Lipoaminoacids Enzyme-Based Production and Application as Gene Delivery Vectors
Abstract
1. Introduction
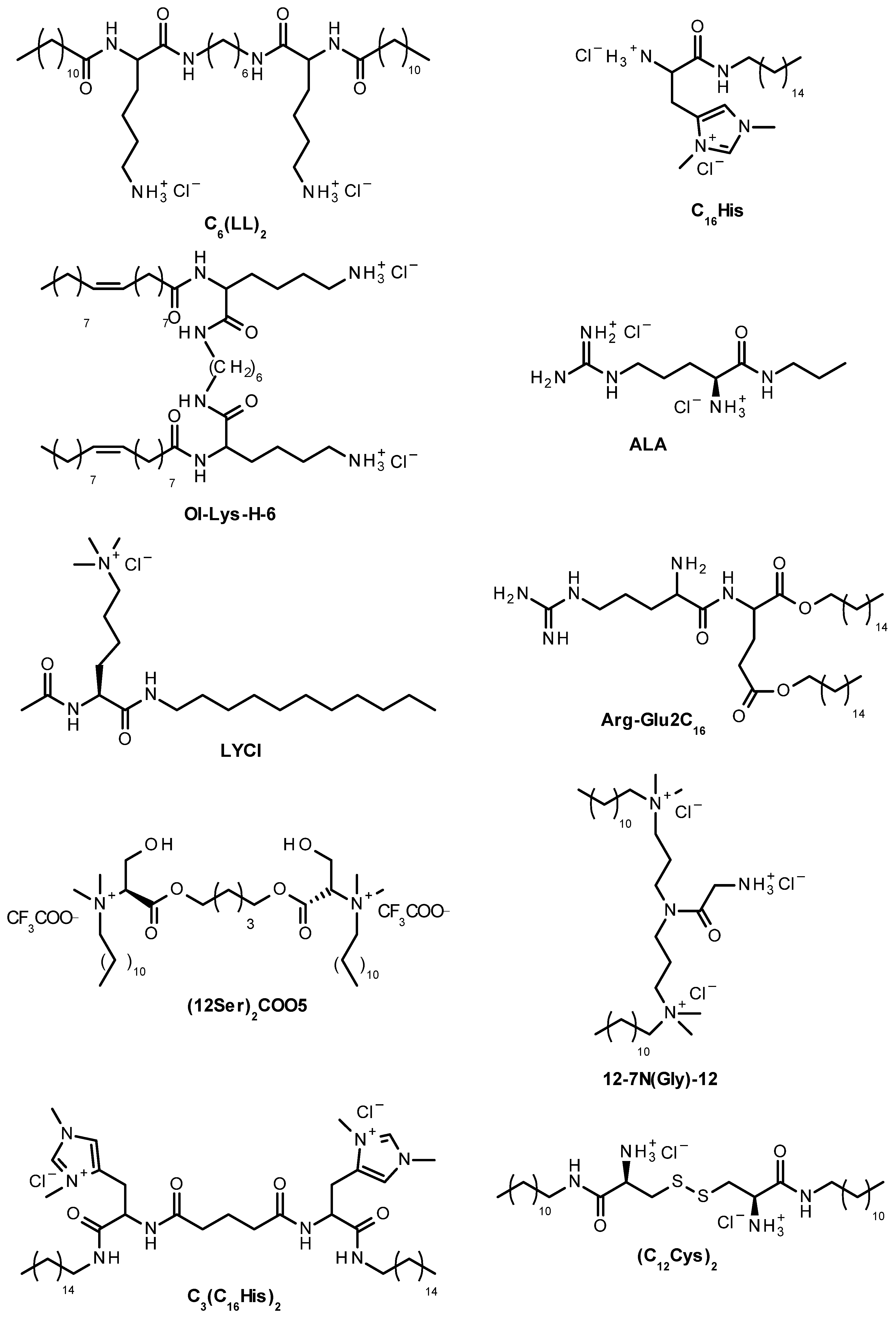
2. Cationic Lipoaminoacids
2.1. Structure and Characterization
2.2. Biocatalytic Production
2.2.1. Enzymes
2.2.2. Solvents
2.2.3. Immobilization
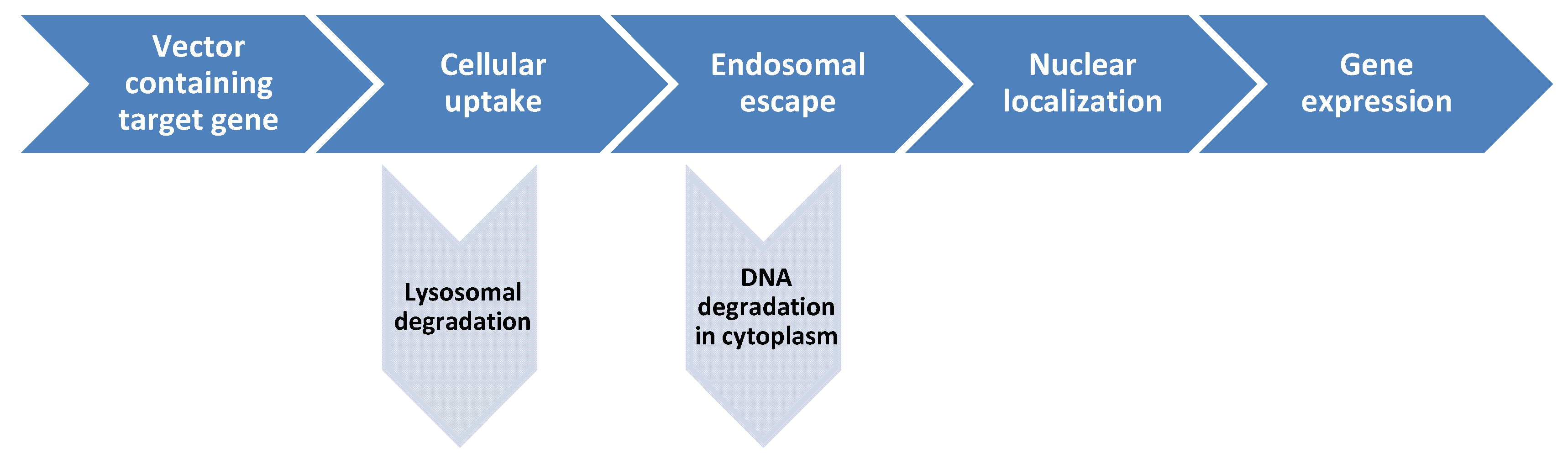
3. Cationic Lipoaminoacid-DNA Interactions
4. Applications of Cationic Gemini Surfactants as Transfection Agents
5. Advantages of Cationic Lipoamino Acids in Gene Delivery
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Gharaei-Fathabad, E. Biosurfactants in Pharmaceutical Industry (A Mini-Review). Am. J. Drug Discov. Dev. 2011, 1, 58–69. [Google Scholar]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.; Pashirova, T.; Doktorovova, S.; Fernandes, A.; Sanchez-Lopez, E.; Silva, A.; Souto, S. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.; Grossen, P.; Cullis, P.R.; Huwyler, J.; Witzigmann, D. Lipid-Based DNA Therapeutics: Hallmarks of Non-Viral Gene Delivery. ACS Nano 2019, 13, 3754–3782. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Bai, Y.; Yang, J.; Cui, S.; Zhao, Y.; Chen, H.; Zhang, S. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 2018, 253, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Puras, G.; Zárate, J.; Sainz-Ramos, M.; Qtaish, N.A.L.; López, T.; Mashal, M.; Attia, N.; Díaz, D.; Pons, R.; et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics 2019, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Ilarduya, C.T.; Sun, Y.; Duzgunes, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Wettig, S.D.; Verrall, R.E.; Foldvari, M. Gemini surfactants: A new family of building blocks for non-viral gene delivery systems. Curr. Gene Ther. 2008, 8, 9–23. [Google Scholar] [CrossRef]
- Singh, J.; Yang, P.; Michel, D.; Verrall, R.E.; Foldvari, M.; Baeda, I. Amino acid-substituted gemini surfactant-based nanoparticles as safety and versatile gene delivery agents. Curr. Drug Deliv. 2011, 8, 299–306. [Google Scholar] [CrossRef]
- Singh, J.; Michel, D.; Chitanda, J.M.; Verrall, R.E.; Badea, I. Evaluation of cellular uptake and intracellular trafficking as determining factors of gene expression for amino acid-substituted gemini surfactant-based DNA nanoparticles. J. Nanobiotechnol. 2012, 10, 7. [Google Scholar] [CrossRef]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef]
- Bhadani, A.; Singh, S. Novel gemini pyridinium surfactants: Synthesis and study of their surface activity, DNA binding, and cytotoxicity. Langmuir 2009, 25, 11703–11712. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Pons, R.; Pérez, L.; Infante, M.R. Amino Acids as raw material for biocompatible surfactants. Ind. Eng. Chem. Res. 2011, 50, 4805–4817. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, G.; Ao, M.; Yang, Y.; Wang, C. DNA compaction and multi-molecular DNA condensation induced by cationic imidazolium gemini surfactants. Colloids Surf. A. 2012, 41, 33–40. [Google Scholar] [CrossRef]
