Passive Solar Photocatalytic Treatment of Emerging Contaminants in Water: A Field Study
Abstract
:1. Introduction
2. Results and Discussion
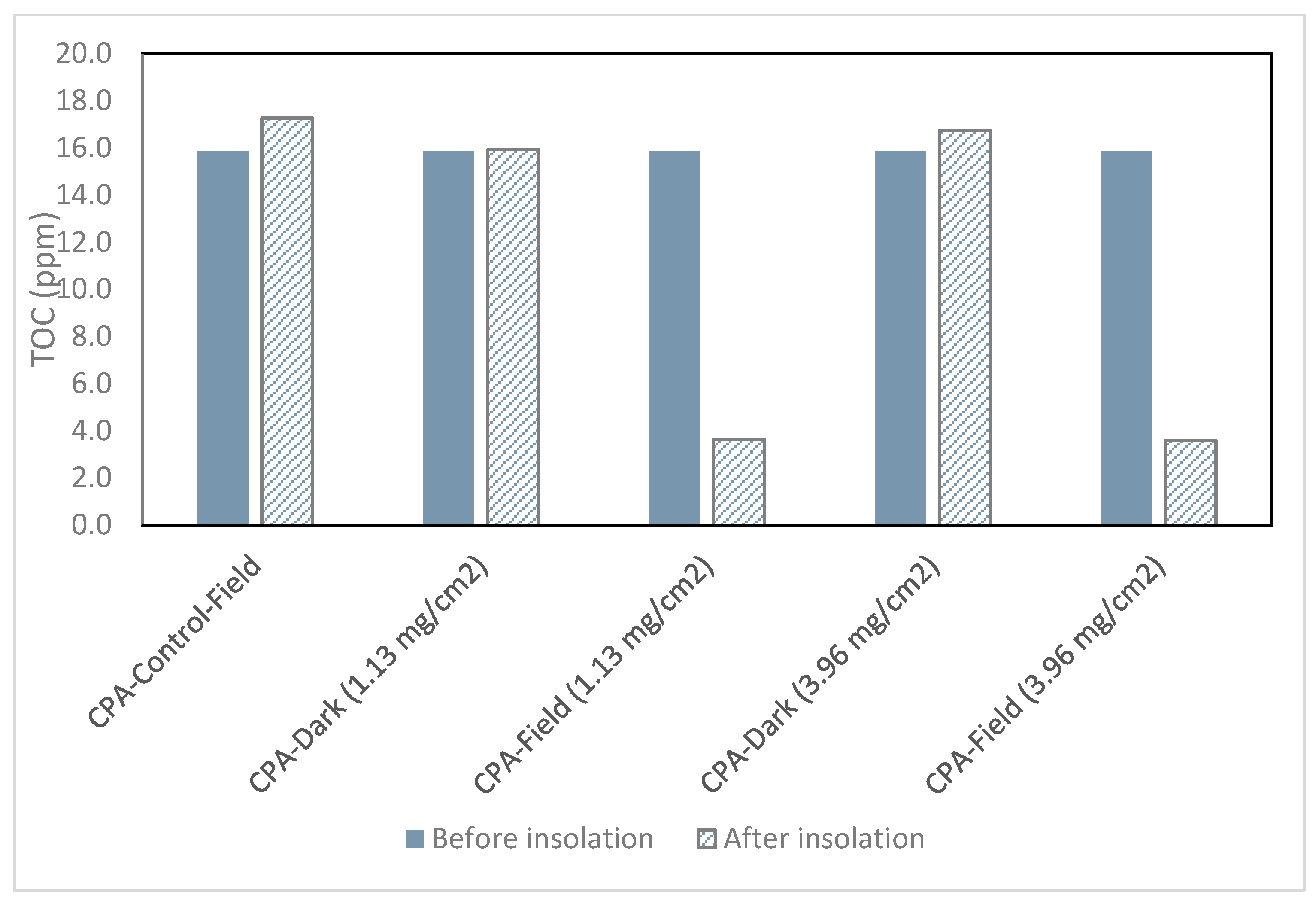
2.1. CPA
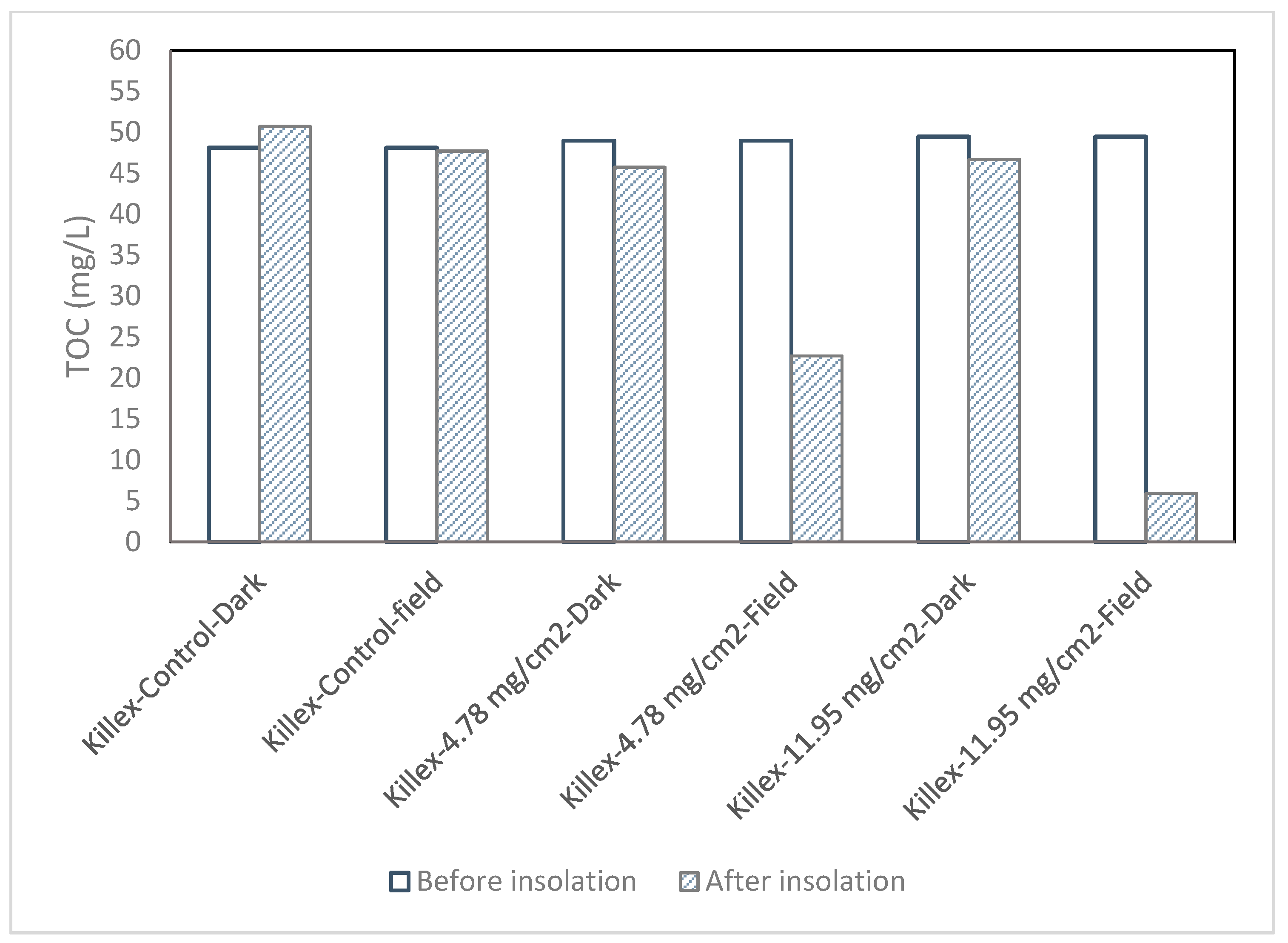
2.2. Killex®
2.3. Sulfolane
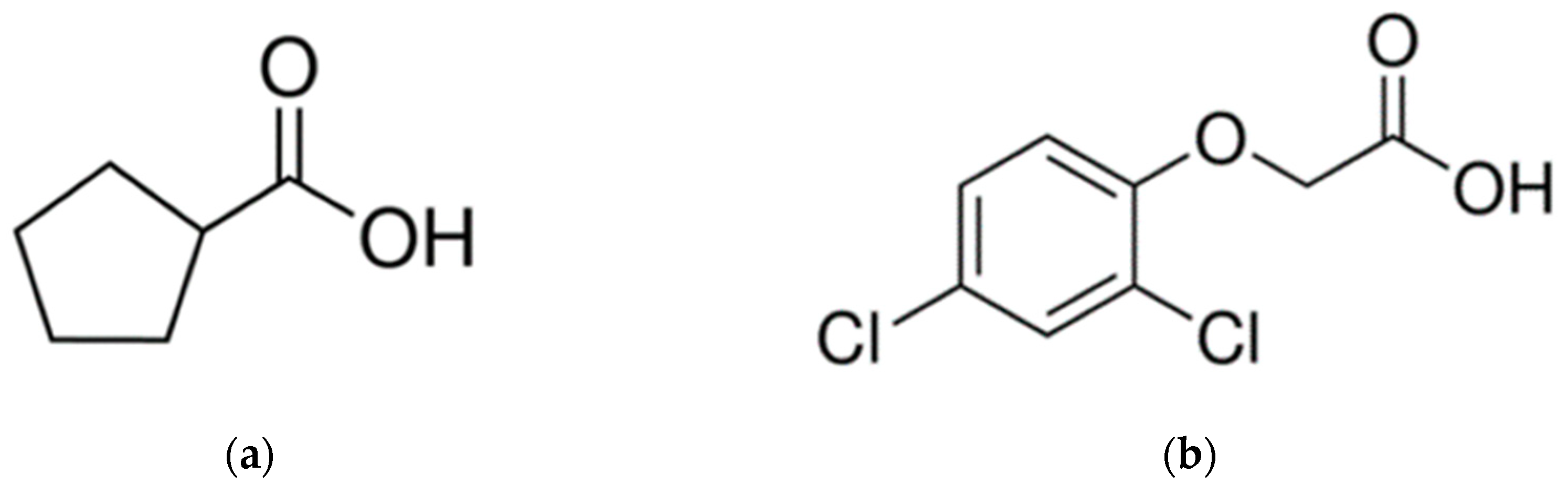
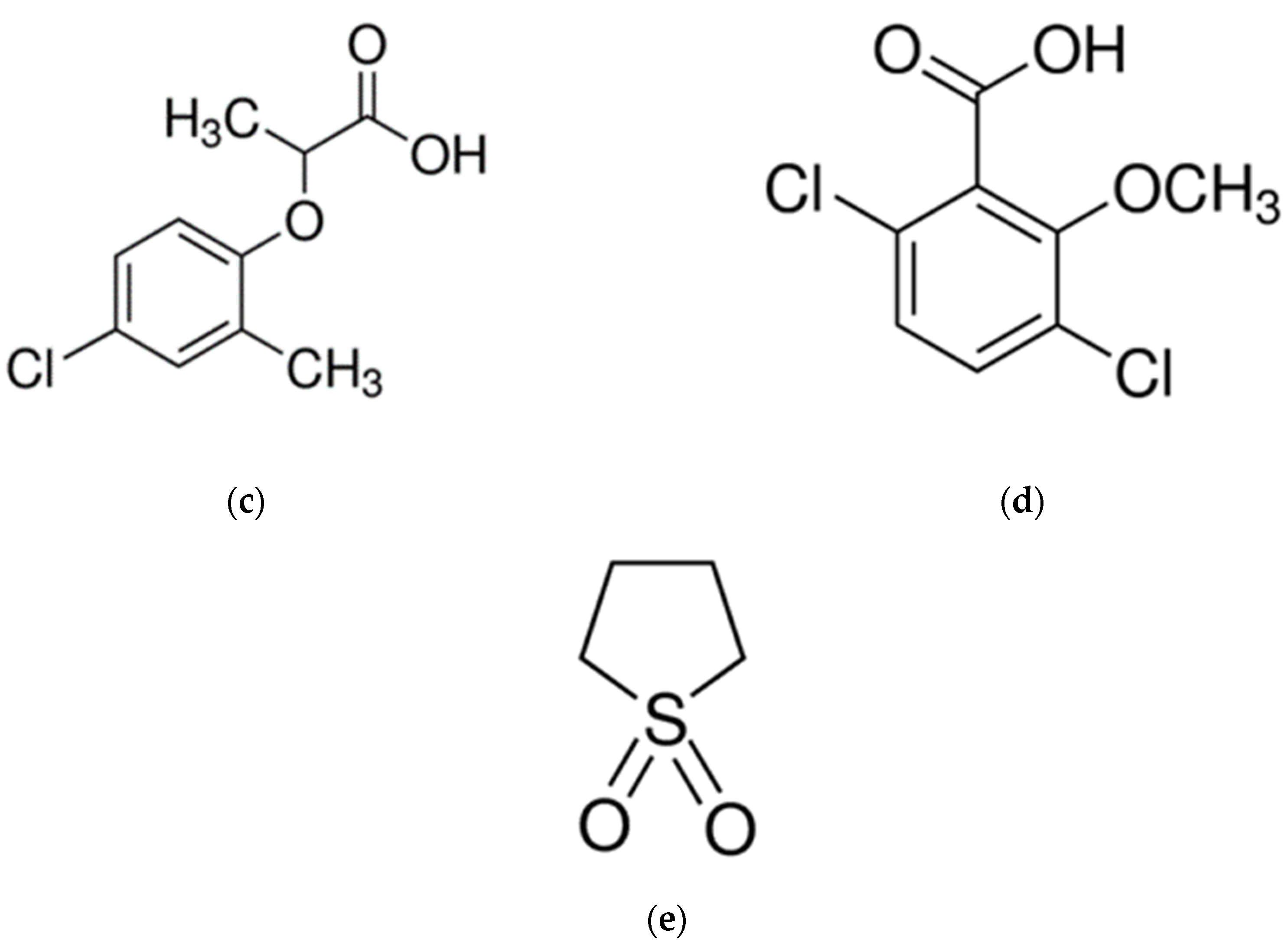
3. Materials
4. Methods and Analysis of Samples
5. Description of Experiments
5.1. Experimental Design
5.2. Sample Preparation
