Immobilization of Stabilized Gold Nanoparticles on Various Ceria-Based Oxides: Influence of the Protecting Agent on the Glucose Oxidation Reaction
Abstract
1. Introduction
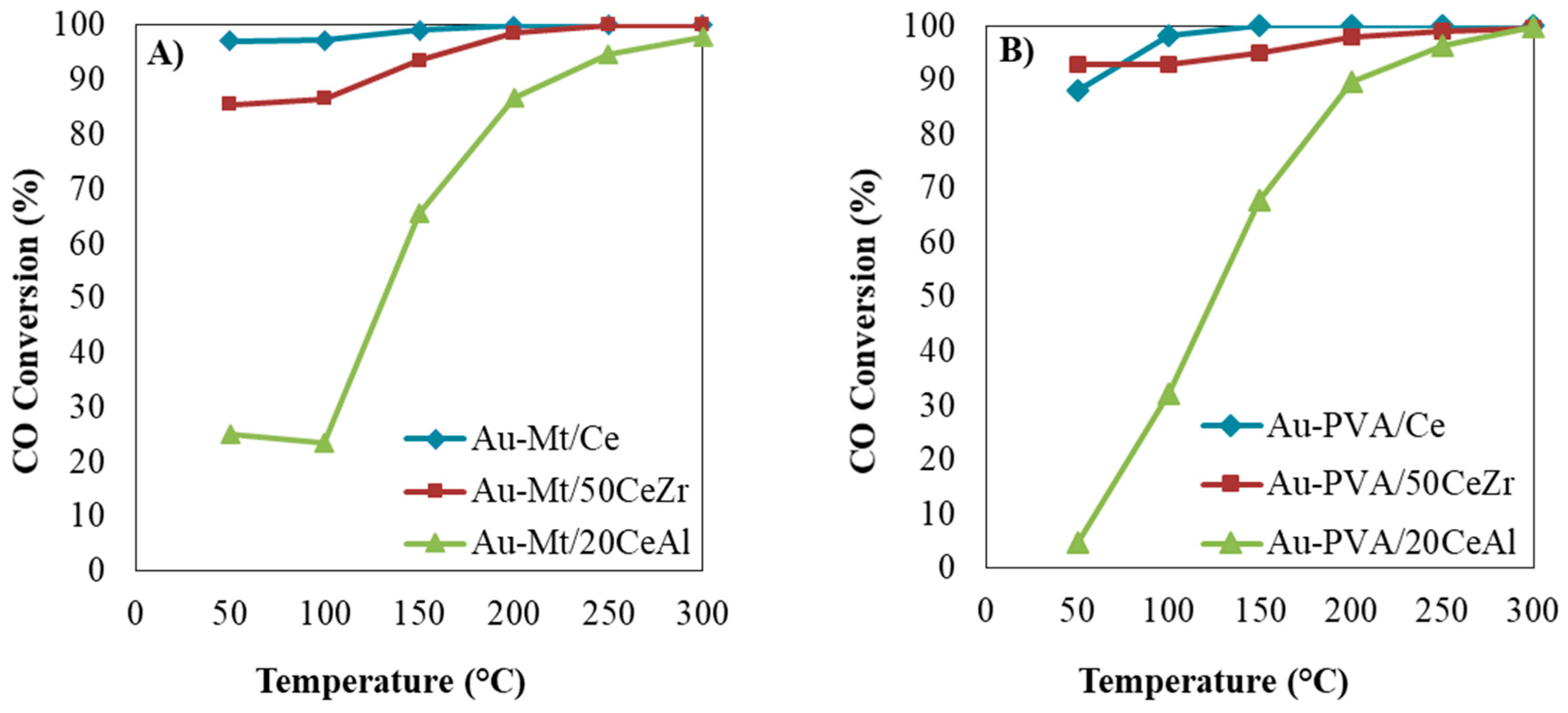
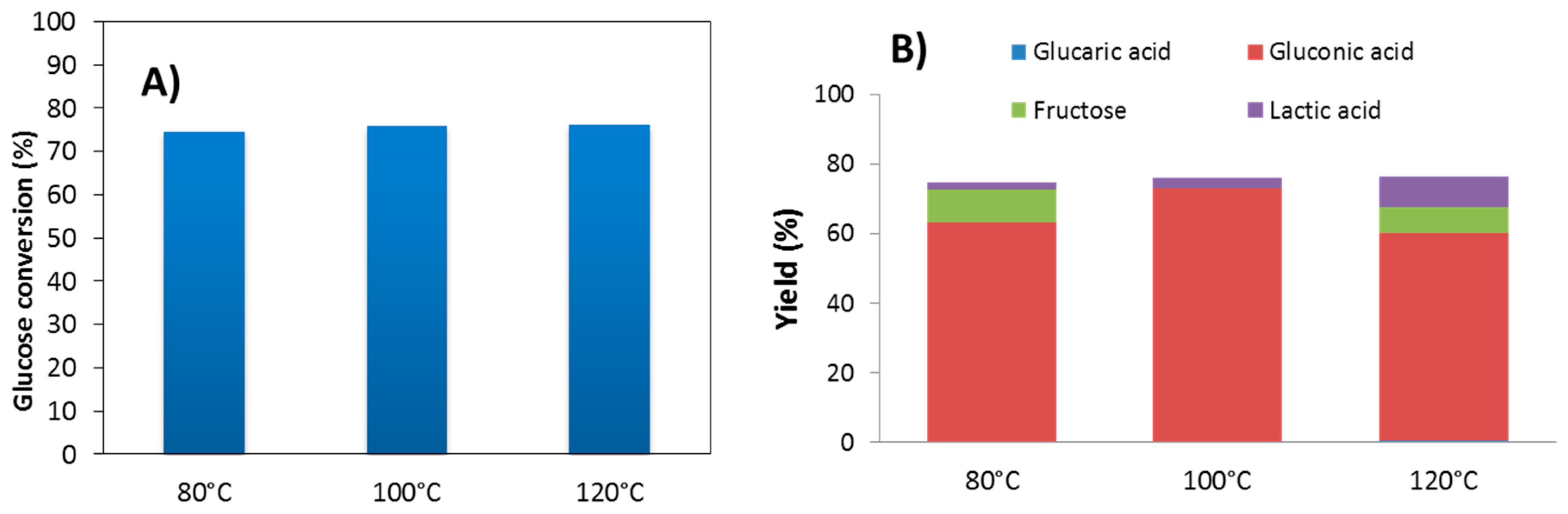
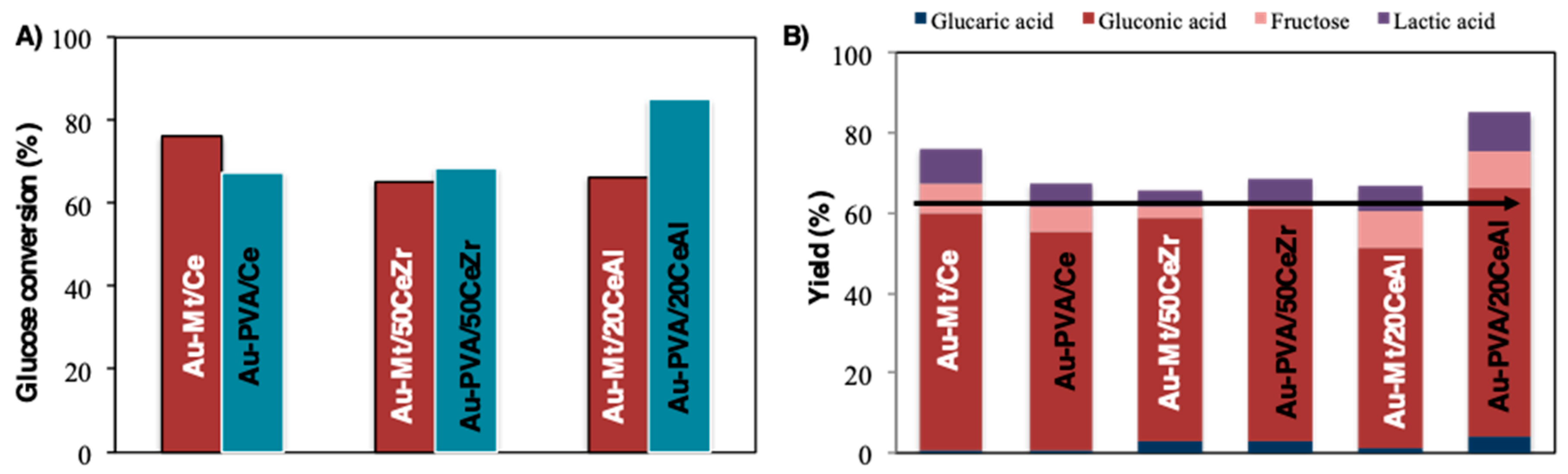
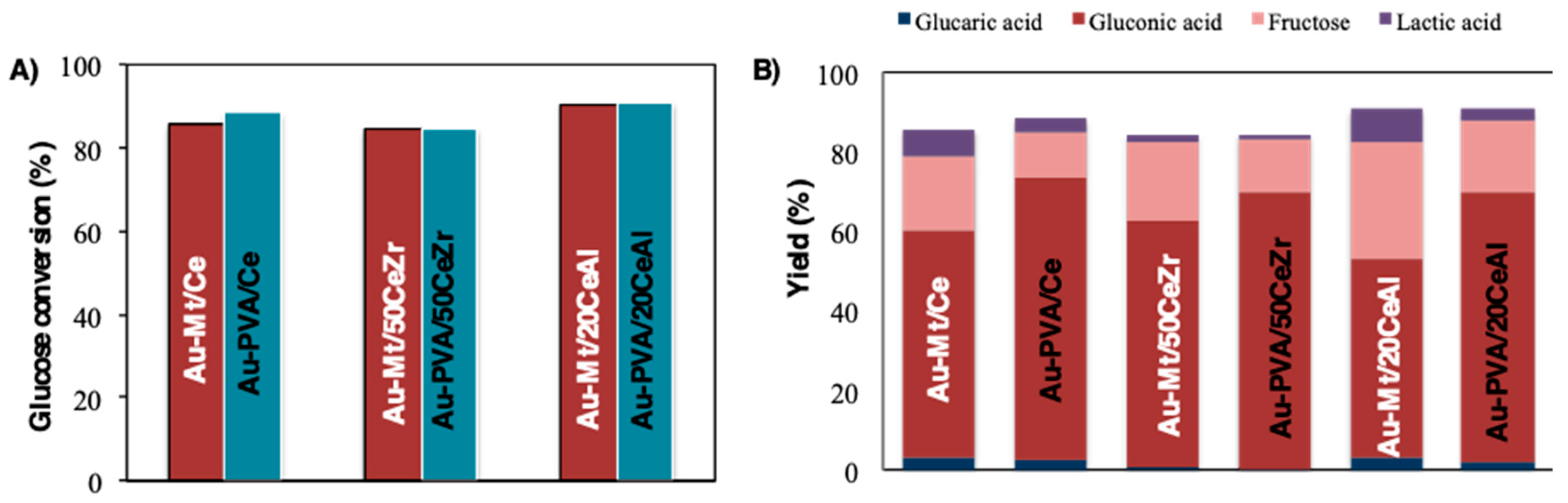
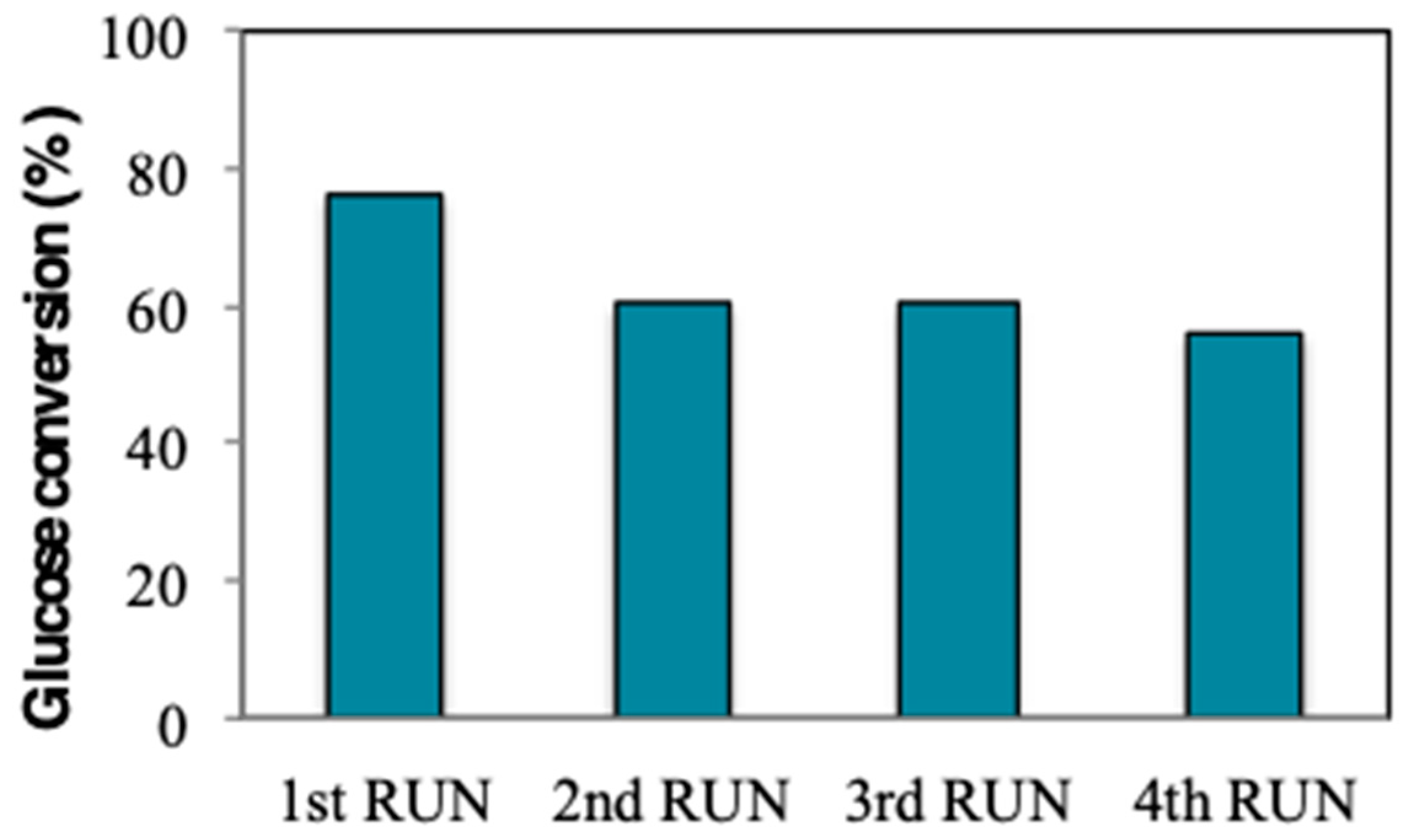
2. Results and Discussion
3. Experimental
3.1. Stabilizing Agents
3.2. Gold Catalysts
3.2.1. Mt as Stabilizing Agent
3.2.2. PVA as Stabilizing Agent
3.3. Catalytic Test
3.3.1. CO Oxidation
3.3.2. Glucose Oxidation
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kinoshita, N.; Okatsu, H.; Akita, T.; Takei, T.; Haruta, M. Influence of the support and the size of gold clusters on catalytic activity for glucose oxidation. Angew. Chem. Int. Ed. 2008, 47, 9265–9268. [Google Scholar] [CrossRef] [PubMed]
- Mirescu, A.; Prüße, U. Selective glucose oxidation on gold colloids. Catal. Commun. 2006, 7, 11–17. [Google Scholar] [CrossRef]
- Rautiainen, S.; Lehtinen, P.; Vehkamäki, M.; Niemelä, K.; Kemell, M.; Heikkilä, M.; Repo, T. Microwave-assisted base-free oxidation of glucose on gold nanoparticle catalysts. Catal. Commun. 2016, 74, 115–118. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Hustede, E.S.H.; Haberstroh, H.J.; Schinzig, E. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Wojcieszak, R.; Cuccovia, I.M.; Silva, M.A.; Rossi, L.M. Selective oxidation of glucose to glucuronic acid by cesium-promoted gold nanoparticle catalyst. J. Mol. Catal. A Chem. 2016, 422, 35–42. [Google Scholar] [CrossRef]
