Fast Catalytic Pyrolysis of Dilaurin in the Presence of Sodium Carbonate Alone or Combined with Alumina
Abstract
:1. Introduction
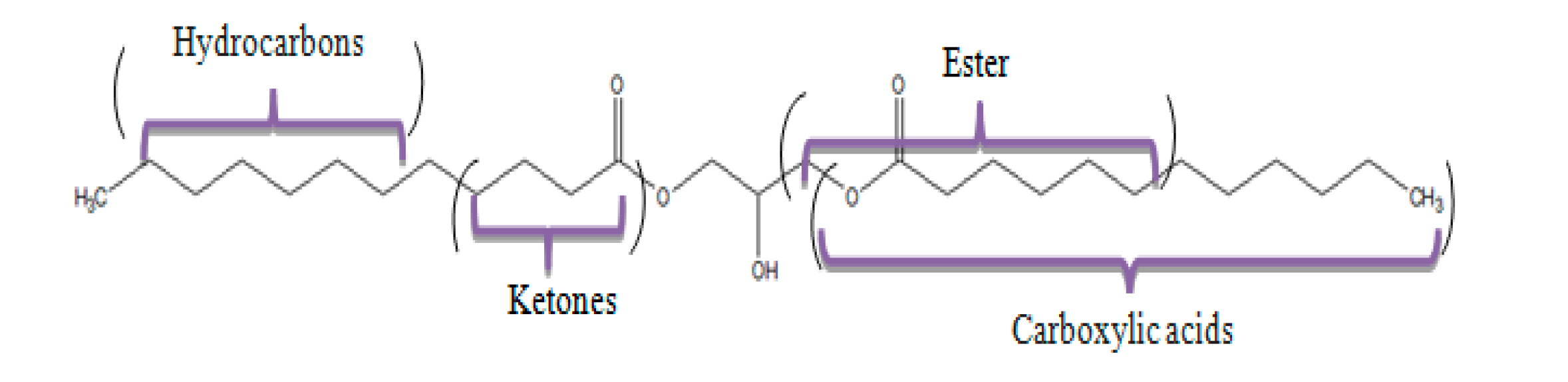
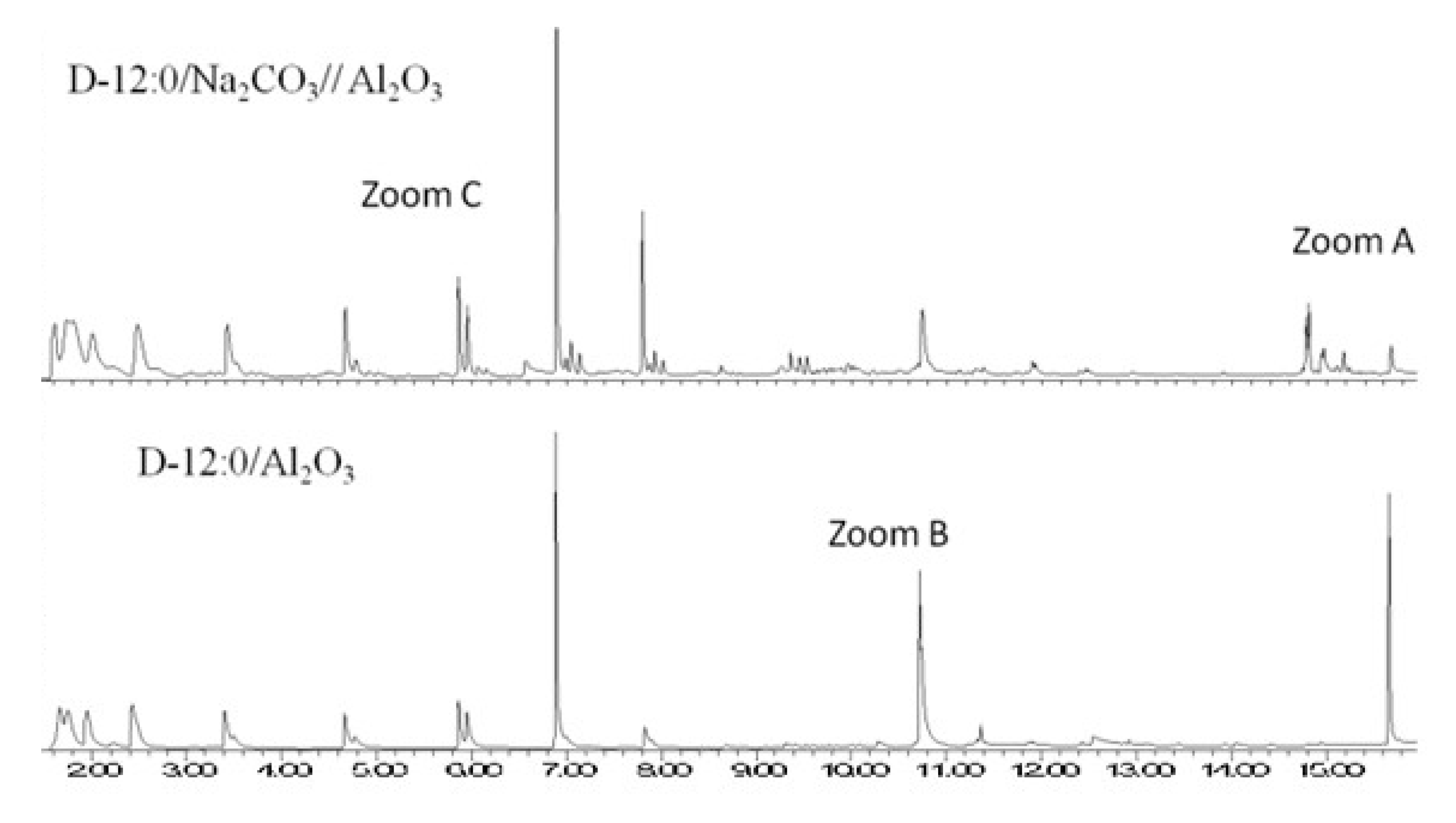
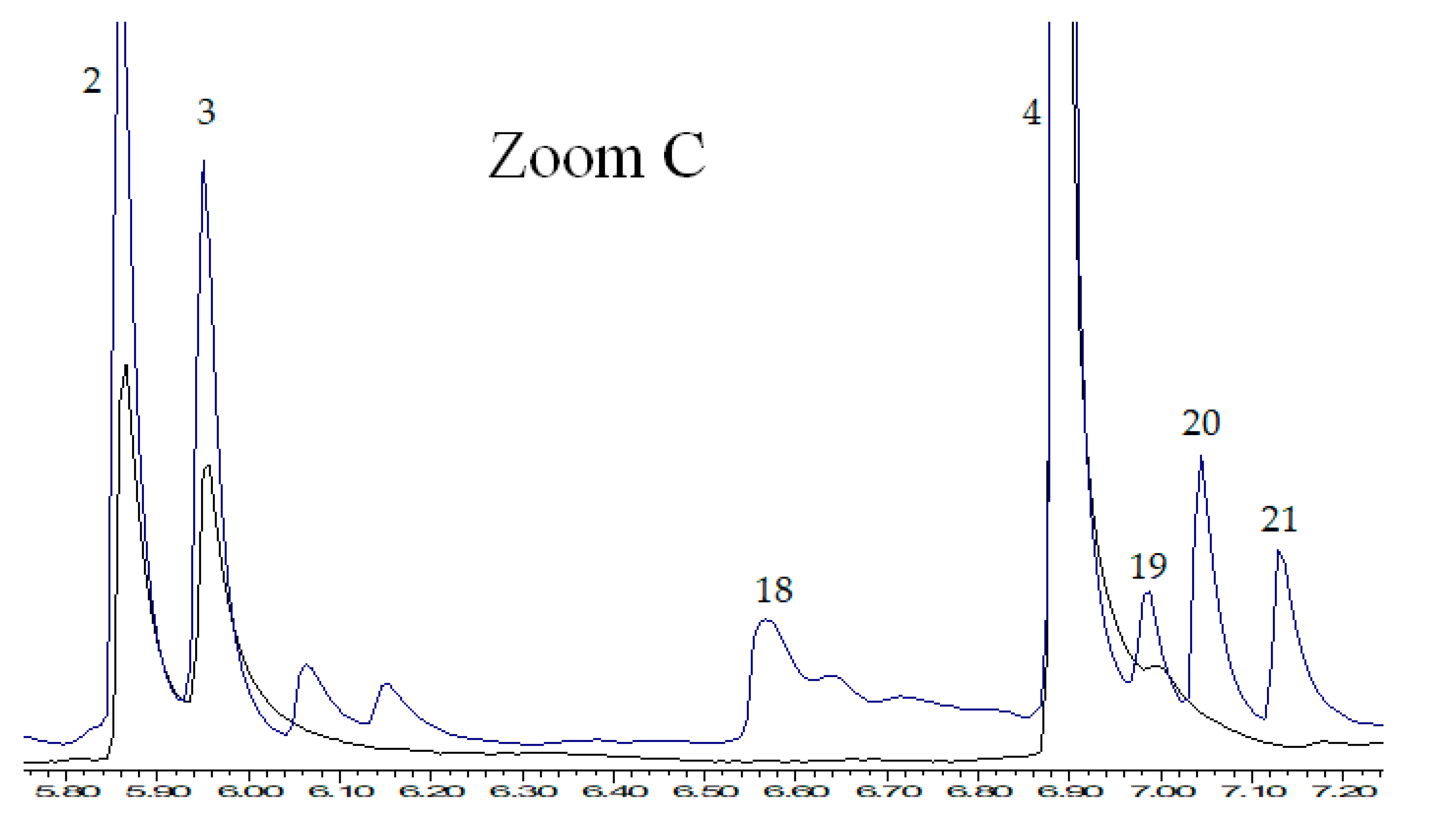
2. Results and Discussion
2.1. Pyrolysis Temperature Effect
2.2. Study of Thermocatalytic Effect
3. Materials and Methods
- ○
- Group 1—pyrolysis of pure dilaurin (D-12: 0);
- ○
- Group 2—pyrolysis of dilaurin adsorbed on sodium carbonate (D-12:0/Na2CO3);
- ○
- Group 3—(i) pyrolysis of dilaurin adsorbed on alumina (D-12:0/Al2O3); (ii) pyrolysis of dilaurin adsorbed on sodium carbonate, coated with an alumina layer (D-12:0/Na2CO3 //Al2O3).
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Feroldi, M.; Feiden, A.; Teleken, J.G.; Gris, D.J.; Dieter, J.; De Rossi, E.; Antonelli, J. Current scenario and prospects of use of liquid biofuels in South America. Renew. Sustain. Energy Rev. 2015, 43, 352–362. [Google Scholar] [CrossRef]
- Lossau, S.; Fischer, G.; Tramberend, S.; Van Velthuizen, H.; Kleinschmit, B.; Schomäcker, R.; Schomaecker, R. Brazil’s current and future land balances: Is there residual land for bioenergy production? Biomass Bioenergy 2015, 81, 452–461. [Google Scholar] [CrossRef]
- Maher, K.; Bressler, D. Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef] [PubMed]
- Frety, R.; Pacheco, J.G.; Santos, M.R.; Padilha, J.F.; Azevedo, A.F.; Brandão, S.T.; Pontes, L.A. Flash pyrolysis of model compounds adsorbed on catalyst surface: A method for screening catalysts for cracking of fatty molecules. J. Anal. Appl. Pyrolysis 2014, 109, 56–64. [Google Scholar] [CrossRef]