5.2.1. CPA
5.2.2. Killex®
5.2.3. Sulfolane
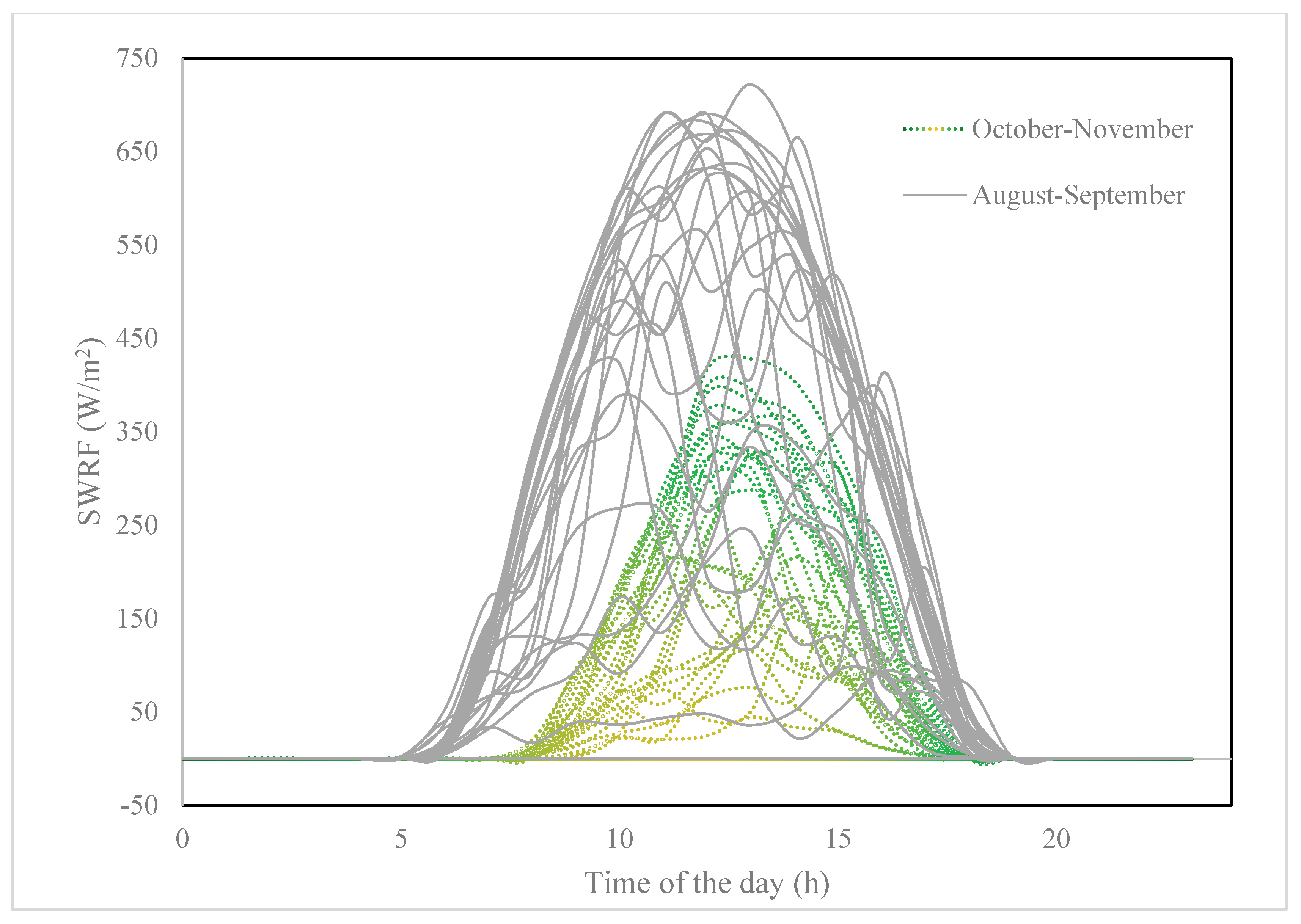
5.3. Field Set-Up under Natural Sunlight and Assumptions
6. Conclusions
- This research investigated the efficacy of the solar based photocatalysis in a passive mode.
- In a passive system, buoyant anatase TiO2 covered hollow glass microspheres were used as a typical photocatalyst. Killex®, CPA and sulfolane were selected as the model contaminants.
- In the Killex® solution, Dicamba and MCPP were completely degraded, 2, 4-D was degraded up to 99.8%, and sulfolane and CPA were also degraded by 97.4% and 100% in aqueous solutions, respectively.
- TOC of Killex® samples were reduced by 53% and 88% with catalyst loadings of 4.78 mg/cm2 and 11.95 mg/cm2, respectively. The same trend was observed in sulfolane samples, where TOC decreased by 28% and 64% at catalyst loadings of 4.78 mg/cm2 and 11.95 mg/cm2, respectively. TOC in CPA solutions also decreased by approximately 77% at the both catalyst loadings.
- The results confirmed the effectiveness of the passive photocatalysis under natural sunlight in the northern climate (latitude: 51°, 4′ N, longitude: 114°, 8′ W; altitude: 1114 m) using buoyant photospheres, and during the late summer and fall season.
- This study established what should be expected of a passive photocatalysis system in terms of duration and reaction rates in the ambient environment.
