Photocatalytic and Antimicrobial Properties of Ga Doped and Ag Doped ZnO Nanorods for Water Treatment
Abstract
:1. Introduction
2. Results
2.1. ZnO Nanoparticle Characterization
2.2. Doped and Undoped ZnO Nanorods Characterization
2.2.1. Morphology
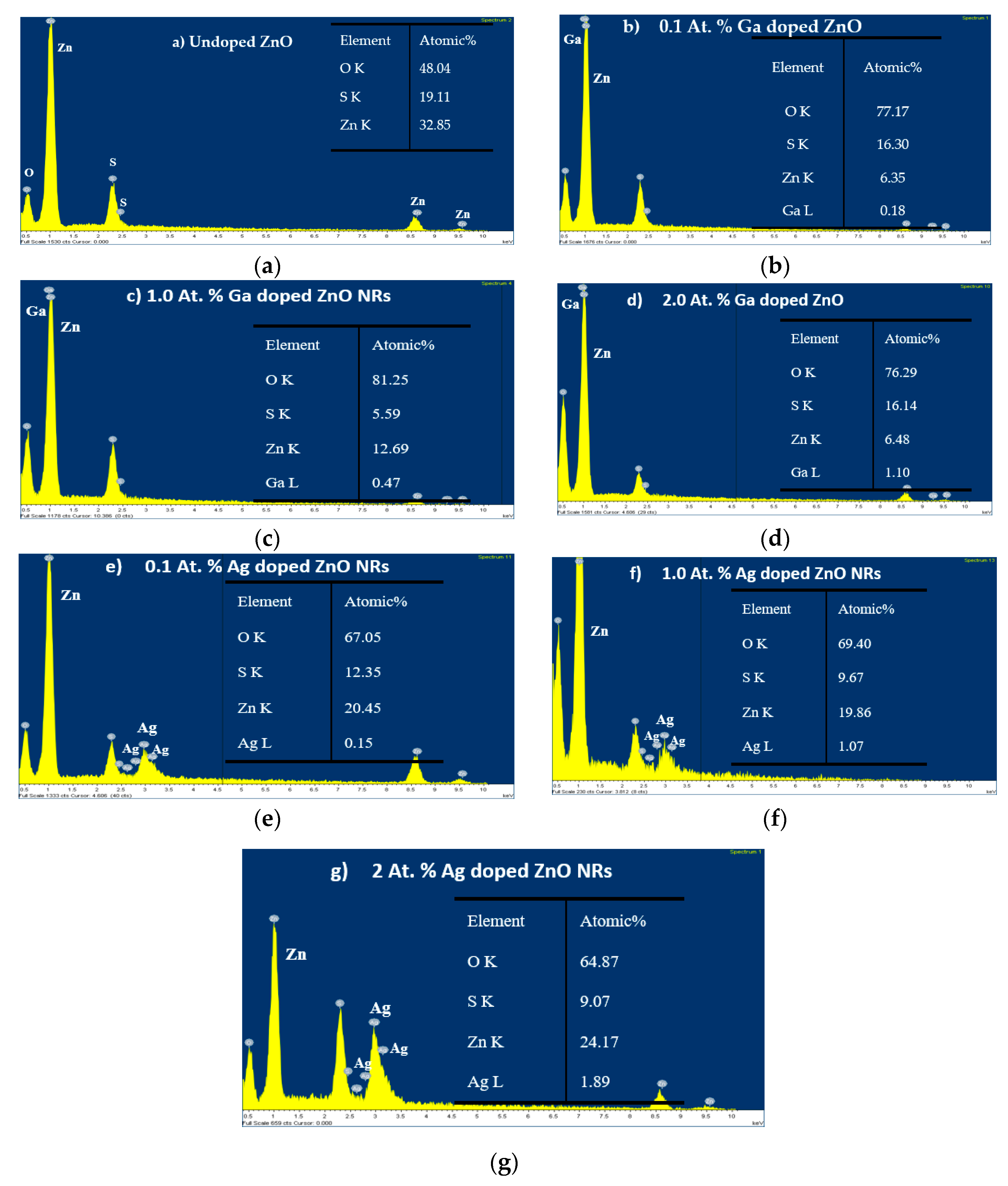
2.2.2. Chemical Composition
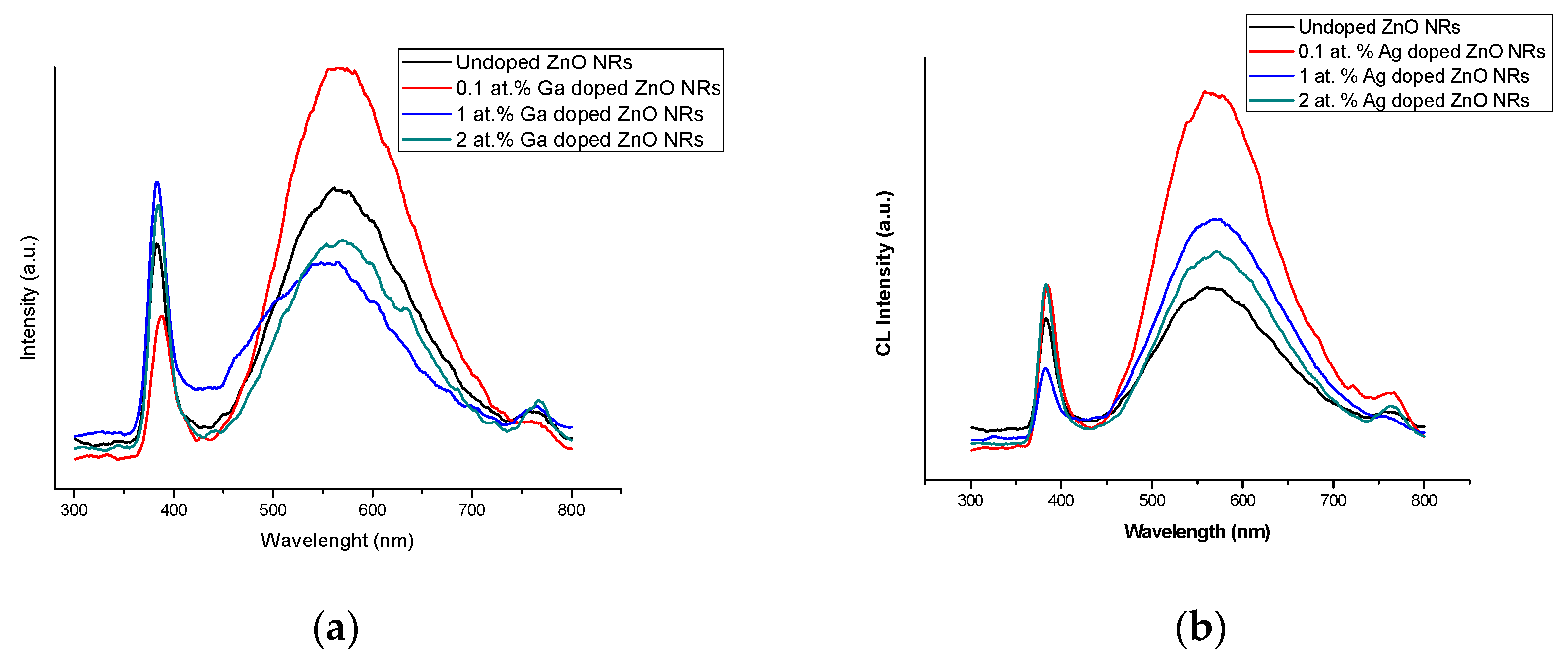
2.2.3. Optical Properties
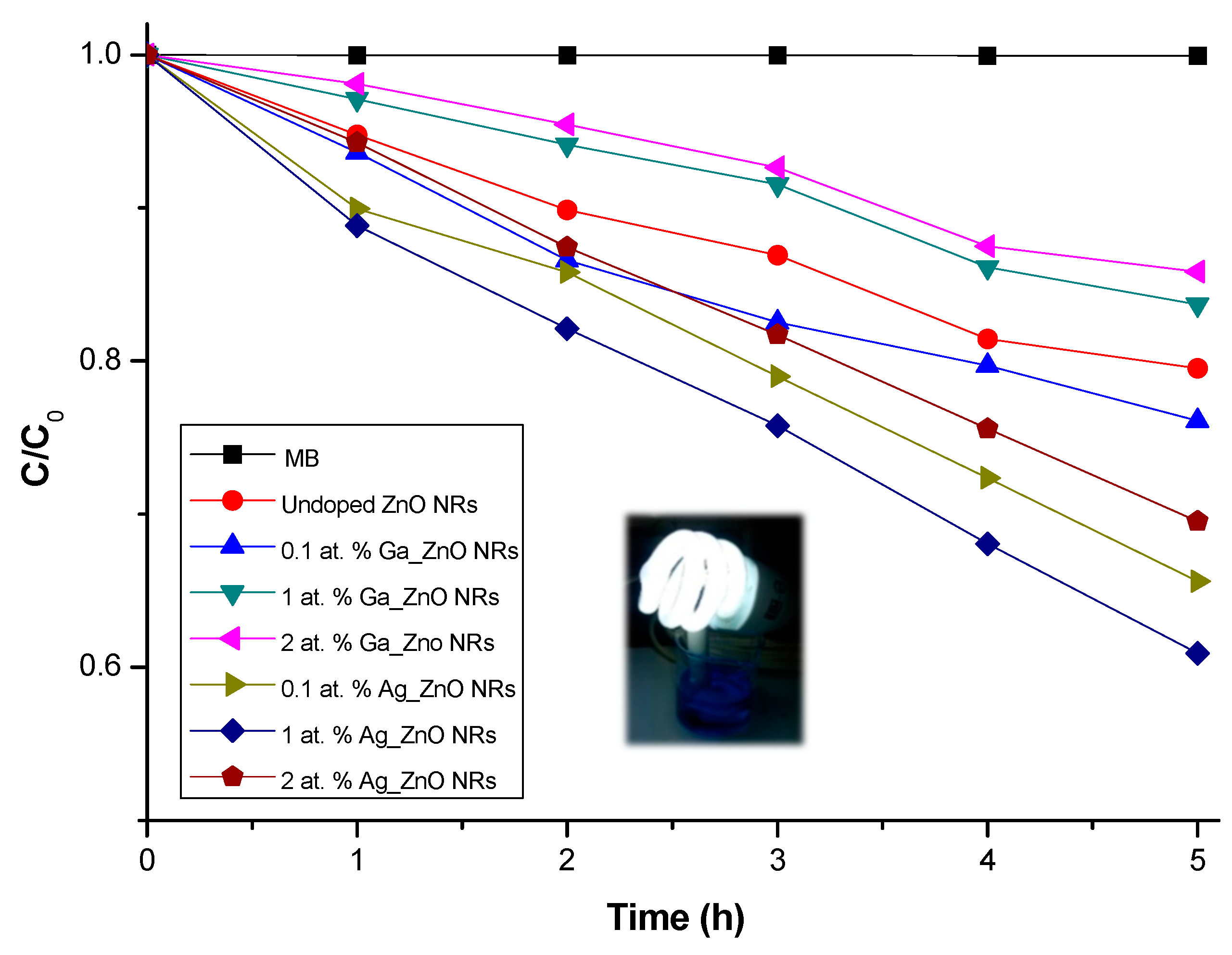
2.3. Photocatalytic Activity
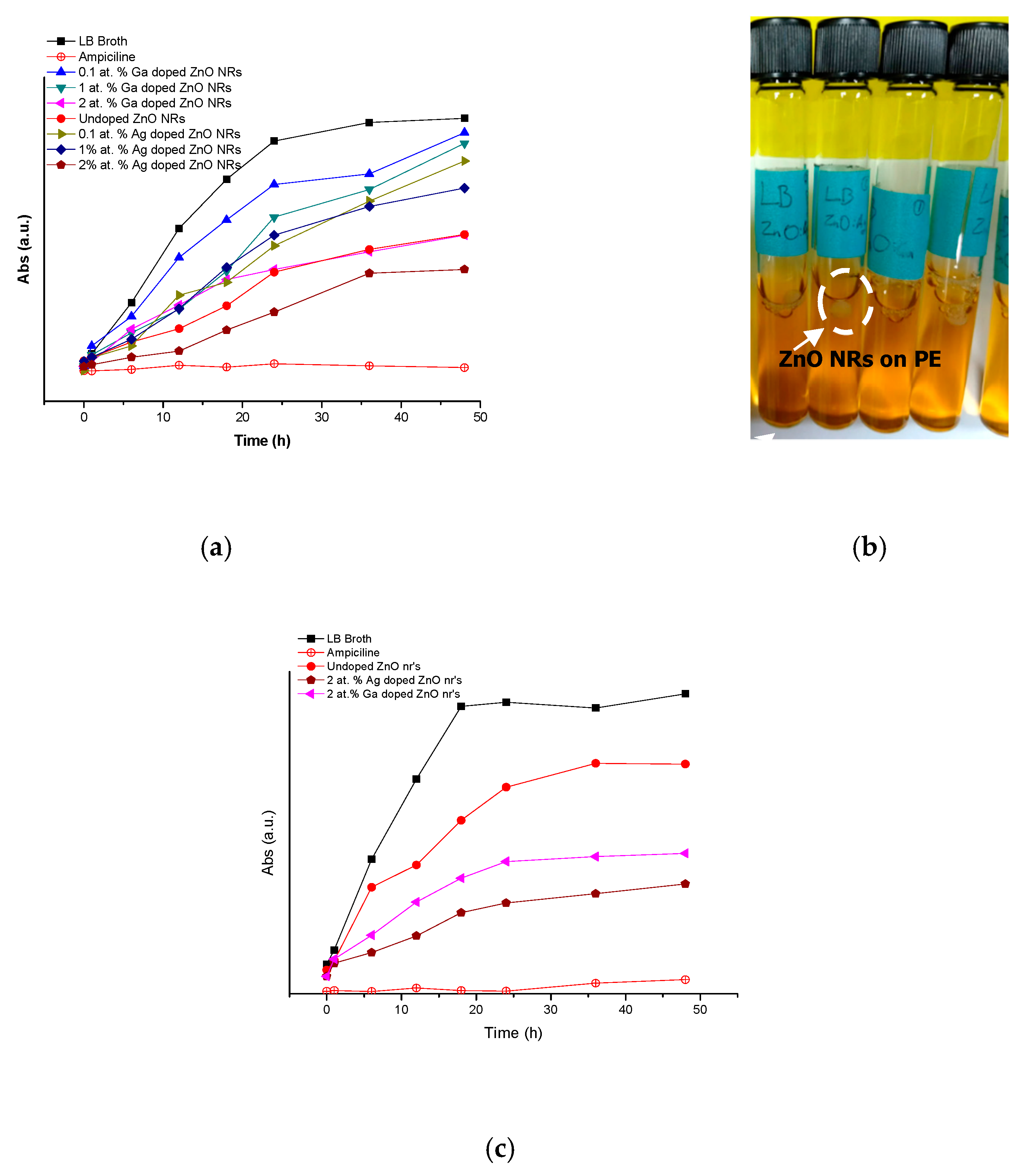
2.4. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. ZnO Nanoparticles Synthesis and Characterization
4.2. ZnO Nanorods Microwave Synthesis on Polyethylene Substrate
4.3. ZnO Nanorod Characterization
4.3.1. SEM
4.3.2. EDS
4.3.3. CL
4.4. Photocatalytic Performance
4.5. Antimicrobial Evaluation
4.5.1. Bacterial Strains
4.5.2. Growth Kinetics of Vibrio spp. and E. coli Using ZnO NRs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chong, M.N.; Jin, B.K.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid Ultrafiltration/Photocatalytic Membranes for Efficient Water Treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Sun, P.; Liua, J.; Ji, Y.; Nakad, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Zhang, Q.; Quan, X.; Wang, H.; Chen, S.; Su, Y.; Li, Z. Constructing a visible-light-driven photocatalytic membrane by g-C3N4 quantum dots and TiO2 nanotube array for enhanced water treatment. Sci. Rep. 2017, 7, 3128. [Google Scholar] [CrossRef]