- Chagas, B.M.; Dorado, C.; Serapiglia, M.J.; Mullen, C.A.; Boateng, A.A.; Melo, M.A.; Ataíde, C.H. Catalytic pyrolysis-GC/MS of Spirulina: Evaluation of a highly proteinaceous biomass source for production of fuels and chemicals. Fuel 2016, 179, 124–134. [Google Scholar] [CrossRef]
- Vonghia, E.; Boocock, D.G.B.; Konar, S.K.; Leung, A. Pathways for the Deoxygenation of Triglycerides to Aliphatic Hydrocarbons over Activated Alumina. Energy Fuels 1995, 9, 1090–1096. [Google Scholar] [CrossRef]
- Leung, A.; Boocock, D.G. Pathway for the catalytic conversion of carboxylic acids to hydrocarbons over activated alumina. Energy Fuels 1995, 9, 913–920. [Google Scholar] [CrossRef]
- Seifi, H.; Sadrameli, S.M. Bound cleavage at carboxyl group-glycerol backbone position in thermal cracking of the triglycerides in sun flower oil. J. Anal. Appl. Pyrolysis 2016, 121, 1–10. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Wang, T.; Xu, Y.; Zhang, Q.; Ma, L.; He, M.; Li, K. Upgrading of Bio-oil by Removing Carboxylic Acids in Supercritical Ethanol. Energy Procedia 2014, 61, 1033–1036. [Google Scholar] [CrossRef]
- Shakya, R.; Adhikari, S.; Mahadevan, R.; Hassan, E.B.; Dempster, T.A. Catalytic upgrading of bio-oil produced from hydrothermal liquefaction of Nannochloropsis sp. Bioresour. Technol. 2018, 252, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Lourenço, R.; De Abreu, D.; Pereira, A.; De Castro, D.; Pereira, M.; Almeida, H.; Mâncio, A.; Lhamas, D.; Da Mota, S.; et al. Gasoline-like hydrocarbons by catalytic cracking of soap phase residue of neutralization process of palm oil ( Elaeis guineensis Jacq). J. Taiwan Inst. Chem. Eng. 2017, 71, 106–119. [Google Scholar] [CrossRef]