- The findings confirmed the efficacy of the passive system in the selected geographical region. However, there are limitations for using a buoyant powdered photocatalyst in the ambient environment including collection after treatment, digestion by wildlife, and spreading in the unwanted streams. These limitations necessitate the additional research for development of a more easily deployable photocatalyst for such applications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khetarpal, D.; Yassaa, N.; Nzotcha, U.; Asthana, A.; Kasture, M.; Bizzarri, F. World Energy Resources: Solar 2016; World Energy Council: London, UK, 2016. [Google Scholar]
- Blanco, E.; González-Leal, J.M.; Ramírez-del Solar, M. Photocatalytic TiO2 sol–gel thin films: Optical and morphological characterization. Sol. Energy 2015, 122, 11–23. [Google Scholar] [CrossRef]
- International Energy Agency. Key World Energy Statistics; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Farrauto, R.J.; Heck, R.M. Environmental catalysis into the 21st century. Catal. Today 2000, 55, 179–187. [Google Scholar] [CrossRef]
- Gazea, B.; Adam, K.; Kontopoulos, A. A review of passive systems for the treatment of acid mine drainage. Miner. Eng. 1996, 9, 23–42. [Google Scholar] [CrossRef]
- Mostafa, S. Sunlight-Induced Photochemical Processes in Natural and Wastewater Treatment Systems. Ph.D. Thesis, University of Colorado, Boulder, CO, USA, 2015. [Google Scholar]
- Mekhilef, S.; Saidur, R.; Safari, A. A review on solar energy use in industries. Renew. Sustain. Energy Rev. 2011, 15, 1777–1790. [Google Scholar] [CrossRef]
- Bradford, T. Solar Revolution: The Economic Transformation of the Global Energy Industry; MIT Press: Cambridge, MA, USA, 2008; ISBN 9780262269094. [Google Scholar]
- Blanco, J.; Malato, S.; Fernández-Ibañez, P.; Alarcón, D.; Gernjak, W.; Maldonado, M.I. Review of feasible solar energy applications to water processes. Renew. Sustain. Energy Rev. 2009, 13, 1437–1445. [Google Scholar] [CrossRef]
- Danwittayakul, S.; Jaisai, M.; Dutta, J. Efficient solar photocatalytic degradation of textile wastewater using ZnO/ZTO composites. Appl. Catal. B Environ. 2015, 163, 1–8. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Yu, L.; Achari, G.; Langford, C.H. LED-based photocatalytic treatment of pesticides and chlorophenols. J. Environ. Eng. 2013, 139, 1146–1151. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Akolekar, D.B.; Bhargava, S.K.; Shirgoankar, I.; Prasad, J. Catalytic wet oxidation: An environmental solution for organic pollutant removal from paper and pulp industrial waste liquor. Appl. Catal. A Gen. 2002, 236, 255–262. [Google Scholar] [CrossRef]
- Ghosh, J.P.; Langford, C.H.; Achari, G. Characterization of an LED based photoreactor to degrade 4-chlorophenol in an aqueous medium using coumarin (C-343) sensitized TiO2. J. Phys. Chem. A 2008, 112, 10310–10314. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S.; Edge, M.; Verran, J.; Stratton, J.; Maltby, J.; Bygott, C. Photocatalytic titania based surfaces: Environmental benefits. Polym. Degrad. Stab. 2008, 93, 1632–1646. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Blake, D.M. Bibliography of work on the heterogeneous photocatalytic removal of hazardous compounds from water and air. Natl. Renew. Energy Lab. 2001, 4, 1–265. [Google Scholar] [CrossRef] [Green Version]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. Standard Tables for Reference Solar Spectral Irradiances: DIRECT, Normal and Hemispherical on 37° Tilted Surface; Standard G173-03; ASTM International: West Conshohocken, PA, USA, 2013; ISBN G 173 03. [Google Scholar]
- Langford, C.H. Photocatalysis—A special issue on a unique hybrid rea of catalysis. Catalysts 2012, 2, 327–329. [Google Scholar] [CrossRef]
- Keane, D.A.; McGuigan, K.G.; Ibáñez, P.F.; Polo-López, M.I.; Byrne, J.A.; Dunlop, P.S.M.; O’Shea, K.; Dionysiou, D.D.; Pillai, S.C. Solar photocatalysis for water disinfection: Materials and reactor design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, F.; Behpour, M.; Ghoreishi, S.M.; Khalilian, H. Photocatalytic degradation of paraquat herbicide in the presence TiO2 nanostructure thin films under visible and sun light irradiation using continuous flow photoreactor. Sol. Energy 2015, 120, 287–295. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Portjanskaja, E.; Krichevskaya, M.; Preis, S.; Kallas, J. Photocatalytic oxidation of humic substances with TiO2-coated glass micro-spheres. Environ. Chem. Lett. 2004, 2, 123–127. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Photocatalytic decomposition of leather dye comparative study of TiO2 supported on alumina and glass beads. J. Photochem. Photobiol. A Chem. 2002, 148, 153–159. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Sirisuk, A.; Hill, C.G.; Anderson, M.A. Photocatalytic degradation of ethylene over thin films of titania supported on glass rings. Catal. Today 1999, 54, 159–164. [Google Scholar] [CrossRef]
- Guillard, C.; Amalric, L.; D’Oliveira, J.C.; Delpart, H.; Hoang-Van, C.; Pichat, P. Heterogeneous photocatalysis: Use in water treatment and involvement in atmopheric chemistry. In Aquatic and Surface Photochemistry; Helz, G.R., Zepp, R.G., Crosby, D.G., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 369–386. ISBN 0-87371-871-2. [Google Scholar]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Chekir, N.; Boukendakdji, H.; Igoud, S.; Taane, W. Solar energy for the benefit of water treatment: Solar photoreactor. Procedia Eng. 2012, 33, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 solar photocatalytic reactor systems: Selection of reactor design for scale-up and commercialization—Analytical review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef] [Green Version]