- Cantarella, M.; Di Mauro, A.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Privitera, V.; Impellizzeri, G. Selective photodegradation of paracetamol by molecularly imprinted ZnO nanonuts. Appl. Catal. B 2018, 238, 509–517. [Google Scholar] [CrossRef]
- Zhou, Q.; Wen, J.Z.; Zhao, P.; Anderson, W.A. Synthesis of Vertically-Aligned Zinc Oxide Nanowires and Their Application as a Photocatalyst. Nanomaterials 2017, 7, 9. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl. Catal. B Environ. 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Baruah, S.; Mahmood, M.A.; Myint, M.T.Z.; Bora, T.; Dutta, J. Enhanced visible light photocatalysis through fast crystallization of zinc oxide nanorods. Beilstein J. Nanotechnol. 2010, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Wiley-VCH Verlag: Weinheim, Germany, 2013. [Google Scholar]
- Cravotto, G.; Carnaroglio, D. Microwave Chemistry; DeGruyterTextbook; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Current Trends in the Development of Microwave Reactors for the Synthesis of Nanomaterials in Laboratories and Industries: A Review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef]
- Türkyılmaz, S.S.; Güy, N.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A Chem. 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Ashebir, M.E.; Tesfamariam, G.M.; Nigussie, G.Y.; Gebreab, T.W. Structural, Optical, and Photocatalytic Activities of Ag-Doped and Mn-Doped ZnO Nanoparticles. J. Nanomater. 2018, 9. [Google Scholar] [CrossRef]
- Park, G.C.; Hwang, S.M.; Choi, J.H.; Kwon, Y.H.; Cho, H.K.; Kim, S.-W.; Lim, J.H.; Joo, J. Effects of In or Ga doping on the growth behavior and optical properties of ZnO nanorods fabricated by hydrothermal process. Phys. Status Solidi A 2013, 210, 1552–1556. [Google Scholar] [CrossRef]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef]
- Rzhepishevska, O.; Ekstrand-Hammarstrom, B.; Popp, M.; Bjorn, E.; Bucht, A.; Sjostedt, A.; Antti, H.; Ramstedt, M. The Antibacterial Activity of Ga3+ Is Influenced by Ligand Complexation as Well as the Bacterial Carbon Source. Antimicrob. Agents Chemother. 2011, 55, 5568–5580. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Longquan, S. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Salomoni, R.; Leo, P.; Montemor, A.F.; Rinaldi, B.G.; Rodrigues, M.F.A. Antibacterial Effect of Silver Nanoparticles in Pseudomonas Aeruginosa. Nanotechnol. Sci. Appl. 2017, 10, 115–121. [Google Scholar]
