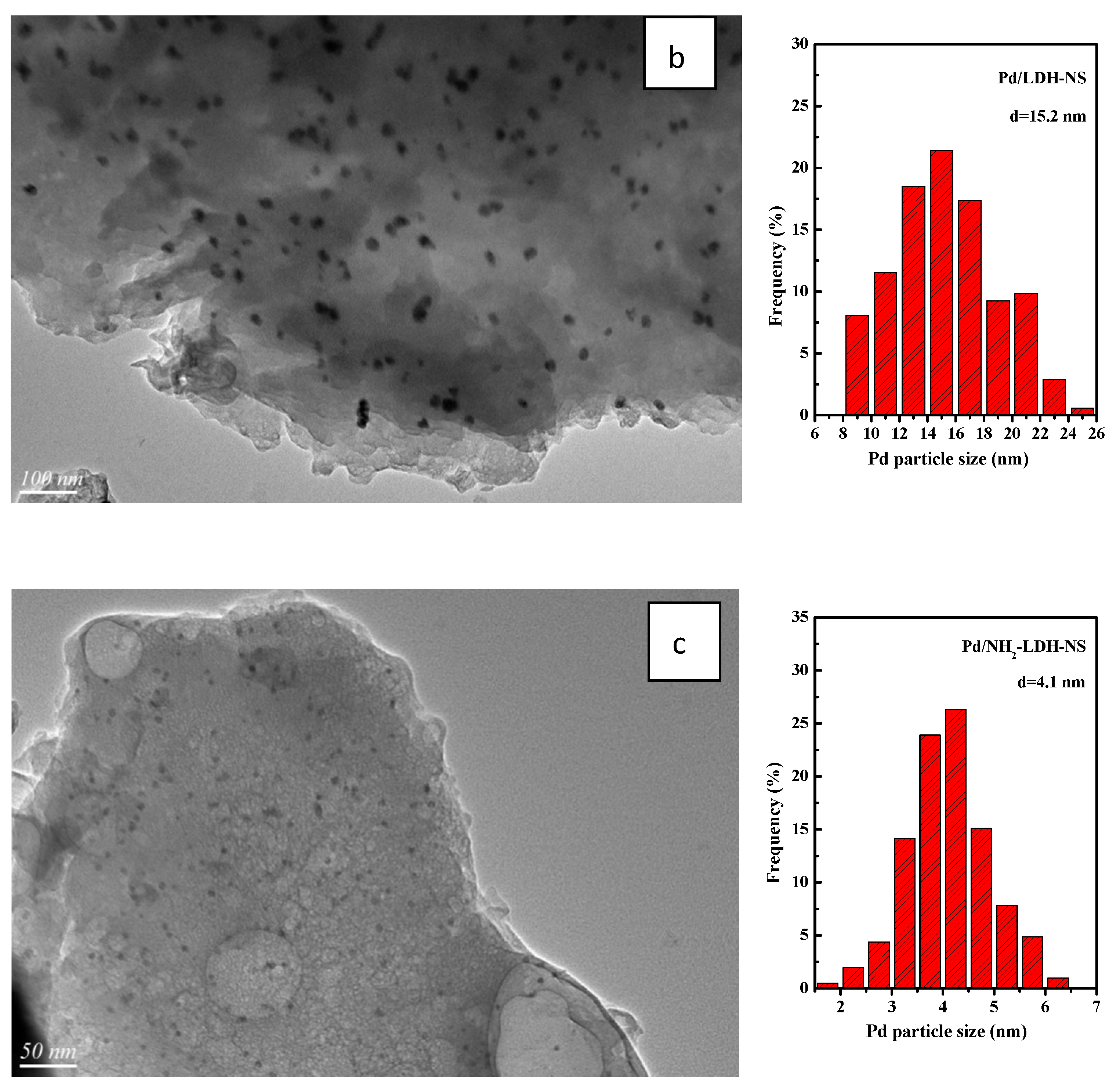
2.2. Characterization of Pd-Supported Catalysts
2.3. Solvent-Free Selective Oxidation of Benzyl Alcohol
2.3.1. Catalytic Performance of the Prepared Different Catalysts
2.3.2. Effect of the Implanted APTS
2.3.3. Effect of Pd Loading Amount
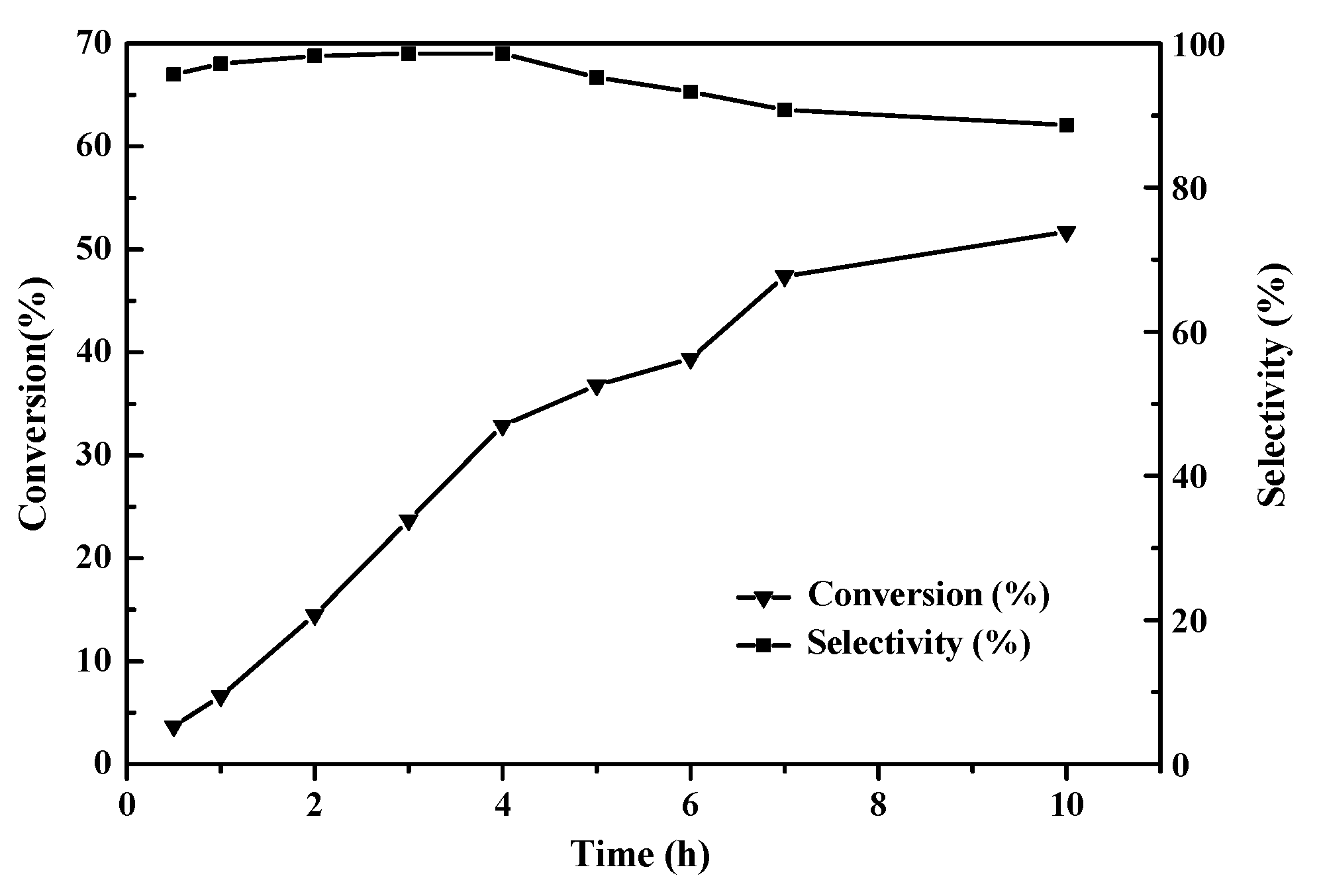
2.3.4. Effect of Reaction Time
2.3.5. Effect of Supports
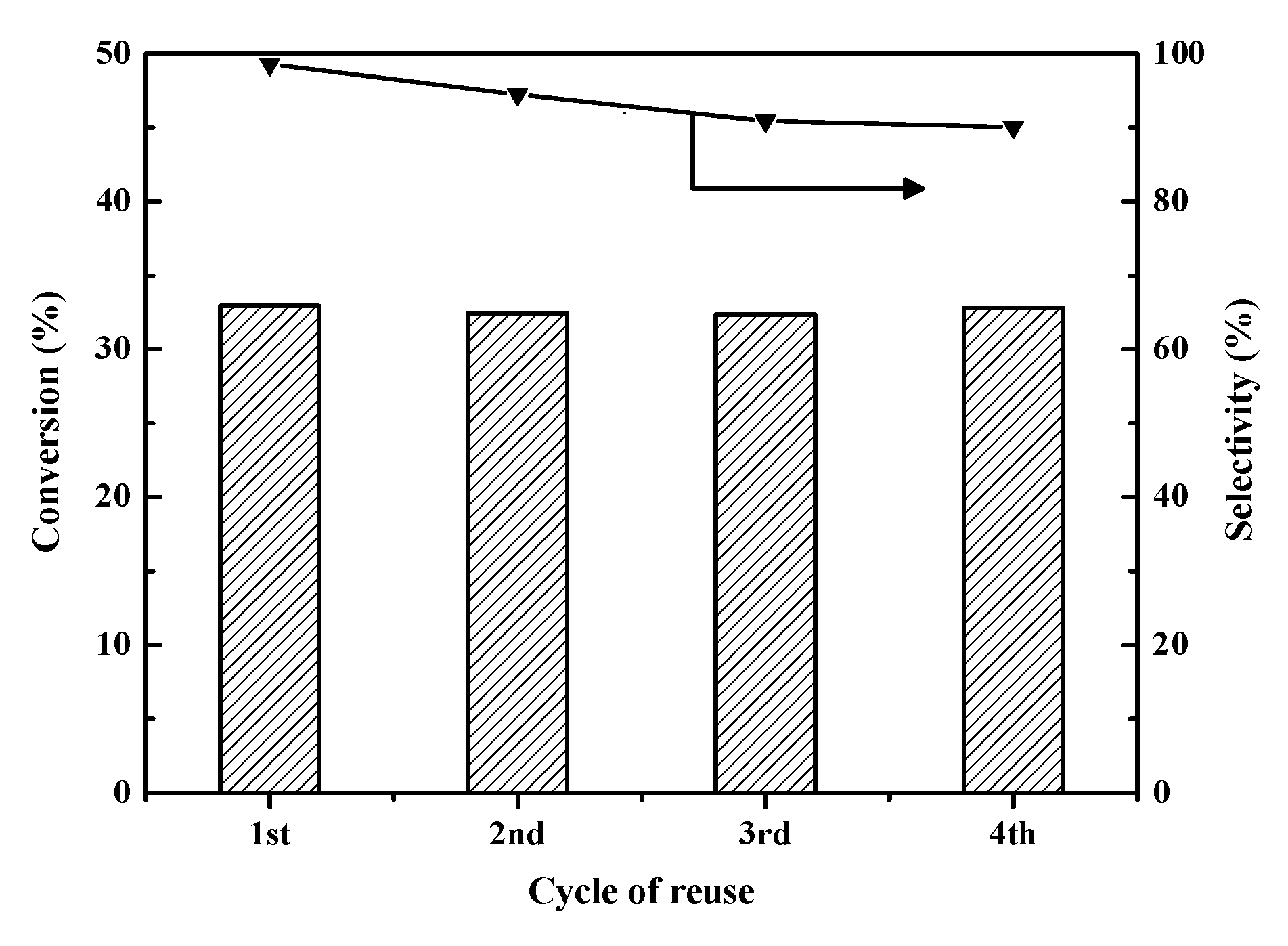
2.3.6. Reusability
2.4. Plausible Mechanism of Enhancement
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Catalyst
3.2.1. Preparation of the Amine-Functionalized MgAl LDH Nanosheets
3.2.2. Supporting Pd NPs on NH2-LDH-NS
3.3. Characterizations
3.4. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; Arends, I.; Dijksman, A. New developments in catalytic alcohol oxidations for fine chemicals synthesis. Catal. Today 2000, 57, 157–166. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Kochi, J.K. Metal-catalyzed oxidations of organic compounds in the liquid phase: A mechanistic approach. Adv. Catal. 1976, 25, 272–413. [Google Scholar]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef]
- Naik, R.; Nizam, A.; Siddekha, A.; Pasha, M. An efficient sonochemical oxidation of benzyl alcohols into benzaldehydes by FeCl3/HNO3 in acetone. Ultrason. Sonochem. 2011, 18, 1124–1127. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Gholamrezapor, E.; Naimi-jamal, M.R. Oxidation of benzyl alcohols to the corresponding carbonyl compounds catalyzed by copper (II) meso-tetra phenyl porphyrin as cytochrome P-450 model reaction. Inorg. Chem. Commun. 2011, 14, 1561–1568. [Google Scholar] [CrossRef]
- Schultz, M.J.; Sigman, M.S. Recent advances in homogeneous transition metal-catalyzed aerobic alcohol oxidations. Tetrahedron 2006, 35, 8227–8241. [Google Scholar] [CrossRef]
- Zheng, N.; Stucky, G.D. Promoting gold nanocatalysts in solvent-free selective aerobic oxidation of alcohols. Chem. Commun. 2007, 37, 3862–3864. [Google Scholar] [CrossRef]