- Goswami, D.Y. Principles of Solar Engineering, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2015; Volume 1, ISBN 1466563796. [Google Scholar]
- Goswami, D.Y.; Kreith, F.; Kreider, J.F. Principles of Solar Engineering, 2nd ed.; Taylor & Francis Group: Philadelphia, PA, USA, 2000. [Google Scholar]
- Feitz, A.J.; Boyden, B.H.; Waite, T.D. Evaluation of two solar pilot scale fixed-bed photocatalytic reactors. Water Res. 2000, 34, 3927–3932. [Google Scholar] [CrossRef]
- Toor, A.P.; Verma, A.; Jotshi, C.K.; Bajpai, P.K.; Singh, V. Photocatalytic degradation of 3, 4-dichlorophenol using TiO2 in a shallow pond slurry reactor. Indian J. Chem. Technol. 2005, 12, 75–81. [Google Scholar]
- Heller, A.; Brock, J. Materials and Methods for Photocatalyzing Oxidation of Organic Compounds on Water. U.S. Patent 4,997,576, 25 September 1989. [Google Scholar]
- Yuan, J.; An, Z.-G.; Zhang, J.-J.; Li, B. Synthesis and properties of hollow glass spheres/TiO2 composite. Imaging Sci. Photochem. 2012, 30, 447–455. [Google Scholar]
- Wang, J.; He, B.; Kong, X.Z. A study on the preparation of floating photocatalyst supported by hollow TiO2 and its performance. Appl. Surf. Sci. 2015, 327, 406–412. [Google Scholar] [CrossRef]
- Maki, Y.; Ide, Y.; Okada, T. Water-floatable organosilica particles for TiO2 photocatalysis. Chem. Eng. J. 2016, 299, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Shifu, C.; Gengyu, C. Photocatalytic degradation of organophosphorus pesticides using floating photocatalyst TiO2 • SiO2/beads by sunlight. Sol. Energy 2005, 79, 1–9. [Google Scholar] [CrossRef]
- Shifu, C.; Gengyu, C. Photocatalytic oxidation of nitrite by sunlight using TiO2 supported on hollow glass microbeads. Sol. Energy 2002, 73, 15–21. [Google Scholar] [CrossRef]
- Hartley, A.C.; Moss, J.B.; Keesling, K.J.; Moore, N.J.; Glover, J.D.; Boyd, J.E. PMMA-titania floating macrospheres for the photocatalytic remediation of agro-pharmaceutical wastewater. Water Sci. Technol. 2017, 75, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Preis, S.; Krichevskaya, M.; Kharchenko, A. Photocatalytic oxidation of aromamtic aminocompounds in aqueous solutions and groundwater from abandoned military bases. Water Sci. Technol. 1997, 35, 265–272. [Google Scholar] [CrossRef]
- Welch, K. Passive purification-effectiveness of photocatalytic titanium dioxide to convert pathogens and pollutants. Am. Ceram. Soc. Bull. 2014, 93, 25–30. [Google Scholar]
- Komtchou, S.; Dirany, A.; Drogui, P. Traitement des eaux contaminées par les pesticides—Pour le treatment des eaux contaminees par les pesticides-revue de litterature. Rev. Sci. l’eau 2018, 29, 231–262. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Irie, H.; Fujishima, A. A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Magalhães, F.; Lago, R.M. Floating photocatalysts based on TiO2 grafted on expanded polystyrene beads for the solar degradation of dyes. Sol. Energy 2009, 83, 1521–1526. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Sboui, M.; Faouzi, M.; Rayes, A.; Ochiai, T.; Houas, A. Application of solar light for photocatalytic degradation of Congo red by a floating salicylic acid-modified TiO2/palm trunk photocatalyst. Comptes Rendus Chim. 2017, 20, 181–189. [Google Scholar] [CrossRef]
- Leshuk, T.; Krishnakumar, H.; de Oliveira Livera, D.; Gu, F. Floating photocatalysts for passive solar degradation of naphthenic acids in oil sands process-affected water. Water 2018, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Robert, D.; Keller, V.; Keller, N. Immobilization of a semi-conductor photocatalyst on solid supports, methods, materials and applications. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications; Lu, M., Pichat, P., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 145–172. ISBN 9783527645411. [Google Scholar]
- Gong, X.-Q.; Selloni, A. Reactivity of Anatase TiO2 Nanoparticles: The Role of the Minority (001) Surface. J. Phys. Chem. B 2005, 109, 19560–19562. [Google Scholar] [CrossRef]
- Nakamura, R.; Ohashi, N.; Imanishi, A.; Osawa, T.; Matsumoto, Y.; Koinuma, H.; Nakato, Y. Crystal-Face Dependences of Surface Band Edges and Hole Reactivity, Revealed by Preparation of Essentially Atomically Smooth and Stable (110) and (100) n-TiO2 (Rutile) Surfaces. J. Phys. Chem. B 2005, 109, 1648–1651. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Highly reactive {001} facets of TiO2-based composites: Synthesis formation mechanism and characterization. Nanoscale 2014, 6, 1946–2008. [Google Scholar] [CrossRef]
- Tian, T.; Hu, J.; Xiao, Z. Research Advances in Photocatalysis of Inorganic Hollow Spheres. World J. Nano Sci. Eng. 2014, 4, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Wang, X.; Wang, H.; Dong, Y.; Ma, Y.; Yao, J. Multi-shelled titania hollow spheres fabricated by a hard template strategy: Enhanced photocatalytic activity. Chem. Commun. 2010, 46, 4312–4314. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Zheng, F.; Li, Y.; Huang, F. Synthesis of the double-shell anatase–rutile TiO2 hollow spheres with enhanced photocatalytic activity. Nanoscale 2013, 5, 12150–12155. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, Y.; Pan, J.; Gu, H.; Qin, C.; Zhou, P. Glycine assisted synthesis of flower-like TiO2 hierarchical spheres and its application in photocatalysis. Mater. Sci. Eng. B 2012, 177, 1664–1671. [Google Scholar] [CrossRef]
- Natural Resources Canada Oil Sands: Water Management, a Strategic Resource for Canada, North America and the Global Market. Available online: https://www.nrcan.gc.ca/energy/publications/18750 (accessed on 8 February 2017).