- Biella, S.; Prati, L.; Rossi, M. Selective oxidation of D-glucose on gold catalyst. J. Catal. 2002, 206, 242–247. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Green, D.; McMillan, E. Efficient oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran using Mn (III)-salen catalysts. Catal. Commun. 2008, 9, 286–288. [Google Scholar] [CrossRef]
- Saliger, R.; Decker, N.; Prüße, U. D-Glucose oxidation with H2O2 on an Au/Al2O3 catalyst. Appl. Catal. B Environ. 2011, 102, 584–589. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bobadilla, L.F.; Ivanova, S.; Penkova, A.; Centeno, M.A.; Odriozola, J.A. Gold catalyst recycling study in base-free glucose oxidation reaction. Catal. Today 2018, 301, 72–77. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Ivanova, S.; López-Cartes, C.; Centeno, M.A.; Odriozola, J.A. Gold catalysts screening in base-free aerobic oxidation of glucose to gluconic acid. Catal. Today 2017, 279, 148–154. [Google Scholar] [CrossRef]
- Miedziak, P.J.; Alshammari, H.; Kondrat, S.A.; Clarke, T.J.; Davies, T.E.; Morad, M.; Morgan, D.J.; Willock, D.J.; Knight, D.W.; Taylor, S.H.; et al. Base-free glucose oxidation using air with supported gold catalysts. Green Chem. 2014, 16, 3132–3141. [Google Scholar] [CrossRef]