- Bordes, R.; Holmberg, K. Amino acid-based surfactants–do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Pinazo, A.; Pons, R.; Infante, M.R. Gemini surfactants from natural amino acids. Adv. Colloid Interface Sci. 2014, 205, 134–155. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.S.; Lo, M.; Araújo, M.J.; Marques, E.F. Temperature-responsive self-assembled nanostructures from lysine-based surfactants with high chain length asymmetry: From tubules and helical ribbons to micelles and vesicles. Soft Matter 2019, 15, 3700–3711. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, D.M.; Deng, X.; Zhang, J.; Zhang, Y.M.; Yu, X.Q. Functionalized asymmetric bola-type amphiphiles for efficient gene and drug delivery. Nanomaterials 2018, 8, 115. [Google Scholar] [CrossRef]
- Haldar, S.; Karmakar, K. Concentration dependent morphological transition of nanostructured self-assembly towards hydrogelation seeding from micellar aggregates through stereochemically optimized H-bonding network of amino acid derived cationic amphiphiles. Colloids Surf. A 2017, 516, 394–404. [Google Scholar] [CrossRef]
- Joondan, N.; Caumul, P.; Jhaumeer-Laulloo, S. Investigation of the physicochemical and biological properties of proline-based surfactants in single and mixed surfactant systems. J. Surfactants Deterg. 2017, 20, 103–115. [Google Scholar] [CrossRef]
- Kaki, S.S.; Arukali, S.; Korlipara, P.V.; Prasad, R.B.; Yedla, P.; Ganesh Kumar, C. Synthesis and biological evaluation of novel lipoamino acid derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 209–212. [Google Scholar] [CrossRef]
- Mohini, Y.; Prasad, R.B.; Karuna, M.S.; Poornachandra, Y.; Ganesh Kumar, C. Synthesis and biological evaluation of ricinoleic acid-based lipoamino acid derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 5198–5202. [Google Scholar] [CrossRef] [PubMed]
- Joondan, N.; Jhaumeer-Laullo, S.; Caumul, P. A study of the antibacterial activity of L-phenylalanine and L-tyrosine esters in relation to their CMCs and their interactions with1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DPPC as model membrane. Microbiol. Res. 2014, 169, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Ménard, N.; Tsapis, N.; Poirier, C.; Arnauld, T.; Moine, L.; Lefoulon, F.; Péan, J.-M.; Fattal, E. Drug solubilization and in vitro toxicity evaluation of lipoamino acid surfactants. Int. J. Pharm. 2012, 423, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, R.; Pérez, L.; Pinazo, A.; Tavano, L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: Skin penetration behavior and controlled drug release. Int. J. Pharm. 2017, 529, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Serafim, C.; Ferreira, I.; Pinheiro, L.; Calado, A. Solubilization power of an amino acid-based gemini surfactant towards the hydrophobic drug amphotericin B. Colloids Surf. A. 2015, 480, 426–432. [Google Scholar] [CrossRef]