- Tan, H.T.; Chen, Y.T.; Zhou, C.M.; Jia, X.L.; Zhu, J.X.; Chen, J.; Rui, X.H.; Yan, Q.Y.; Yang, Y.H. Palladium nanoparticles supported on manganese oxide-CNT composites for solvent-free aerobic oxidation of alcohols: Tuning the properties of Pd active sites using MnOx. APPL. Catal. B Environ. 2012, 119-120, 166–174. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopezsanchez, J.A.; Morgan, D.; Carley, A.F.; Tiruvalam, R.; Kiely, C.J.; Bethell, D.; Hutchings, G.J. Solvent-free oxidation of benzyl alcohol using Au-Pd catalysts prepared by sol immobilization. Phys. Chem. Chem. Phys. 2009, 11, 5142–5153. [Google Scholar] [CrossRef]
- Wang, L.C.; Liu, Y.M.; Chen, M.; Cao, Y.; He, H.Y.; Fan, K.N. MnO2 nanorod supported gold nanoparticles with enhanced activity for solvent-free aerobic alcohol oxidation. J. Phys. Chem. C 2008, 112, 6981–6987. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Wang, Y.; Wan, H. Size-dependent catalytic cctivity of supported palladium nanoparticles for aerobic oxidation of alcohols. Adv. Synth. Catal. 2008, 350, 453–464. [Google Scholar] [CrossRef]
- Choi, K.M.; Akita, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Highly selective oxidation of allylic alcohols catalysed by monodispersed 8-shell Pd nanoclusters in the presence of molecular oxygen. New J. Chem. 2003, 27, 324–328. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, G.; Xu, L.; Yang, J.; Zhang, B.; Xiang, X. Preparation of ternary Pd/CeO2-nitrogen doped graphene composites as recyclable catalysts for solvent-free aerobic oxidation of benzyl alcohol. Appl. Surf. Sci. 2019, 471, 852–861. [Google Scholar] [CrossRef]
- Ma, C.; Du, Y.; Feng, J.; Cao, X.; Yang, J.; Li, D. Fabrication of supported Pd Au nanoflower catalyst for partial hydrogenation of acetylene. J. Catal. 2014, 317, 263–271. [Google Scholar] [CrossRef]