- Qi, P.; Chen, S.; Chen, J.; Zheng, J.; Zheng, X.; Yuan, Y. Catalysis and reactivation of ordered mesoporous carbon-supported gold nanoparticles for the base-free oxidation of glucose to gluconic acid. ACS Catal. 2015, 5, 2659–2670. [Google Scholar] [CrossRef]
- Prüße, U.; Herrmann, M.; Baatz, C.; Decker, N. Gold-catalyzed selective glucose oxidation at high glucose concentrations and oxygen partial pressures. Appl. Catal. A Gen. 2011, 406, 89–93. [Google Scholar] [CrossRef]
- Schrekker, H.S.; Gelesky, M.A.; Stracke, M.P.; Schrekker, C.M.L.; Machado, G.; Teixeira, S.R.; Rubim, J.C.; Dupont, J. Disclosure of the imidazolium cation coordination and stabilization mode in ionic liquid stabilized gold (0) nanoparticles. J. Colloid Interface Sci. 2007, 316, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Geng, Y.; Xu, X.; Wang, C.; Lee, Y.I.; Hao, J.; Liu, H.G. Unique self-assembly behavior of a triblock copolymer and fabrication of catalytically active gold nanoparticle/polymer thin films at the liquid/liquid interface. Mater. Chem. Phys. 2014, 146, 88–98. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Zhang, B.; Bian, G.; Qi, Y.; Yang, X.; Li, C. Synthesis of monodisperse magnetic sandwiched gold nanoparticle as an easily recyclable catalyst with a protective polymer shell. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 210–218. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A.; Illas, F.; Radilla, J.; Ródenas, T.; Sabater, M.J. Mechanism of selective alcohol oxidation to aldehydes on gold catalysts: Influence of surface roughness on reactivity. J. Catal. 2011, 278, 50–58. [Google Scholar] [CrossRef]