- Serafim, C.; Ferreira, I.; Rijo, P.; Pinheiro, L.; Faustino, C.; Calado, A.; Garcia-Rio, L. Lipoamino acid-based micelles as promising delivery vehicles for monomeric amphotericin B. Int. J. Pharm. 2016, 497, 23–35. [Google Scholar] [CrossRef]
- Bustelo, M.; Pinazo, A.; Manresa, M.; Mitjans, M.; Vinardell, M.; Perez, L. Monocatenary histidine-based surfactants: Role of the alkyl chain length in antimicrobial activity and their selectivity over red blood cells. Colloids Surf. A 2017, 532, 501–509. [Google Scholar] [CrossRef]
- Katiyar, S.; Kushwah, V.; Dora, C.P.; Patil, R.Y.; Jain, S. Design and toxicity evaluation of novel fatty acid-amino acid-based biocompatible surfactants. AAPS Pharm. Sci. Tech. 2019, 20, 186. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathy, D.B.; Clark, J.; Farmer, T. Synthesis, chemistry, physicochemical properties and industrial applications of amino acid surfactants: A review. Comptes Rendus Chim. 2018, 21, 112–130. [Google Scholar]
- Foley, P.; Kermanshahi, A.; Beach, E.S.; Zimmerman, J.B. Derivation and synthesis of renewable surfactants. Chem. Soc. Rev. 2012, 41, 1499–1518. [Google Scholar] [CrossRef] [PubMed]
- Franssen, M.C.R.; Steunenberg, P.; Scott, E.L.; Zuilhofac, H.; Sanders, J.P.M. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [PubMed]
- Morcelle, S.R.; Liggieri, C.S.; Bruno, M.A.; Priolo, N.; Clapés, P. Screening of plant peptidases for the synthesis of arginine-based surfactants. J. Mol. Catal. B 2009, 57, 177–182. [Google Scholar] [CrossRef]
- Faustino, C.; Martins, T.; Duarte, N.; Ribeiro, M.H. Self-assembly of lipoaminoacids-DNA based on thermodynamic and aggregation properties. J. Surfactants Deterg. 2019, Submitted. [Google Scholar]
- Villeneuve, P. Lipases in lipophilization reactions. Biotechnol. Adv. 2007, 25, 515–536. [Google Scholar] [CrossRef]
- Bautista, M.E.; Pérez, L.; García, M.T.; Cuadros, S.; Marsal, A. Valorization of tannery wastes: Lipoamino acid surfactant mixtures from the protein fraction of process wastewater. Chem. Eng. J. 2015, 262, 399–408. [Google Scholar] [CrossRef]
- Scott, E.; Peter, F.; Sanders, J. Biomass in the manufacture of industrial products–the use of proteins and amino acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. [Google Scholar] [CrossRef]
- Paranthaman, R.; Alagusundaram, K.; Indhumathi, J. Production of protease from rice mill wastes by Aspergillus niger in solid state fermentation. World J. Agric. Sci. 2009, 5, 308–312. [Google Scholar]
- Shu, Z.-Y.; Jiang, H.; Lin, R.-F.; Jiang, Y.-M.; Lin, L.; Huang, J.-Z. Technical methods to improve yield, activity and stability in the development of microbial lipases. J. Mol. Catal. B 2010, 62, 1–8. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Drepper, T.; Eggert, T.; Hummel, W.; Leggewie, C.; Pohl, M.; Rosenau, F.; Jaeger, K.-E. Novel biocatalysts for white biotechnology. Biotechnol. J. 2006, 1, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Brockman, H.L. Lipases Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2004; pp. 571–576. [Google Scholar]
