Microalgae Cultivation for the Biotransformation of Birch Wood Hydrolysate and Dairy Effluent
Abstract
:1. Introduction
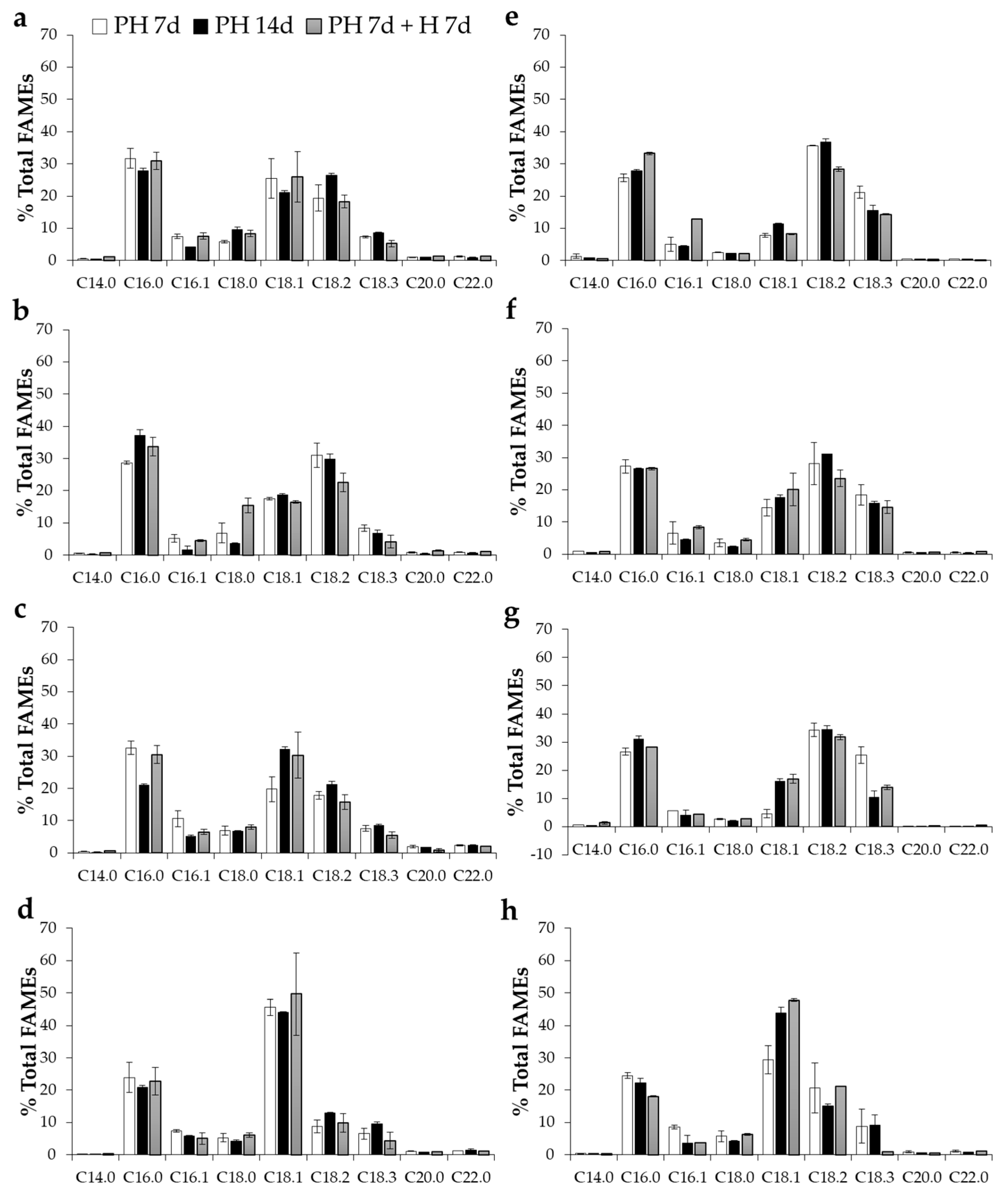
2. Results and Discussion
3. Materials and Methods
3.1. Collection and Cultivation of Microalgal Strains
3.2. Assessment of Birch Hydrolysate and Dairy Effluent as Growth Medium
3.3. Analytical Methods
3.3.1. Nutrients Analyses
3.3.2. Biomass Concentration
3.3.3. Total Lipids Extraction
3.3.4. Fatty Acid Methyl Esters Analysis
3.3.5. Total N and Total C Analyses
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Chen, F. Growing phototrophic cells without light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Tabernero, A.; del Valle, E.M.M.; Galán, M.A. Evaluating the industrial potential of biodiesel from a microalgae heterotrophic culture: Scale-up and economics. Biochem. Eng. J. 2012, 63, 104–115. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Shi, X.M.; Jiang, Y.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef]
- Qtae, J.; Eun, J.C.; Doo, W.P.; Benoît, V. Sterol dynamics of heterotrophic Tetraselmis suecica and its nutritional implication in the bivalve aquaculture. Aquac. Res. 2004, 35, 371–377. [Google Scholar] [CrossRef]
- Wen, Z.-Y.; Chen, F. Production potential of eicosapentaenoic acid by the diatom Nitzschia laevis. Biotechnol. Lett. 2000, 22, 727–733. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Chen, F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol. Adv. 2003, 21, 273–294. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Chojnacka, K.; Marquez-Rocha, F.J. Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 2004, 3, 21–34. [Google Scholar]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef]
- Bassi, A.; Saxena, P.; Aguirre, A.-M. Mixotrophic algae cultivation for energy production and other applications. In Algal Biorefineries; Springer: Dordrecht, The Netherlands, 2014; pp. 177–202. [Google Scholar]
- Kaur, A.; Vats, S.; Rekhi, S.; Bhardwaj, A.; Goel, J.; Goel, J.; Tanwar, R.S.; Gaur, K.K. Physico-chemical analysis of the industrial effluents and their impact on the soil microflora. Procedia Environ. Sci. 2010, 2, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Russell, P. Effluent and waste water treatment. Milk Ind. Int. 1998, 100, 36–39. [Google Scholar]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr. 2011, 51, 442–452. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Solvent Pretreatments of Lignocellulosic Materials to Enhance Biogas Production: A Review. Energy Fuels 2016, 30, 1892–1903. [Google Scholar] [CrossRef]