- Mancio, A.; Da Costa, K.; Ferreira, C.; Santos, M.; Lhamas, D.; Da Mota, S.; Leão, R.; De Souza, R.; Araújo, M.; Borges, L.; et al. Thermal Catalytic Cracking of Crude Palm Oil at Pilot Scale: Effect of the Percentage of Na2CO3 on the Quality of Biofuels. Crop. Prod. 2016, 91, 32–43. [Google Scholar] [CrossRef]
- Mâncio, A.; Da Costa, K.; Ferreira, C.; Santos, M.; Lhamas, D.; Da Mota, S.; Leão, R.; De Souza, R.; Araújo, M.; Borges, L.; et al. Process analysis of physico chemical properties and chemical composition of organic liquid products obtained by thermochemical conversion of palm oil. J. Anal. Appl. Pyrolysis 2017, 123, 284–295. [Google Scholar] [CrossRef]
- Smets, K.; Roukaerts, A.; Czech, J.; Reggers, G.; Schreurs, S.; Carleer, R.; Yperman, J. Slow catalytic pyrolysis of rapeseed cake: Product yield and characterization of the pyrolysis liquid. J. Biomass Bioenergy 2013, 57, 180–190. [Google Scholar] [CrossRef]
- Long, F.; Li, F.; Zhai, Q.; Wang, F.; Xu, J.; Feng, L.; Fanglin, L.; Qiaolong, Z. Thermochemical conversion of waste acidic oil into hydrocarbon products over basic composite catalysts. J. Clean. Prod. 2019, 234, 105–112. [Google Scholar] [CrossRef]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. Catalytic flash pyrolysis of oil-impregnated-wood and jatropha cake using sodium-based catalysts. J. Anal. Appl. Pyrolysis 2016, 117, 236–246. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.H.; Abu-Elyazeed, O.S.M.; El Mawla, E.A.; Abdelazeem, M.A. On biodiesels from castor raw oil using catalytic pyrolysis. Energy 2018, 143, 950–960. [Google Scholar] [CrossRef]
- Dos Anjos, J.R.; Gonzales, W.A.; Lam, Y.L.; Fréty, R. Catalytic decomposition of vegetable oil. Appl. Catal. 1983, 5, 299–308. [Google Scholar] [CrossRef]