- López-Esparza, J.; Espinosa-Cristóbal, L.F.; Donohue-Cornejo, A.; Reyes-López, S.Y. Antimicrobial Activity of Silver Nanoparticles in Polycaprolactone Nanofibers against Gram-Positive and Gram-Negative Bacteria. Ind. Eng. Chem. Res. 2016, 55, 12532–12538. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, Z.; Ma, Y.; Zhang, W. Effect of Na Doping on the Nanostructures and Electrical Properties of ZnO Nanorod Arrays. J. Nanomater. 2016, 22, 1–5. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a chemical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407. [Google Scholar] [CrossRef]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurisic, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Phillips, D.L.; Ding, L.; et al. Defects in ZnO nanorods prepared by a hydrothermal method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef]
- Ye, J.D.; Gu, S.L.; Zhu, S.M.; Liu, S.M.; Zheng, Y.D.; Zhang, R.; Shi, Y. Fermi-level band filling and band-gap renormalization in Ga-doped ZnO. Appl. Phys. Lett. 2005, 86, 192111. [Google Scholar] [CrossRef]
- Kuo, S.-T.; Tuan, W.-H.; Shieh, J.; Wang, S.-F. Effect of Ag on the microstructure and electrical properties of ZnO. J. Eur. Ceram. Soc. 2007, 27, 4521–4527. [Google Scholar] [CrossRef]