- Ivanova, S.; Pitchon, V.; Zimmermann, Y.; Petit, C. Preparation of alumina supported gold catalysts: Influence of washing procedures, mechanism of particles size growth. Appl. Catal. A Gen. 2006, 298, 57–64. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Shin, C.H.; Henry, C.R.; Louis, C. Characterization and reactivity in CO oxidation of gold nanoparticles supported on TiO2 prepared by deposition-precipitation with NaOH and urea. J. Catal. 2004, 222, 357–367. [Google Scholar] [CrossRef]
- Agarwal, S.; Ganguli, J.N. Selective hydrogenation of monoterpenes on rhodium (0) nanoparticles stabilized in Montmorillonite K-10 clay. J. Mol. Catal. A Chem. 2013, 372, 44–50. [Google Scholar] [CrossRef]
- Sarmah, P.P.; Dutta, D.K. Stabilized Rh0-nanoparticles-Montmorillonite clay composite: Synthesis and catalytic transfer hydrogenation reaction. Appl. Catal. A Gen. 2014, 470, 355–360. [Google Scholar] [CrossRef]
- Eren, E.; Afsin, B. An investigation of Cu (II) adsorption by raw and acid-activated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA study. J. Hazard. Mater. 2008, 151, 682–691. [Google Scholar] [CrossRef]