- Santos, M.R.; Arias, S.; Padilha, J.F.; Carneiro, M.C.N.; Sales, E.A.; Pacheco, J.G.A.; Fréty, R. Catalytic cracking of palmitic and oleic acids pre-adsorbed on γ-alumina Catal. Today 2019, in press. [Google Scholar] [CrossRef]
- Billaud, F.; Minh, A.T.; Lozano, P.; Pioch, D. Catalytic cracking of octanoic acid. J. Anal. Appl. Pyrolysis 2001, 58–59, 605–616. [Google Scholar] [CrossRef]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. High quality bio-oil from catalytic flash pyrolysis of lignocellulosic biomass over alumina-supported sodium carbonate. Fuel Process. Technol. 2014, 127, 72–79. [Google Scholar] [CrossRef]
- Renz, M. Ketonization of carboxylic acids by decarboxylation: Mechanism and scope. Eur. J. Org. Chem. 2005, 6, 979–988. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Wolf, C.R.; Suarez, P.A.Z. Transformação de triglicerídeos em combustíveis, materiais poliméricos e insumos químicos: Algumas aplicações da catálise na oleoquímica. Química Nova 2007, 30, 667–676. [Google Scholar] [CrossRef]
- Kumar, R.; Enjamuri, N.; Shah, S.; Al-Fatesh, A.S.; Bravo-Suárez, J.J.; Chowdhury, B. Ketonization of oxygenated hydrocarbons on metal oxide-based catalysts. Catal. Today 2018, 302, 16–49. [Google Scholar] [CrossRef]
- Lappi, H.; Alén, R. Pyrolysis of vegetable oil soaps—Palm, olive, rapeseed and castor oils. J. Anal. Appl. Pyrolysis 2011, 91, 154–158. [Google Scholar] [CrossRef]
- Almeida, H.D.S.; Corrêa, O.; Eid, J.; Ribeiro, H.; De Castro, D.; Pereira, M.; Pereira, L.; Aâncio, A.D.A.; Santos, M.; Da Mota, S.; et al. Performance of thermochemical conversion of fat oils, and grease into kerosene-like hydrocarbons in different production scales J. Anal. Appl. Pyrolysis 2016, 120, 126–143. [Google Scholar] [CrossRef]