- Kakhki, R.M.; Tayebee, R.; Ahsani, F. New and highly efficient Ag doped ZnO visible nano photocatalyst for removing of methylene blue. J. Mater. Sci. Mater. Electron. 2017, 28, 5941–5952. [Google Scholar]
- Lu, W.; Gao, S.; Wang, J. One-pot synthesis of Ag/ZnO self-assembled 3D hollow microspheres with photocatalytic performance. J. Phys. Chem. C 2008, 112, 16792–16800. [Google Scholar] [CrossRef]
- Whang, T.-J.; Hsieh, M.-T.; Chen, H.-H. Visible-light photocatalytic degradation of methylene blue with laser-induced Ag/ZnO nanoparticles. Appl. Surf. Sci. 2012, 258, 2796–2801. [Google Scholar] [CrossRef]
- Liu, H.R.; Shao, G.X.; Zhao, J.F.; Zhang, Z.X.; Zhang, Y.; Liang, J.; Liu, X.G.; Jia, H.S.; Xu, B.S. Worm-like Ag/ZnO core-shell heterostructural composites: fabrication, characterization, and photocatalysis. J. Phys. Chem. C 2012, 116, 16182–16190. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.-A.; Kim, K.-W.; Lee, B.-T. Comparative toxicity of silver nanoparticles and silver ions to Escherichia coli. J. Environ. Sci. 2018, 66, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, D.W.; Kormann, C.; Hofmann, M.R. Preparation and characterization of quantum size zinc oxide: A detailed spectroscopic study. J. Phys. Chem. 1987, 91, 3789–3798. [Google Scholar] [CrossRef]