- Meher, S.; Rana, R.K. A rational design of a Pd-based catalyst with a metal–metal oxide interface influencing molecular oxygen in the aerobic oxidation of alcohols. Green Chem. 2019, 21, 2494–2503. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Guan, N.; Li, L. Supported Pd catalysts for solvent-free benzyl alcohol selective oxidation: Effects of calcination pretreatments and reconstruction of Pd sites. Appl. Catal. B-Environ. 2012, 115, 7–15. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.K.; Chen, Y.; Wang, Y.; Li, Y.X. Palladium nanoparticles supported on mesoporous carbon nitride for efficiently selective oxidation of benzyl alcohol with molecular oxygen. Appl. Catal. A-Gen. 2017, 542, 380–388. [Google Scholar] [CrossRef]
- Cao, E.; Sankar, M.; Nowicka, E.; He, Q.; Morad, M.; Miedziak, P.J.; Taylor, S.H.; Knight, D.W.; Bethell, D.; Kiely, C.J.; et al. Selective suppression of disproportionation reaction in solvent-less benzyl alcohol oxidation catalysed by supported Au–Pd nanoparticles. Catal. Today 2013, 203, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Sankar, M.; Nowicka, E.; Tiruvalam, R.; He, Q.; Taylor, S.H.; Kiely, C.J.; Bethell, D.; Knight, D.W.; Hutchings, G.J. Controlling the duality of the mechanism in liquid-phase oxidation of benzyl alcohol catalysed by supported Au–Pd nanoparticles. Chem. Eur. J. 2011, 17, 6524–6532. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; He, Y.; Liu, Y.; Du, Y.; Li, D. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: General functionality and promising application prospects. Chem. Soc. Rev. 2015, 44, 5291–5319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, L.; Williams, K.; Han, W.Y.; Drage, T.; Snape, C.; Wood, J.; Wang, J. Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route. Chem. Eng. J. 2013, 215, 699–708. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Yang, Q. Accelerated catalytic activity of Pd NPs supported on amine-rich silica hollow nanospheres for quinoline hydrogenation. Catal. Rev.-Sci. Eng. 2017, 7, 2221–2227. [Google Scholar]