- Lotti, M.; Alberghina, L. Lipases: Molecular Structure and Function. In Industrial Enzymes; Polaina, J., MacCabe, A., Eds.; Springer: Amsterdam, The Netherlands, 2007; pp. 263–281. [Google Scholar]
- Hamza, T. Bacterial Protease Enzyme: Safe and Good Alternative for Industrial and Commercial Use. Int. J. Chem. Biomol. Sci. 2017, 3, 1–10. [Google Scholar]
- Gorke, J.; Srienc, F.; Kazlauskas, R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol. Bioprocess Eng. 2010, 15, 40–53. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Crittle, T.D. Choline-based deep eutectic solvents for enzymatic preparation of biodiesel from soybean oil. J. Mol. Catal. B 2012, 85, 243–247. [Google Scholar] [CrossRef]
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Infante, M.R. “Green” amino acid-based surfactants. Green Chem. 2004, 6, 233–240. [Google Scholar] [CrossRef]
- Clapes, P.; Infante, M.R. Amino Acid-based Surfactants: Enzymatic Synthesis, Properties and Potential Applications. J. Biocatal. Biotransform. 2002, 20, 215–233. [Google Scholar] [CrossRef]
- Christensen, M.W.; Andersen, L.; Husum, T.L.; Kirk, O. Industrial lipase immobilization. Eur. J. Lipid Sci. Technol. 2003, 105, 318–321. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Rocha-Martin, J.; Guisán, J.M.; Favela-Torres, E. Improving enantioselectivity of lipase from Candida rugosa by carrier bound and carrier-free immobilization. J. Mol. Catal. B Enzym. 2016, 130, 32–39. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, C.; Xia, H.; Mahmood, I.; Liu, C.; Liu, H. Magnetic nanoparticles supported ionic liquids for lipase immobilization: Enzyme activity in catalyzing esterification. J. Mol. Catal. B 2009, 58, 103–109. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Bernal, C.; Guzman, F.; Illanes, A.; Wilson, L. Selective and eco-friendly synthesis of lipoaminoacid-based surfactants for food, using immobilized lipase and protease biocatalysts. Food Chem. 2018, 239, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Fait, M.; Garrote, G.; Clapés, P.; Tanco, S.; Lorenzo, J.; Morcelle, S. Biocatalytic synthesis, antimicrobial properties and toxicity studies of arginine derivative surfactants. Amino Acids 2015, 47, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Martins, T.S.; Faustino, C. Enzymatic development of lipoaminoacids towards nanostructured gene delivery systems. In Proceedings of the European Symposium on Biochemical Engineering Sciences, Lisbon, Portugal, 9–12 Sepetember 2018. Book of Abstracts ESBES 2018. [Google Scholar]
- Faustino, C.M.C.; Calado, A.R.T.; Garcia-Rio, L. New urea-based surfactants derived from α, ω-amino acids. J. Phys. Chem. B 2009, 113, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.M.C.; Calado, A.R.T.; Garcia-Rio, L. Gemini surfactant-protein interactions: Effect of pH, temperature and surfactant stereochemistry. Biomacromolecules 2009, 10, 2508–2514. [Google Scholar] [CrossRef]
- Husale, S.; Grange, W.; Karle, M.; Burgi, S.; Hegner, M. Interaction of cationic surfactants with DNA: A single-molecule study. Nucleic Acids Res. 2008, 36, 1443–1449. [Google Scholar] [CrossRef]