- Baruah, S.; Thanachayanont, C.; Dutta, J. Growth of ZnO nanowires on nonwoven polyethylene fibers. Sci. Technol. Adv. Mater. 2008, 9, 025009. [Google Scholar] [CrossRef]


| % At. | Ga Doped ZnO NRs Diameter (nm) | Ag Doped ZnO NRs Diameter (nm) |
|---|---|---|
| 0.0 | 150–250 | 150–250 |
| 0.1 | 100–200 | 150–250 |
| 1.0 | 150–250 | 200–300 |
| 2.0 | 300–500 | 500–800 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoza-Contreras, M.N.; Vásquez-Gallegos, A.; Vidal-Limon, A.; Romo-Herrera, J.M.; Águila, S.; Contreras, O.E. Photocatalytic and Antimicrobial Properties of Ga Doped and Ag Doped ZnO Nanorods for Water Treatment. Catalysts 2019, 9, 165. https://doi.org/10.3390/catal9020165
Cardoza-Contreras MN, Vásquez-Gallegos A, Vidal-Limon A, Romo-Herrera JM, Águila S, Contreras OE. Photocatalytic and Antimicrobial Properties of Ga Doped and Ag Doped ZnO Nanorods for Water Treatment. Catalysts. 2019; 9(2):165. https://doi.org/10.3390/catal9020165
Chicago/Turabian StyleCardoza-Contreras, Marlene N., Adrián Vásquez-Gallegos, Abraham Vidal-Limon, José M. Romo-Herrera, Sergio Águila, and Oscar E. Contreras. 2019. "Photocatalytic and Antimicrobial Properties of Ga Doped and Ag Doped ZnO Nanorods for Water Treatment" Catalysts 9, no. 2: 165. https://doi.org/10.3390/catal9020165