- Saadatmand, S.; Edlund, U.; Albertsson, A.-C.; Danielsson, S.; Dahlman, O.; Karlstrom, K. Turning hardwood dissolving pulp polysaccharide residual material into barrier packaging. Biomacromolecules 2013, 14, 2929–2936. [Google Scholar] [CrossRef]
- Li, P.; Miao, X.; Li, R.; Zhong, J. In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. BioMed Res. Int. 2011, 2011, 141207. [Google Scholar]
- Wei, A.; Zhang, X.; Wei, D.; Chen, G.; Wu, Q.; Yang, S.-T. Effects of cassava starch hydrolysate on cell growth and lipid accumulation of the heterotrophic microalgae Chlorella protothecoides. J. Ind. Microbiol. Biotechnol. 2009, 36, 1383. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef]
- Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour. Technol. 2017, 230, 122–131. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Liu, P.; Xia, J.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.; Qiu, G. The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef]
- Devi, M.P.; Subhash, G.V.; Mohan, S.V. Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: Effect of nutrient supplementation. Renew. Energy 2012, 43, 276–283. [Google Scholar] [CrossRef]
- Farooq, W.; Lee, Y.C.; Ryu, B.G.; Kim, B.H.; Kim, H.S.; Choi, Y.E.; Yang, J.W. Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. Bioresour. Technol. 2013, 132, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Chiranjeevi, P.; Venkata Mohan, S. Diverse acidogenic effluents as feedstock for microalgae cultivation: Dual phase metabolic transition on biomass growth and lipid synthesis. Bioresour. Technol. 2017, 242, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Sashidhar, B.; Adholeya, A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016, 211, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Fábregas, J.; Maseda, A.; Domínguez, A.; Ferreira, M.; Otero, A. Changes in the cell composition of the marine microalga, Nannochloropsis gaditana, during a light: Dark cycle. Biotechnol. Lett. 2002, 24, 1699–1703. [Google Scholar] [CrossRef]
- Hu, Q.; Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Science: Oxford, UK, 2004. [Google Scholar]
- Aoki, S.; Matsuka, M.; Hase, E. De-and re-generation of chloroplasts in the cells of Chlorella protothecoides: V. Degeneration of chloroplasts induced by different carbon sources, and effects of some antimetabolites upon the process induced by glucose. Plant Cell Physiol. 1965, 6, 487–498. [Google Scholar]