- Park, A.Y.; Kwon, H.; Woo, A.J.; Kim, S.J. Layered double hydroxide surface modified with (3-aminopropyl) triethoxysilane by covalent bonding. Adv. Mater. 2005, 17, 106–109. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Large-scale synthesis of highly dispersed layered double hydroxide powders containing delaminated single layer nanosheets. Chem. Commun. 2013, 49, 6301–6303. [Google Scholar] [CrossRef]
- Yang, L.; Fan, B.; Cui, X.; Shi, X.; Li, R. Solvent-free aerobic oxidation of ethylbenzene over Mn-containing silylated MgAl layered double hydroxides. J. Ind. Eng. Chem. 2015, 21, 689–695. [Google Scholar] [CrossRef]
- Chai, H.; Lin, Y.; Evans, D.G.; Li, D. Synthesis and UV absorption properties of 2-naphthylamine-1, 5-disulfonic acid intercalated Zn−Al layered double hydroxides. Ind. Eng. Chem. Res. 2008, 47, 2855–2860. [Google Scholar] [CrossRef]
- Tao, Q.; He, H.; Li, T.; Frost, R.L.; Zhang, D.; He, Z. Tailoring surface properties and structure of layered double hydroxides using silanes with different number of functional groups. J. Solid State Chem. 2014, 213, 176–181. [Google Scholar] [CrossRef]
- Tao, Q.; Zhu, J.; Wellard, R.M.; Bostrom, T.E.; Frost, R.L.; Yuan, P.; He, H. Silylation of layered double hydroxides via an induced hydrolysis method. J. Mater. Chem. 2011, 21, 10711–10719. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Zhao, Y.; Niu, P.; Lu, N.; Fan, B.; Li, R. Amine-functionalized MgAl LDH nanosheets as efficient solid base catalysts for Knoevenagel condensation. Mol. Catal. 2018, 449, 31–37. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Zou, J.; Li, L.; Yu, Y.; Wu, L. The cooperation effect in the Au–Pd/LDH for promoting photocatalytic selective oxidation of benzyl alcohol. Catal. Sci. Technol. 2018, 8, 268–275. [Google Scholar] [CrossRef]