- Province of Alberta. Environmental Protection and Enhancement Act, Revised Statutes of Alberta 2000; Chapter E-12; Alberta Queen’s Printer: Edmonton, AB, Canada, 2000; pp. 1–164. [Google Scholar]
- Kannel, P.R.; Gan, T.Y. Naphthenic acids degradation and toxicity mitigation in tailings wastewater systems and aquatic environments: A review. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2012, 47, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Grewer, D.M.; Young, R.F.; Whittal, R.M.; Fedorak, P.M. Naphthenic acids and other acid-extractables in water samples from Alberta: What is being measured? Sci. Total Environ. 2010, 408, 5997–6010. [Google Scholar] [CrossRef] [PubMed]
- Leshuk, T.; Wong, T.; Linley, S.; Peru, K.M.; Headley, J.V.; Gu, F. Solar photocatalytic degradation of naphthenic acids in oil sands process-affected water. Chemosphere 2016, 144, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Health Canada Special Review of 2,4-D: Proposed Decision for Consultation. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/consultations/re-evaluation-note/2016/special-review-2-4-d/document.html#s2 (accessed on 8 February 2017).
- Krieger, R. Hayes’ Handbook of Pesticide Toxicology; Krieger, R., Ed.; Elsevier Science & Technology: Saint Louis, MS, USA, 2010; ISBN 9780080922010. [Google Scholar]
- Giri, R.R.; Ozaki, H.; Taniguchi, S.; Takanami, R. Photocatalytic ozonation of 2,4-dichlorophenoxyacetic acid in water with a new TiO2 fiber. Int. J. Environ. Sci. Technol. 2008, 5, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Ikehata, K.; El-Din, M.G.; Snyder, S.A. Ozonation and advanced oxidation treatment of emerging organic pollutants in water and wastewater. Ozone Sci. Eng. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Anderson, A.; Byrtus, G.; Thompson, J.; Humphries, D.; Hill, B.; Bilyk, M.; Alberta. Dept. of Environment. Water Research User Group. Baseline Pesticide Data for Semi-Permanent Wetlands in the Aspen Parkland of Alberta; Alberta Environment: Edmonton, Alberta, 2002. [Google Scholar]
- Canadian Council of Ministers of Envivronment. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health—Sulfolane; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2006. [Google Scholar]
- Leigh, M.B.; Barnes, D. Water and Environmental Research Center, annual technical report. In Toxicity of Sulfolane Breakdown Products in Contaminated Groundwater; Water and Environmental Research Center: University of Alaska Fairbanks, AK, USA, 2013. [Google Scholar]
- Greene, E.A.; Beatty, P.H.; Fedorak, P.M. Sulfolane degradation by mixed cultures, and a bacterial isolate identified as a Variovorax sp. Arch. Microbiol. 2000, 174, 111–119. [Google Scholar] [CrossRef]