- Hörtensteiner, S.; Chinner, J.; Matile, P.; Thomas, H.; Donnison, I.S. Chlorophyll breakdown in Chlorella protothecoides: Characterization of degreening and cloning of degreening-related genes. Plant Mol. Biol. 2000, 42, 439–450. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H. Microalgae for the production of bulk chemicals and biofuels. Biofuelsbioproducts Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef]
- Reitan, K.I.; Rainuzzo, J.R.; Olsen, Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J. Phycol. 1994, 30, 972–979. [Google Scholar] [CrossRef]
- Matsakas, L.; Rova, U.; Christakopoulos, P. Sequential parametric optimization of methane production from different sources of forest raw material. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Nitsos, C.; Vörös, D.; Rova, U.; Christakopoulos, P. High-Titer Methane from Organosolv-Pretreated Spruce and Birch. Energies 2017, 10, 263. [Google Scholar] [CrossRef]
- Matsakas, L.; Novak, K.; Enman, J.; Christakopoulos, P.; Rova, U. Acetate-detoxification of wood hydrolysates with alkali tolerant Bacillus sp. as a strategy to enhance the lipid production from Rhodosporidium toruloides. Bioresour. Technol. 2017, 242, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Axelsson, M.; Gentili, F. A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [PubMed]
- Lage, S.; Gentili, F.G. Quantification and characterisation of fatty acid methyl esters in microalgae: Comparison of pretreatment and purification methods. Bioresour. Technol. 2018, 257, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.; Han, X. Lipid Analysis-Isolation, Separation, Identification and Lipidomic Analysis; Oily Press: Bridgwater, UK, 2010; 446p. [Google Scholar]
- Werner, R.A.; Bruch, B.A.; Brand, W.A. ConFlo III—An interface for high precision δ13C and δ15N analysis with an extended dynamic range. Rapid Commun. Mass Spectrom. 1999, 13, 1237–1241. [Google Scholar] [CrossRef]


| Without NPK Supplementation | With NPK Supplementation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NH4+-N | NO3−-N | TP | TOC (mg/L) | TOC Reduction (%) | NH4+-N | NO3−-N | TP | TOC (mg/L) | TOC Reduction (%) | ||
| Day 0 | 72.70 ± 0.00 | ≤0.50 | 8.56 ± 0.01 | 663 ± 89 | - | 98.40 ± 1.60 | 247.60 ± 2.00 | 16.15 ± 0.15 | 654 ± 66 | - | |
| C. sorokiniana | MT 7d | 0.85 ± 0.00 | 7.07 ± 0.07 | 1.58 ± 0.04 | 282 ± 20 | 57.5 | 6.58 ± 0.03 | 5.48 ± 0.12 | 4.15 ± 0.00 | 250 ± 27 | 61.8 |
| MT 14d | 2.12 ± 0.03 | 5.85 ± 0.19 | 4.49 ± 0.07 | 232.5 ± 23.5 | 64.9 | 1.67 ± 0.01 | 5.00 ± 0.01 | 6.15 ± 0.04 | 198 ± 3 | 69.7 | |
| MT 7d + H 7d | 1.47 ± 0.00 | 6.10 ± 0.53 | 2.20 ± 0.09 | 245 ± 30 | 63 | 39.15 ± 0.15 | 4.91 ± 0.16 | 4.00 ± 0.01 | 202 ± 8 | 69.1 | |