- He, Y.; Shang, Y.; Shao, S.; Liu, H.; Hu, Y. Micellization of cationic gemini surfactant and its interaction with DNA in dilute brine. J. Colloid Interface Sci. 2011, 358, 513–520. [Google Scholar] [CrossRef]
- Dias, R.S.; Magno, L.M.; Valente, A.J.; Das, D.; Das, P.K.; Maiti, S.; Miguel, M.G.; Lindman, B. Interaction between DNA and cationic surfactants: Effect of DNA conformation and surfactant headgroup. J. Phys. Chem. B 2008, 112, 14446–14452. [Google Scholar] [CrossRef]
- Petrov, A.I.; Khalil, D.N.; Kazaryan, R.L.; Savintsev, I.V.; Sukhorukov, B.I. Structural and thermodynamic features of complexes formed by DNA and synthetic polynucleotides with dodecylamine and dodecyltrimethylammonium bromide. Bioelectrochemistry 2002, 58, 75–85. [Google Scholar] [CrossRef]
- Zhu, D.M.; Evans, R.K. Molecular mechanism and thermodynamics study of plasmid DNA and cationic surfactants interactions. Langmuir 2006, 22, 3735–3743. [Google Scholar] [CrossRef]
- Faustino, C.M.C.; Calado, A.R.T.; Garcia-Rio, L. Dimeric and monomeric surfactants derived from sulfur-containing amino acids. J. Colloid Interface Sci. 2010, 351, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.M.C.; Calado, A.R.T.; Garcia-Rio, L. Mixed micelle formation between amino acid-based surfactants and phospholipids. J. Colloid Interface Sci. 2011, 359, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Shortall, S.M.; Wettig, S.D. Cationic gemini surfactant-plasmid deoxyribonucleic acid condensates as a single amphiphilic entity. J. Phys. Chem. B 2018, 122, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.A.; Pinheiro, L.; Faustino, C. Amino acid-based cationic gemini surfactant-protein interactions. Colloids Surf. A 2015, 480, 105–112. [Google Scholar] [CrossRef]
- Miguel, M.G.; Pais, A.A.C.C.; Dias, R.S.; Leal, C.; Rosa, M.; Lindman, B. DNA-cationic amphiphile interactions. Colloids Surf. A 2003, 228, 43–55. [Google Scholar] [CrossRef]
- Matulis, D.; Rouzina, I.; Bloomfield, V.A. Thermodynamics of cationic lipid binding to DNA and DNA condensation: Roles of electrostatics and hydrophobicity. J. Am. Chem. Soc. 2002, 124, 7331–7342. [Google Scholar] [CrossRef]
- Zhao, X.; Shang, Y.; Hu, J.; Liu, H.; Hu, Y. Biophysical characterization of complexation of DNA with oppositely charged Gemini surfactant 12-3-12. Biophys. Chem. 2008, 138, 144–149. [Google Scholar] [CrossRef]
- Ansari, A.A.; Kamil, M.; Kabir-ud-Din. Polymer-surfactant interactions and the effect of tail size variation on micellization process of cationic ATAB surfactants in aqueous medium. J. Disper. Sci. Technol. 2013, 34, 722–730. [Google Scholar] [CrossRef]
- Chaterjee, A.; Moulik, S.P.; Majhi, P.R.; Sanyal, S.K. Studies on surfactant–biopolymer interaction. I. Microcalorimetric investigation on the interaction of cetyltrimetylammonium bromide (CTAB) and sodium dodecylsulfate (SDS) with gelatin (Gn), lysozyme (Lz) and deoxyribonucleic acid (DNA). Biophys. Chem. 2002, 98, 313–327. [Google Scholar] [CrossRef]