- Sarma, G.K.; Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J. Environ. Manag. 2016, 171, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, C.H.; Wu, L.M.; Lou, J.Y.; Li, N.; Yang, H.M.; Tong, D.S.; Yu, W.H. Catalytic dehydration of glycerol to acrolein over sulfuric acid-activated montmorillonite catalysts. Appl. Clay Sci. 2013, 74, 154–162. [Google Scholar] [CrossRef]
- Letaief, S.; Grant, S.; Detellier, C. Phenol acetylation under mild conditions catalyzed by gold nanoparticles supported on functional pre-acidified sepiolite. Appl. Clay Sci. 2011, 53, 236–243. [Google Scholar] [CrossRef]
- Álvarez, A.; Moreno, S.; Molina, R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Gold supported on pillared clays for CO oxidation reaction: Effect of the clay aggregate size. Appl. Clay Sci. 2012, 69, 22–29. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Martínez, L.M.; Odriozola, J.A.; Moreno, S.; Molina, R.; Centeno, M.A. Gold supported on Fe, Ce, and Al pillared bentonites for CO oxidation reaction. Appl. Catal. B Environ. 2007, 72, 157–165. [Google Scholar] [CrossRef]
- Martínez, L.M.T.; Domínguez, M.I.; Sanabria, N.; Hernández, W.Y.; Moreno, S.; Molina, J.A.; Odriozola, J.A.; Centeno, M.A. Deposition of Al-Fe pillared bentonites and gold supported Al-Fe pillared bentonites on metallic monoliths for catalytic oxidation reactions. Appl. Catal. A Gen. 2009, 364, 166–173. [Google Scholar] [CrossRef]