| C. saccharophila | MT 7d | 0.33 ± 0.00 | 6.64 ± 0.15 | 1.52 ± 0.03 | 312 ± 38 | 52.9 | 13.65 ± 0.05 | 5.50 ± 0.13 | 3.67 ± 0.02 | 234.5 ± 14.5 | 64.1 |
| MT 14d | 1.52 ± 0.05 | 5.82 ± 0.24 | 3.81 ± 0.09 | 258 ± 22 | 61.1 | 1.28 ± 0.02 | 4.86 ± 0.13 | 6.97 ± 0.30 | 269 ± 11 | 58.9 | |
| MT 7d + H 7d | 0.84 ± 0.00 | 5.68 ± 0.52 | 1.87 ± 0.20 | 241.5 ± 2.5 | 63.6 | 54.70 ± 0.40 | 4.76 ± 0.06 | 5.63 ± 0.01 | 229 ± 18.5 | 65.0 | |
| C. vulgaris | MT 7d | 6.69 ± 0.02 | 7.00 ± 0.20 | 1.65 ± 0.06 | 298 ± 32 | 55.1 | 8.88 ± 0.03 | 6.89 ± 0.20 | 3.05 ± 0.00 | 221.5 ± 7.5 | 66.1 |
| MT 14d | 0.96 ± 0.00 | 5.39 ± 0.22 | 4.70 ± 0.12 | 239.5 ± 0.5 | 63.9 | 20.05 ± 0.15 | 6.30 ± 0.15 | 8.03 ± 0.67 | 200.5 ± 9.5 | 69.3 | |
| MT 7d + H 7d | 0.42 ± 0.00 | 6.10 ± 0.57 | 1.64 ± 0.22 | 268.5 ± 9.5 | 59.5 | 15.35 ± 0.05 | 5.08 ± 0.02 | 3.43 ± 0.04 | 187.5 ± 13.5 | 71.3 | |
| Coelastrella sp. | MT 7d | 0.41 ± 0.00 | 6.88 ± 0.14 | 1.37 ± 0.04 | 304 ± 23.5 | 54.1 | 9.30 ± 0.04 | 42.95 ± 0.55 | 2.98 ± 0.01 | 258.5 ± 2.5 | 60.5 |
| MT 14d | 0.42 ± 0.00 | 5.86 ± 0.22 | 3.44 ± 0.08 | 274 ± 22 | 58.7 | 3.92 ± 0.04 | 16.15 ± 1.95 | 6.75 ± 0.12 | 227.5 ± 2.5 | 65.2 | |
| MT 7d + H 7d | 1.97 ± 0.01 | 5.47 ± 0.44 | 2.15 ± 0.21 | 231.5 ± 17.5 | 65.1 | 0.78 ± 0.00 | 5.12 ± 0.03 | 5.31 ± 0.01 | 197 ± 9 | 69.9 | |
| Supplementation | Growth Regime | TSS (mg L−1) |
|---|---|---|
| With NPK | MT 7d | 15 ± 0.0 |
| MT 14d | 27 ± 1.0 | |
| MT 7d + H 7d | 16 ± 0.0 | |
| Without NPK | MT 7d | 16 ± 1.0 |
| MT 14d | 15 ± 0.0 | |
| MT 7d + H 7d | 27 ± 1.0 |
| Without NPK Supplementation | With NPK Supplementation | ||||||
|---|---|---|---|---|---|---|---|
| Biomass | Lipids | FAMEs | Biomass | Lipids | FAMEs | ||
| C. sorokiniana | MT 7d | 0.13 ± 0.03 | 24.75 ± 4.16 | NA | 0.26 ± 0.00 | 24.50 ± 2.33 | 13.71 ± 3.94 |
| MT 14d | 0.06 ± 0.04 | 21.36 ± 0.66 | 9.79 ± 0.82 | 0.14 ± 0.00 | 15.97 ± 1.40 | 9.75 ± 0.56 | |
| MT 7d + H 7d | 0.04 ± 0.00 | 18.85 ± 2.07 | 11.03 ± 1.36 | 0.10 ± 0.00 | 13.77 ± 1.94 | 9.74 ± 0.48 | |
| C. saccharophila | MT 7d | 0.23 ± 0.29 | 25.58 ± 2.31 | 25.37 ± 0.56 | 0.23 ± 0.06 | 31.51 ± 8.66 | 19.96 ± 3.50 |
| MT 14d | 0.09 ± 0.05 | 16.73 ± 1.25 | 11.09 ± 0.73 | 0.13 ± 0.00 | 14.21 ± 1.35 | 11.05 ± 0.00 | |
| MT 7d + H 7d | 0.06 ± 0.00 | 25.94 ± 1.03 | 5.00 ± 0.15 | 0.07 ± 0.00 | 12.98 ± 1.93 | 9.61 ± 0.38 | |
| C. vulgaris | MT 7d | 0.09 ± 0.02 | NA | 31.57 ± 2.39 | 0.24 ± 0.03 | 28.36 ± 1.97 | 23.20 ± 0.58 |
| MT 14d | 0.05 ± 0.00 | 17.98 ± 1.60 | 11.54 ± 0.25 | 0.12 ± 0.00 | 16.63 ± 1.61 | 13.70 ± 0.10 | |
| MT 7d + H 7d | 0.04 ± 0.00 | 17.76 ± 4.08 | 15.83 ± 1.69 | 0.11 ± 0.00 | 20.33 ± 1.95 | 12.60 ± 0.34 | |