- Das, S.; Mondal, S.; Ghosh, S. Interaction of cationic gemini surfactant tetramethylene-1,4-bis(dimethyltetradecylammonium bromide) with anionic polyelectrolyte sodium carboxymethyl cellulose, with two different molar masses, in aqueous and aquo-organic (isopropanol) media. RSC Adv. 2016, 6, 30795–30803. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y. Aggregation behavior of gemini surfactants and their interaction with macromolecules in aqueous solution. Phys. Chem. Chem. Phys. 2011, 13, 1939–1956. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; van Eijk, M.C.P.; Söderman, O. Compaction of DNA by gemini surfactants: Effects of surfactant architecture. J. Colloid Interface Sci. 2002, 252, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, J.; Wang, Y.; Yan, H.; Thomas, R.K. Microcalorimetric study on the interaction of dissymmetric gemini surfactants with DNA. J. Colloid Interface Sci. 2005, 284, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic lipid saturation influences intracelular delivery of encapsulated nucleic acids. J. Control. Release 2005, 107, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Damen, M.; Cristobal-Lecina, E.; Snmarti, G.C.; van Dongen, S.F.M.; Garcia Rodriguez, C.L.; Dolbnya, I.P.; Nolte, R.J.M.; Feiters, M.C. Structure-delivery relationships of lysine-based gemini surfactants and their lipoplexes. Soft Matter 2014, 10, 5702–5714. [Google Scholar] [CrossRef] [PubMed]
- Pi-Boleda, B.; Bouzas, M.; Gaztelumendi, N.; Illa, O.; Nogués, C.; Branchadell, V.; Pons, R.; Ortuño, R.M. Chiral pH-sensitive cyclobutane β-amino acid-based cationic amphiphiles: Possible candidates for use in gene therapy. J. Mol. Liq. 2019, 111856, in press. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wettig, S.D.; Badea, I.; Foldvari, M.; Verrall, R.E. Investigation of complexes formed by interaction of cationic gemini surfactants with deoxyribonucleic acid. Phys. Chem. Chem. Phys. 2007, 9, 1616–1628. [Google Scholar] [CrossRef]
- García, J.P.; Marrón, E.; Martín, V.I.; Moyá, M.L.; Lopez-Cornejo, P. Conformational changes of DNA in the presence of 12-s-12 gemini surfactants (s = 2 and 10). Role of the spacer’s length in the interaction surfactant-polynucleotide. Colloids Surf. B 2014, 118, 90–100. [Google Scholar] [CrossRef]
- Sarrión, B.; Bernal, E.; Martín, V.I.; López-López, M.; López-Cornejo, P.; García-Calderón, M.; Moyá, M.L. Binding of 12-s-12 dimeric surfactants to calf thymus DNA: Evaluation of the spacer length influence. Colloids Surf. B 2016, 144, 311–318. [Google Scholar] [CrossRef]
- Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R.K. Micellization of cationic gemini surfactants with various counterions and their interaction with DNA in aqueous solution. J. Phys. Chem. B 2004, 108, 15385–15391. [Google Scholar] [CrossRef]