- Zhu, L.; Letaief, S.; Liu, Y.; Gervais, F.; Detellier, C. Clay mineral-supported gold nanoparticles. Appl. Clay Sci. 2009, 43, 439–446. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Silva, A.M.T.; Draić, G.; Tavares, P.B.; Figueiredo, J.L. Gold nanoparticles on ceria supports for the oxidation of carbon monoxide. Catal. Today 2010, 154, 21–30. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Kiener, C.; Wögerbauer, C.; Baiker, A. Preparation of supported gold catalysts for low-temperature CO oxidation via “size-controlled” gold colloids. J. Catal. 1999, 181, 223–232. [Google Scholar] [CrossRef]
- Ivanova, S.; Pitchon, V.; Petit, C.; Caps, V. Support effects in the gold-catalyzed preferential oxidation of CO. ChemCatChem 2010, 2, 556–563. [Google Scholar] [CrossRef]
- Ivanova, S.; Pitchon, V.; Petit, C. Application of the direct exchange method in the preparation of gold catalysts supported on different oxide materials. J. Mol. Catal. A Chem. 2006, 256, 278–283. [Google Scholar] [CrossRef]
- Madier, Y.; Descorme, C.; Le Govi, A.M.; Duprez, D. Oxygen mobility in CeO2 and Ce x Zr (1-x) O2 compounds: Study by CO transient oxidation and 18O/16O isotopic exchange. J. Phys. Chem. B 1999, 103, 10999–11006. [Google Scholar] [CrossRef]
- Comotti, M.; Pina, C.; Della Rossi, M. Mono- and bimetallic catalysts for glucose oxidation. J. Mol. Catal. A Chem. 2006, 251, 89–92. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Santos, J.L.; Ammari, F.; Chenouf, M.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Influence of gold particle size in Au/C catalysts for base-free oxidation of glucose. Cat. Today 2018, 306, 183–190. [Google Scholar] [CrossRef]