| Coelastrella sp. | MT 7d | 0.19 ± 0.05 | 33.95 ± 5.56 | NA | 0.11 ± 0.04 | 41.80 ± 8.13 | NA |
| MT 14d | 0.09 ± 0.01 | 17.10 ± 1.85 | 11.63 ± 0.79 | 0.13 ± 0.00 | 19.02 ± 0.70 | 15.89 ± 0.90 | |
| MT 7d + H 7d | 0.06 ± 0.00 | 28.51 ± 2.27 | NA | 0.08 ± 0.00 | 12.30 ± 1.82 | 16.28 ± 0.22 | |
| Species | Supplementation | Growth Regime | N% | C% | C/N Ratio |
|---|---|---|---|---|---|
| C. sorokiniana | With NPK | MT 14d | 6.90 ± 0.34 | 50.73 ± 0.45 | 7.37 ± 0.41 |
| MT 7d + H 7d | 9.04 ± 0.57 | 49.81 ± 2.06 | 5.52 ± 0.18 | ||
| Without NPK | MT 14d | 3.89 ± 0.88 | 56.03 ± 1.37 | 15.34 ± 4.23 | |
| MT 7d + H 7d | 8.13 ± 0.10 | 51.10 ± 1.35 | 6.28 ± 0.09 | ||
| C. saccharophila | With NPK | MT 14d | 6.84 ± 0.14 | 51.50 ± 0.46 | 7.53 ± 0.18 |
| MT 7d + H 7d | 9.33 ± 0.06 | 50.27 ± 0.06 | 5.38 ± 0.03 | ||
| Without NPK | MT 14d | 3.77 ± 0.17 | 56.34 ± 0.06 | 14.98 ± 0.69 | |
| MT 7d + H 7d | 5.54 ± 1.01 | 55.99 ± 0.33 | 10.42 ± 1.70 | ||
| C. vulgaris | With NPK | MT 14d | 7.15 ± 0.07 | 52.86 ± 0.21 | 7.39 ± 0.10 |
| MT 7d + H 7d | 8.70 ± 0.19 | 52.59 ± 1.02 | 6.05 ± 0.01 | ||
| Without NPK | MT 14d | 7.21 ± 0.34 | 53.82 ± 0.14 | 7.49 ± 0.33 | |
| MT 7d + H 7d | 11.04 ± 0.03 | 52.47 ± 0.15 | 4.73 ± 0.02 | ||
| Coelastrella sp. | With NPK | MT 14d | 6.75 ± 0.14 | 50.66 ± 0.91 | 7.51 ± 0.12 |
| MT 7d + H 7d | 7.59 ± 0.34 | 52.47 ± 0.72 | 6.93 ± 0.40 | ||
| Without NPK | MT 14d | 3.38 ± 0.11 | 58.18 ± 0.62 | 17.21 ± 0.17 | |
| MT 7d + H 7d | 5.87 ± 0.22 | 55.27 ± 1.57 | 9.43 ± 0.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lage, S.; Kudahettige, N.P.; Ferro, L.; Matsakas, L.; Funk, C.; Rova, U.; Gentili, F.G. Microalgae Cultivation for the Biotransformation of Birch Wood Hydrolysate and Dairy Effluent. Catalysts 2019, 9, 150. https://doi.org/10.3390/catal9020150
Lage S, Kudahettige NP, Ferro L, Matsakas L, Funk C, Rova U, Gentili FG. Microalgae Cultivation for the Biotransformation of Birch Wood Hydrolysate and Dairy Effluent. Catalysts. 2019; 9(2):150. https://doi.org/10.3390/catal9020150
Chicago/Turabian StyleLage, Sandra, Nirupa P. Kudahettige, Lorenza Ferro, Leonidas Matsakas, Christiane Funk, Ulrika Rova, and Francesco G. Gentili. 2019. "Microalgae Cultivation for the Biotransformation of Birch Wood Hydrolysate and Dairy Effluent" Catalysts 9, no. 2: 150. https://doi.org/10.3390/catal9020150
APA StyleLage, S., Kudahettige, N. P., Ferro, L., Matsakas, L., Funk, C., Rova, U., & Gentili, F. G. (2019). Microalgae Cultivation for the Biotransformation of Birch Wood Hydrolysate and Dairy Effluent. Catalysts, 9(2), 150. https://doi.org/10.3390/catal9020150