- Singha, S.; Sahoo, M.; Parida, K.M. Highly active Pd nanoparticles dispersed on amine functionalized layered double hydroxide for Suzuki coupling reaction. Dalton Trans. 2011, 40, 7130–7132. [Google Scholar] [CrossRef] [PubMed]
- Guadix-Montero, S.; Alshammari, H.; Dalebout, R.; Nowicka, E.; Morgan, D.J.; Shaw, G.; He, Q.; Sankar, M. Deactivation studies of bimetallic AuPd nanoparticles supported on MgO during selective aerobic oxidation of alcohols. Appl. Catal. A-Gen. 2017, 546, 58–66. [Google Scholar] [CrossRef]
- Lackmann, A.; Mahr, C.; Schowalter, M.; Fitzek, L.; Weissmüller, J.; Rosenauer, A.; Wittstock, A. A comparative study of alcohol oxidation over nanoporous gold in gas and liquid phase. J. Catal. 2017, 353, 99–106. [Google Scholar] [CrossRef]
- Savara, A.; Chan-Thaw, C.E.; Sutton, J.E.; Wang, D.; Prati, L.; Villa, A. Molecular origin of the selectivity differences between palladium and gold–palladium in benzyl alcohol oxidation: Different oxygen adsorption properties. ChemCatChem 2017, 9, 253–257. [Google Scholar] [CrossRef]
- Feng, J.T.; Ma, C.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Li, D.Q.; Du, Y.Y.; Morgan, D.J.; Hutchings, G.J. Au–Pd nanoalloys supported on Mg–Al mixed metal oxides as a multifunctional catalyst for solvent-free oxidation of benzyl alcohol. Dalton Trans. 2013, 42, 14498–14508. [Google Scholar] [CrossRef]


| Sample | SBET (m2/g) | APTS Amount (mmol/g) a |
|---|---|---|
| Pd/LDH | 48 | - |
| Pd/LDH-NS | 35 | - |
| Pd/NH2-LDH-NS-1 | 39 | 0.136 |
| Pd/NH2-LDH-NS-2 | 40 | 0.250 |
| Pd/NH2-LDH-NS | 41 | 0.436 |
| Pd/NH2-LDH-NS-3 | 40 | 0.702 |
| Pd/SiO2 | 203 | - |
| Pd/Al2O3 | 215 | - |
| Sample | Pd Amount (%) b | Conversion (%) | Selectivity (%) | TON | |||
|---|---|---|---|---|---|---|---|
| Benzaldehyde | Toluene | Benzoic Acid | Others | ||||