- Kooyman, C.; Vellenga, K.; De Wilt, H.G.J. The isomerization of D-glucose into D-fructose in aqueous alkaline solutions. Carbohydr. Res. 1977, 54, 33–44. [Google Scholar] [CrossRef]
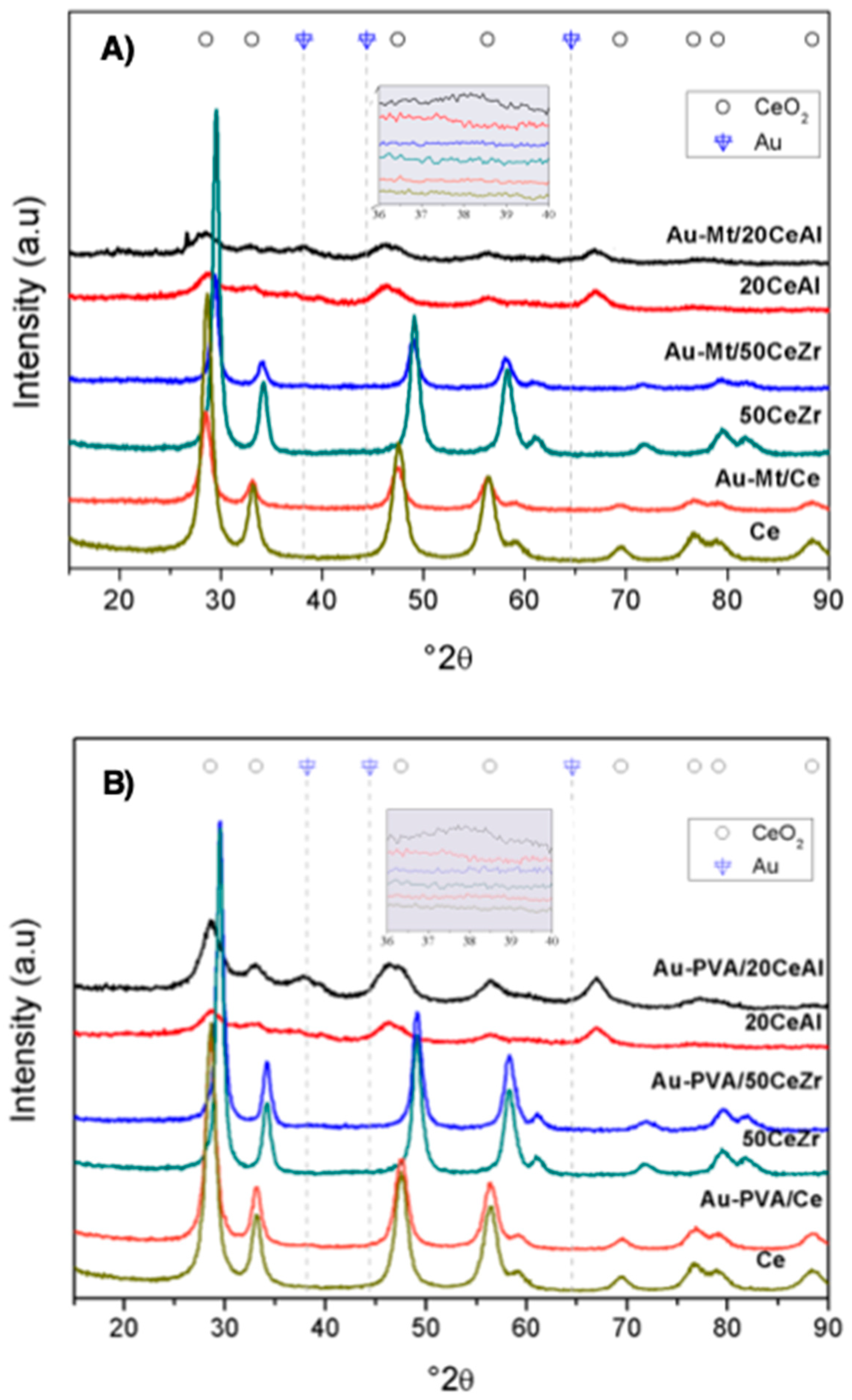
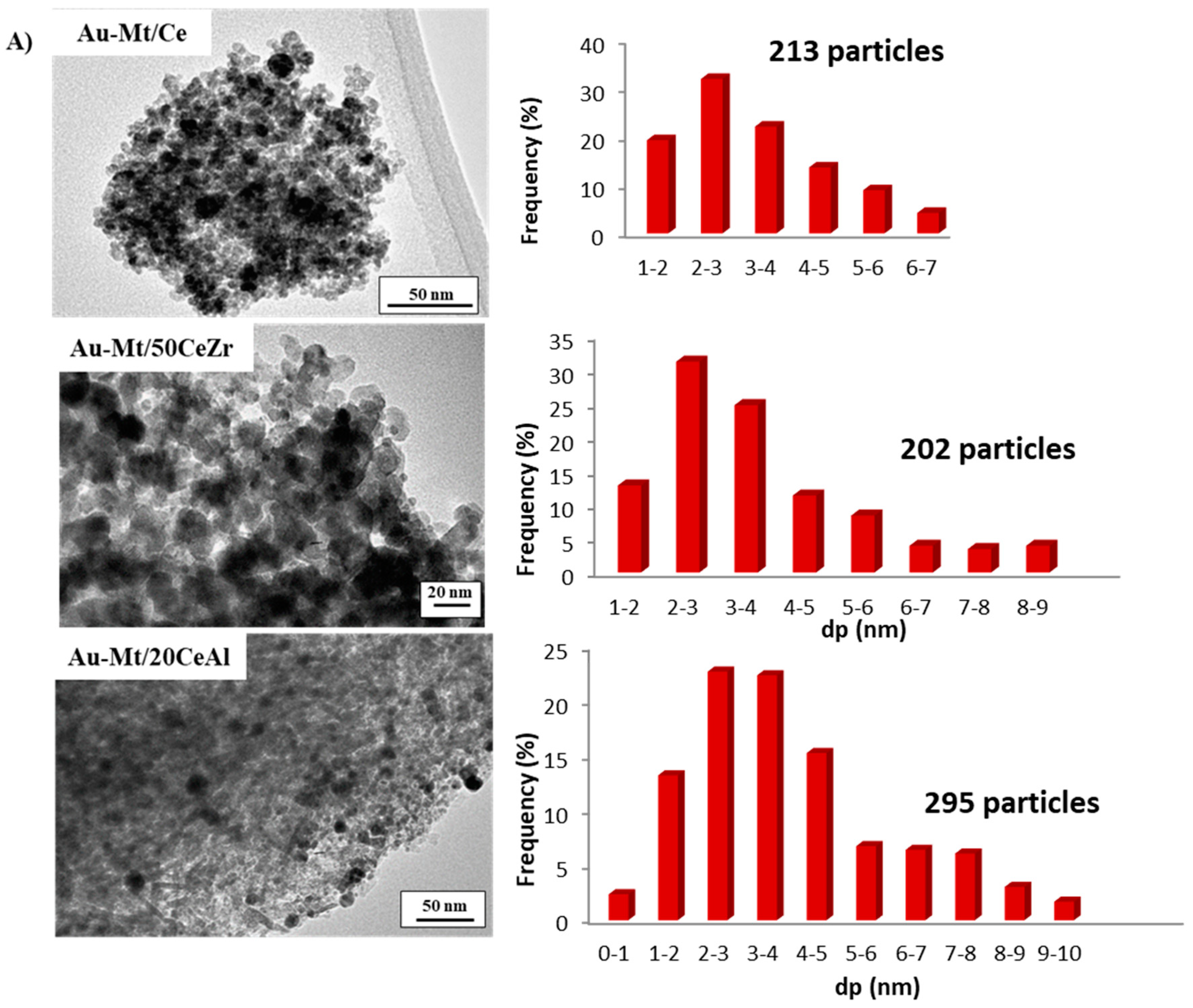
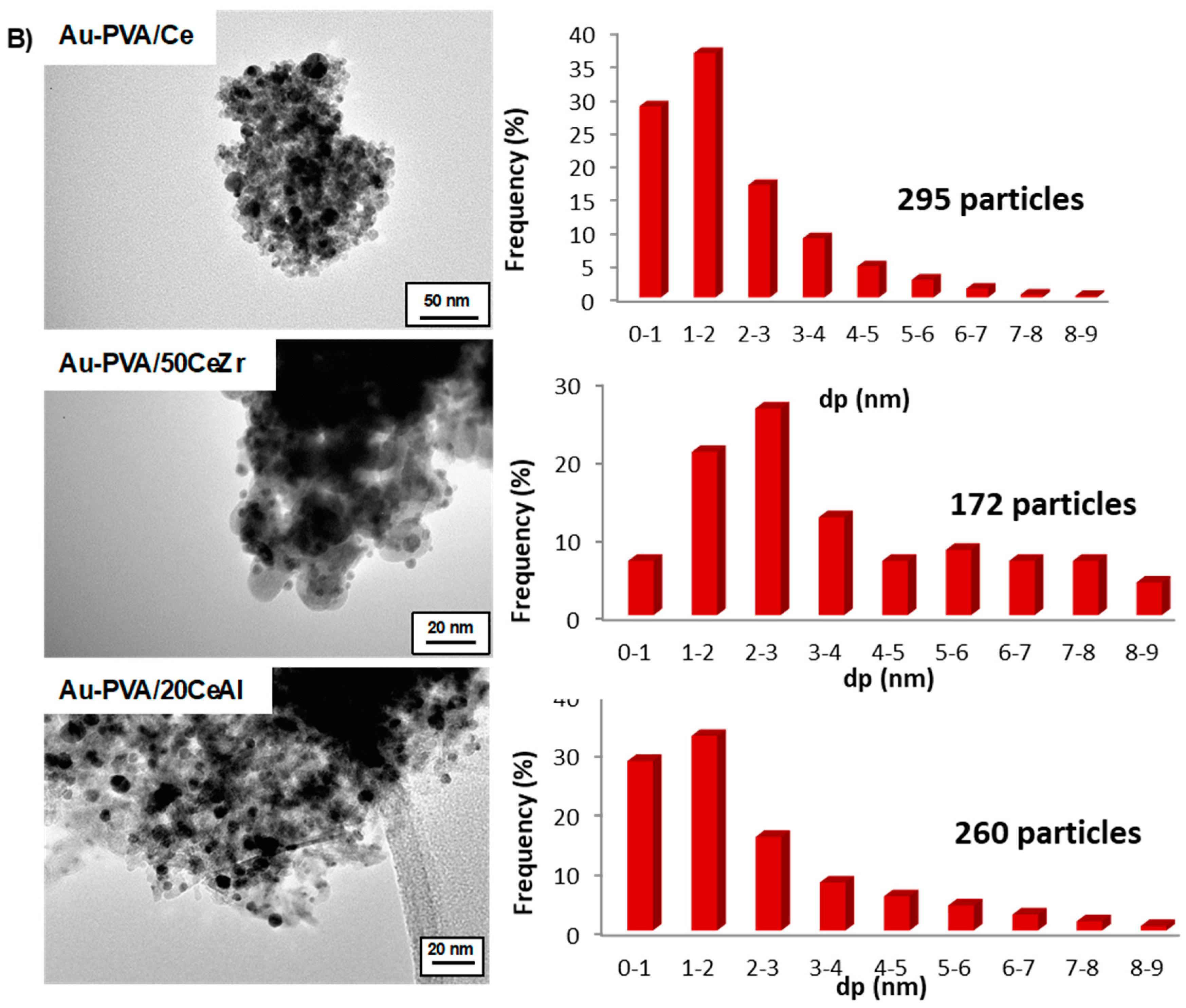
| Samples | Au Loading, wt.% | BET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Mean Particle Diameter dp(nm) TEM ± 1.2 |
|---|---|---|---|---|---|
| CeO2 | - | 137 | 6.5 | 0.23 | - |
| Au-Mt/Ce | 1.5 | 128 | 6 | 0.21 | 4 (6.8 *) |
| Au-PVA/Ce | 1.6 | 137 | 5.5 | 0.21 | 3.9 (7 *) |
| 50Ce50Zr | - | 55 | 11 | 0.18 | - |
| Au-Mt/50CeZr | 1.2 | 50 | 10.4 | 0.16 | 5.1 |
| Au-PVA/50CeZr | 2.2 | 47 | 10.6 | 0.15 | 5.7 |
| 20CeAl | - | 186 | 6.9 | 0.42 | - |
| Au-Mt/20CeAl | 1 | 168 | 7.7 | 0.36 | 5.6 |
| Au-PVA/20CeAl | 1.7 | 154 | 8.1 | 0.37 | 5.7 |
| Sample | Specific Reaction Rate, mmolesCOconv. gcat−1. s−11 | TOF(CO), s−11 ×102 | TOF(Glucose) ×103, s−11 | Dispersion |
|---|---|---|---|---|
| Au-Mt/Ce | 0.69 | 41.3 | 4.7 | 0.32 |
| Au-PVA/Ce | 0.58 | 34.4 | 3.8 | 0.33 |
| Au-Mt/50CeZr | 0.77 | 58.5 | 6.3 | 0.26 |
| Au-PVA/50CeZr | 0.45 | 37.9 | 3.9 | 0.23 |
| Au-Mt/20CeAl | 0.13 | 10.5 | 8.5 | 0.23 |
| Au-PVA/20CeAl | 0.031 | 2.6 | 6.5 | 0.23 |