- Suh, S.; Tomar, S.; Leighton, M.; Kneifel, J. Environmental performance of green building code and certification systems. Environ. Sci. Technol. 2014, 48, 2551–2560. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Hasan, N.; Lintang, H.O.; Shamsuddin, M.; Yuliati, L. Photocatalytic removal of 2,4-dichlorophenoxyacetic acid herbicide on copper oxide/titanium dioxide prepared by co-precipitation method. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yu, L.; Achari, G. Case Study: Evaluation of Pure and Mixed Bacterial Cultures on Sulfolane Biodegradation in Aqueous Media. In Proceedings of the CSCE Annual Conference, Montreal, QC, Canada, 12–15 June 2019. [Google Scholar]
- Yu, L.; Mehrabani-Zeinabad, M.; Achari, G.; Langford, C. Application of UV based advanced oxidation to treat sulfolane in an aqueous medium. Chemosphere 2016, 160, 155–161. [Google Scholar] [CrossRef]
- Izadifard, M. Oxidation of Sulfolane in Aqueous Systems by Chemical and Photochemical Processes. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2019. [Google Scholar]
- Brandão, M.; Yu, L.; Garcia, C.; Achari, G. Advanced oxidation based treatment of soil wash water contaminated with sulfolane. Water 2019, 11, 2152. [Google Scholar] [CrossRef] [Green Version]
- Mehrabani-Zeinabad, M. Advanced Oxidative Processes for Treatment of Emerging Contaminants in Water. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2016. [Google Scholar]
- Versace, F.; Uppugunduri, C.R.S.; Krajinovic, M.; The, Y.; Gumy, F.P.; Mangin, P.; Staub, C.; Ansari, M. A novel method for quantification of sulfolane (a metabolite of busulfan) in plasma by gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 404, 1831–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, Z. Solar Energy Fundamentals and Modeling Techniques; Springer-Verlag: London, UK, 2008; ISBN 9781848001336. [Google Scholar]


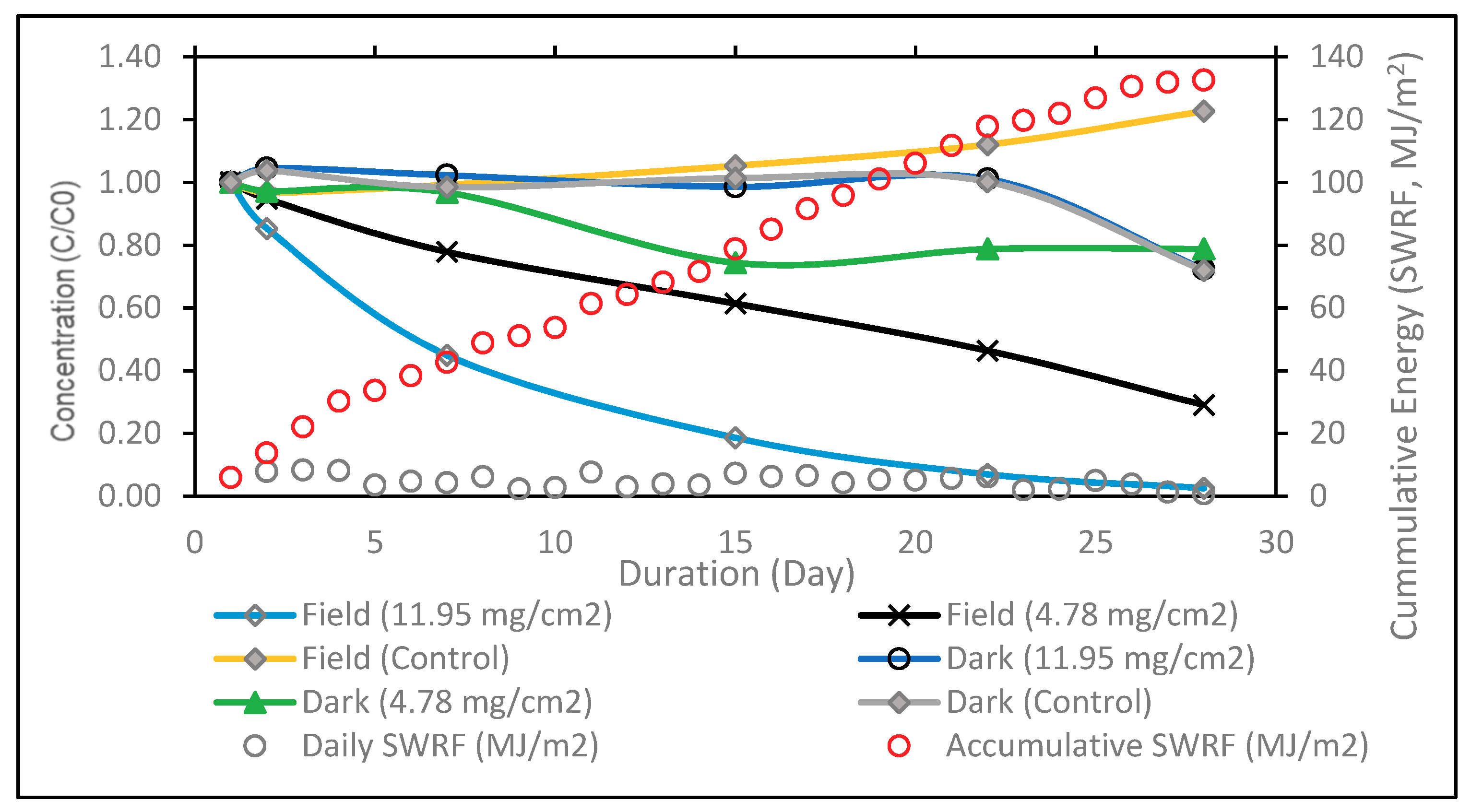

| Catalyst Loading (mg/cm2) | 11.95 | 4.78 |
| Insolation (day) | 22 | 28 |
| Degradation of 2, 4-D (%) | 99.8 | 90 |
| Degradation of Dicamba (%) | Not detectable | 74 |
| Degradation of MCPP (%) | Not detectable | 94 |
| TOC reduction in 28 days (%) | 88 | 53 |
| Kinetic rate constant (day−1) | 0.60 | 0.17 |
| UV Energy (MJ/cm2) | 1.89 | 3.18 |
| Catalyst Loading (mg/cm2) | Duration (Day) | UV Energy (MJ/cm2) | Degradation of Sulfolane (%) | TOC Reduction (%) | K (day−1) |
|---|---|---|---|---|---|
| 11.95 | 28 | 3.18 | 97.4 | 64.4 | 0.35 |
| 4.78 | 70.1 | 27.75 | 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydari, G.; Langford, C.H.; Achari, G. Passive Solar Photocatalytic Treatment of Emerging Contaminants in Water: A Field Study. Catalysts 2019, 9, 1045. https://doi.org/10.3390/catal9121045
Heydari G, Langford CH, Achari G. Passive Solar Photocatalytic Treatment of Emerging Contaminants in Water: A Field Study. Catalysts. 2019; 9(12):1045. https://doi.org/10.3390/catal9121045
Chicago/Turabian StyleHeydari, Gisoo, Cooper H. Langford, and Gopal Achari. 2019. "Passive Solar Photocatalytic Treatment of Emerging Contaminants in Water: A Field Study" Catalysts 9, no. 12: 1045. https://doi.org/10.3390/catal9121045
APA StyleHeydari, G., Langford, C. H., & Achari, G. (2019). Passive Solar Photocatalytic Treatment of Emerging Contaminants in Water: A Field Study. Catalysts, 9(12), 1045. https://doi.org/10.3390/catal9121045