| Pd/LDH | 0.53 | 12.3 | 98.5 | 0.4 | 1.1 | 0.1 | 5989 |
| Pd/LDH-NS | 0.74 | 7.2 | 90.9 | 0.1 | 8.9 | 0.1 | 2511 |
| Pd/NH2-LDH-NS | 0.55 | 31.6 | 98.6 | 0.5 | 0.8 | 0.1 | 14827 |
| Catalyst | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| Benzaldehyde | Toluene | Benzoic Acid | Others | ||
| Pd/LDH-NS | 7.2 | 90.9 | 0.1 | 8.9 | 0.1 |
| Pd/NH2-LDH-NS-1 | 20.8 | 96.2 | 1.2 | 2.4 | 0.2 |
| Pd/NH2-LDH-NS-2 | 25.5 | 98.1 | 1.1 | 0.5 | 0.3 |
| Pd/NH2-LDH-NS | 31.6 | 98.6 | 0.5 | 0.8 | 0.1 |
| Pd/NH2-LDH-NS-3 | 32.7 | 97.9 | 0.5 | 1.4 | 0.2 |
| Pd Loading (%) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| Benzaldehyde | Toluene | Benzoic Acid | Others | ||
| 0 | 0.3 | 100 | 0 | 0 | 0 |
| 0.25 | 16.4 | 96.0 | 0.4 | 3.5 | 0.1 |
| 0.55 | 31.6 | 98.6 | 0.5 | 0.8 | 0.1 |
| 1.0 | 37.1 | 98.2 | 0.4 | 1.0 | 0.4 |
| Catalyst | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| Benzaldehyde | Toluene | Benzoic Acid | Others | ||
| Pd/NH2-LDH-NS | 31.6 | 98.6 | 0.5 | 0.8 | 0.1 |
| Pd/Al2O3 | 24.0 | 94.9 | 3.6 | 1.2 | 0.3 |
| Pd/SiO2 | 18.8 | 93.8 | 0.3 | 5.8 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Xing, B.; Guo, W.; Zhang, H.; Fan, B.; Li, R. Pd Nanoparticles Supported on Amine-Functionalized MgAl Layered Double Hydroxides for Solvent-Free Aerobic Oxidation of Benzyl Alcohol. Catalysts 2019, 9, 1038. https://doi.org/10.3390/catal9121038
Shi X, Xing B, Guo W, Zhang H, Fan B, Li R. Pd Nanoparticles Supported on Amine-Functionalized MgAl Layered Double Hydroxides for Solvent-Free Aerobic Oxidation of Benzyl Alcohol. Catalysts. 2019; 9(12):1038. https://doi.org/10.3390/catal9121038
Chicago/Turabian StyleShi, Xiufeng, Bin Xing, Wenya Guo, Huifang Zhang, Binbin Fan, and Ruifeng Li. 2019. "Pd Nanoparticles Supported on Amine-Functionalized MgAl Layered Double Hydroxides for Solvent-Free Aerobic Oxidation of Benzyl Alcohol" Catalysts 9, no. 12: 1038. https://doi.org/10.3390/catal9121038