| Au/Ce [13] | - | - | 1.7 | 0.33 |
| Au/20CeAl [13] | - | - | 2.6 | 0.38 |
| Au/50CeZr [13] | - | - | 1.6 | 0.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chenouf, M.; Megías-Sayago, C.; Ammari, F.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Immobilization of Stabilized Gold Nanoparticles on Various Ceria-Based Oxides: Influence of the Protecting Agent on the Glucose Oxidation Reaction. Catalysts 2019, 9, 125. https://doi.org/10.3390/catal9020125
Chenouf M, Megías-Sayago C, Ammari F, Ivanova S, Centeno MA, Odriozola JA. Immobilization of Stabilized Gold Nanoparticles on Various Ceria-Based Oxides: Influence of the Protecting Agent on the Glucose Oxidation Reaction. Catalysts. 2019; 9(2):125. https://doi.org/10.3390/catal9020125
Chicago/Turabian StyleChenouf, Meriem, Cristina Megías-Sayago, Fatima Ammari, Svetlana Ivanova, Miguel Angel Centeno, and José Antonio Odriozola. 2019. "Immobilization of Stabilized Gold Nanoparticles on Various Ceria-Based Oxides: Influence of the Protecting Agent on the Glucose Oxidation Reaction" Catalysts 9, no. 2: 125. https://doi.org/10.3390/catal9020125
APA StyleChenouf, M., Megías-Sayago, C., Ammari, F., Ivanova, S., Centeno, M. A., & Odriozola, J. A. (2019). Immobilization of Stabilized Gold Nanoparticles on Various Ceria-Based Oxides: Influence of the Protecting Agent on the Glucose Oxidation Reaction. Catalysts, 9(2), 125. https://doi.org/10.3390/catal9020125