- Dasgupta, A.; Das, P.K.; Dias, R.S.; Miguel, M.G.; Lindman, B.; Jadhav, V.M.; Gnanamani, M.; Maiti, S. Effect of headgroup on DNA-cationic surfactant interactions. J. Phys. Chem. B 2007, 111, 8502–8508. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Miguel, M.G.; Lindman, B. DNA encapsulation by biocompatible catanionic vesicles. J. Colloid Interface Sci. 2007, 312, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Morán, M.C.; Miguel, M.G.; Lindman, B. The association of DNA and stable catanionic amino acid-based vesicles. Colloids Surf. A 2007, 301, 361–375. [Google Scholar] [CrossRef]
- Rosa, M.; Penacho, N.; Simões, S.; Lima, M.C.; Lindman, B.; Miguel, M.G. DNA pre-condensation with an amino acid-based cationic amphiphile. A viable approach for liposome-based gene delivery. Mol. Membr. Biol. 2008, 25, 23–34. [Google Scholar] [CrossRef]
- Santhiya, D.; Dias, R.S.; Dutta, S.; Das, P.K.; Miguel, M.G.; Lindman, B.; Maiti, S. Kinetic studies of amino acid-based surfactant binding to DNA. J. Phys. Chem. B 2012, 116, 5831–5837. [Google Scholar] [CrossRef]
- Martín, V.I.; Sarrión, B.; López-López, M.; López-Cornejo, P.; Robina, I.; Moyá, M.L. Reversibility of the interactions between a novel surfactant derived from lysine and biomolecules. Colloids Surf. B 2015, 135, 346–356. [Google Scholar] [CrossRef]
- Santhiya, D.; Dias, R.S.; Shome, A.; Das, P.K.; Miguel, M.G.; Lindman, B.; Maiti, S. Role of linker groups between hydrophilic and hydrophobic moieties of cationic surfactants on oligonucleotide-surfactant interactions. Langmuir 2009, 25, 13770–13775. [Google Scholar] [CrossRef]
- Jadhav, V.; Maiti, S.; Dasgupta, A.; Das, P.K.; Dias, R.S.; Miguel, M.G.; Lindman, B. Effect of the headgroup geometry of amino acid-based cationic surfactants on interaction with plasmid DNA. Biomacromolecules 2008, 9, 1852–1859. [Google Scholar] [CrossRef]
- Obata, Y.; Saito, S.; Takeda, N.; Takeoka, S. Plasmid DNA-encapsulating liposomes: Effect of a spacer between the cationic headgroup and hydrophobic moieties of the lipids on gene expression efficiency. Biochim. Biophys. Acta 2009, 1788, 1148–1158. [Google Scholar] [CrossRef]
- Obata, Y.; Ciofani, G.C.; Raffa, V.; Cuschieri, A.; Menciassi, A.; Dario, P.; Takeoka, S. Evaluation of cationic liposomes composed of an amino acid-based lipid for neuronal transfection. Nanomedicine 2010, 6, 70–77. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Silva, S.G.; do Vale, M.L.; Marques, E.F.; Pedroso de Lima, M.C.; Jurado, A.S. New serine-derived gemini surfactants as gene delivery systems. Eur. J. Pharm. Biopharm. 2015, 89, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.R.; Arai, S.; Murate, M.; Takahashi, H.; Takata, M.; Kobayashi, T.; Takeoka, S. Evaluation of the influence of ionization states and spacers in the thermotropic phase behavior of amino acid-based cationic lipids and the transfection efficiency of their assemblies. Int. J. Pharm. 2012, 422, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Faustino, C. Amino Acid-Based Surfactants for Biomedical Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Yang, P.; Singh, J.; Wettig, S.; Foldvari, M.; Verral, R.E.; Badea, I. Enhanced gene expression in epithelial cells transfected with amino acid-substituted gemini nanoparticles. Eur. J. Pharm. Biopharm. 