- Mukhambetov, I.N.; Egorova, S.R.; Mukhamed’Yarova, A.N.; Lamberov, A.A. Hydrothermal modification of the alumina catalyst for the skeletal isomerization of n-butenes. Appl. Catal. AGen. 2018, 554, 64–70. [Google Scholar] [CrossRef]
- Freitas, C.; Pereira, M.; Souza, D.; Fonseca, N.; Sales, E.; Frety, R.; Felix, C.; Azevedo, A.; Brandao, S. Thermal and Catalytic Pyrolysis of Dodecanoic Acid on SAPO-5 and Al-MCM-41. Catalysts 2019, 9, 418. [Google Scholar] [CrossRef] [Green Version]



| Temperature (°C) | 500 | 550 | 600 |
|---|---|---|---|
| Hydrocarbons (%) | - | 3.7 | 41.3 |
| Dilaurin/Trilaurin (%) | 17.6/- | 9.0/18.9 | 1.9/- |
| Ketones/Aldehydes (%) | 11.0/- | 24.8/- | 13.9/6.4 |
| Acids (%) | - | 1.2 | 7.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, N.; Pereira, A.; Fréty, R.; Sales, E. Fast Catalytic Pyrolysis of Dilaurin in the Presence of Sodium Carbonate Alone or Combined with Alumina. Catalysts 2019, 9, 993. https://doi.org/10.3390/catal9120993
Fonseca N, Pereira A, Fréty R, Sales E. Fast Catalytic Pyrolysis of Dilaurin in the Presence of Sodium Carbonate Alone or Combined with Alumina. Catalysts. 2019; 9(12):993. https://doi.org/10.3390/catal9120993
Chicago/Turabian StyleFonseca, Noyala, Aline Pereira, Roger Fréty, and Emerson Sales. 2019. "Fast Catalytic Pyrolysis of Dilaurin in the Presence of Sodium Carbonate Alone or Combined with Alumina" Catalysts 9, no. 12: 993. https://doi.org/10.3390/catal9120993
APA StyleFonseca, N., Pereira, A., Fréty, R., & Sales, E. (2019). Fast Catalytic Pyrolysis of Dilaurin in the Presence of Sodium Carbonate Alone or Combined with Alumina. Catalysts, 9(12), 993. https://doi.org/10.3390/catal9120993