2010, 75, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Negro, M.; Blanco-Fernández, L.; Tentori, P.M.; Pérez, L.; Pinazo, A.; Tros de Ilarduya, C.; Aicart, E.; Junquera, E. A gemini cationic lipid with histidine residues as a novel lipid-based gene nanocarrier: A biophysical and biochemical study. Nanomaterials 2018, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Pons, R.; Bustelo, M.; Manresa, M.A.; Morán, C.; Raluy, M.; Pérez, L. Gemini histidine based surfactants: Characterization; surface properties and biological activity. J. Mol. Liq. 2019, 289, 111156. [Google Scholar] [CrossRef]
- Zheng, L.T.; Yi, W.J.; Su, R.C.; Liu, Q.; Zhao, Z.G. Reducible amino acid based cationic lipids as highly efficient and serum-tolerant gene vectors. Chem. Plus Chem. 2016, 81, 125–134. [Google Scholar] [CrossRef]
- Su, R.C.; Liu, Q.; Yi, W.J.; Zheng, L.T.; Zhao, Z.G. Lipoic acid functionalized amino acids cationic lipids as gene vectors. Bioorg. Med. Chem. Lett. 2016, 26, 4692–4697. [Google Scholar] [CrossRef]
- Jiang, Q.; Yue, D.; Nie, Y.; Xu, X.; He, Y.; Zhang, S.; Wagner, E.; Gu, Z. Specially-made lipid-based assemblies for improving transmembrane gene delivery: Comparison of basic amino acid residue rich periphery. Mol. Pharm. 2016, 13, 1809–1821. [Google Scholar] [CrossRef]
- Bogacheva, M.; Egorova, A.; Slita, A.; Maretina, M.; Baranov, V.; Kiselev, A. Arginine-rich cross-linking peptides with different SV40 nuclear localization signal content as vectors for intranuclear DNA delivery. Bioorg. Med. Chem. Lett. 2017, 27, 4781–4785. [Google Scholar] [CrossRef]
| Reactions | |
|---|---|
| Hydrolysis | R1COOR2 + H2O ↔ R1COOH + R2OH |
| Esterification | R1COOH + R2OH ↔ R1COOR2 + H2O |
| (a) Acidolysis | |
| R1COOR2 + R3COOH ↔ R3COOR2 + R1COOH | |
| Transesterification | (b) Alcoholysis |
| R1COOR2 + R3OH ↔ R1COOR3 + R2OH | |
| (c) Aminolysis | |
| R1COOR2 + R3NH2 ↔ R1CONHR3 + R2OH | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.H.L.; Carvalho, P.; Martins, T.S.; Faustino, C.M.C. Lipoaminoacids Enzyme-Based Production and Application as Gene Delivery Vectors. Catalysts 2019, 9, 977. https://doi.org/10.3390/catal9120977
Ribeiro MHL, Carvalho P, Martins TS, Faustino CMC. Lipoaminoacids Enzyme-Based Production and Application as Gene Delivery Vectors. Catalysts. 2019; 9(12):977. https://doi.org/10.3390/catal9120977
Chicago/Turabian StyleRibeiro, Maria H. L., Patricia Carvalho, Tiago Santos Martins, and Célia M. C. Faustino. 2019. "Lipoaminoacids Enzyme-Based Production and Application as Gene Delivery Vectors" Catalysts 9, no. 12: 977. https://doi.org/10.3390/catal9120977
APA StyleRibeiro, M. H. L., Carvalho, P., Martins, T. S., & Faustino, C. M. C. (2019). Lipoaminoacids Enzyme-Based Production and Application as Gene Delivery Vectors. Catalysts, 9(12), 977. https://doi.org/10.3390/catal9120977






