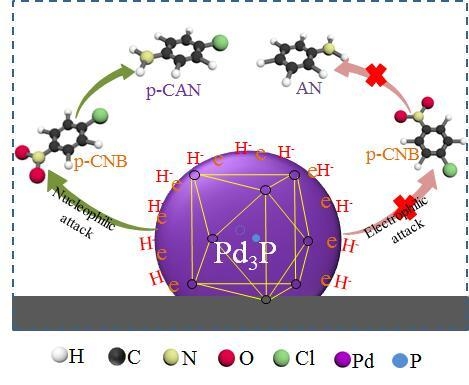
Preparation and Catalytic Performance of Metal-Rich Pd Phosphides for the Solvent-Free Selective Hydrogenation of Chloronitrobenzene
Abstract
:1. Introduction
2. Results and Discussion
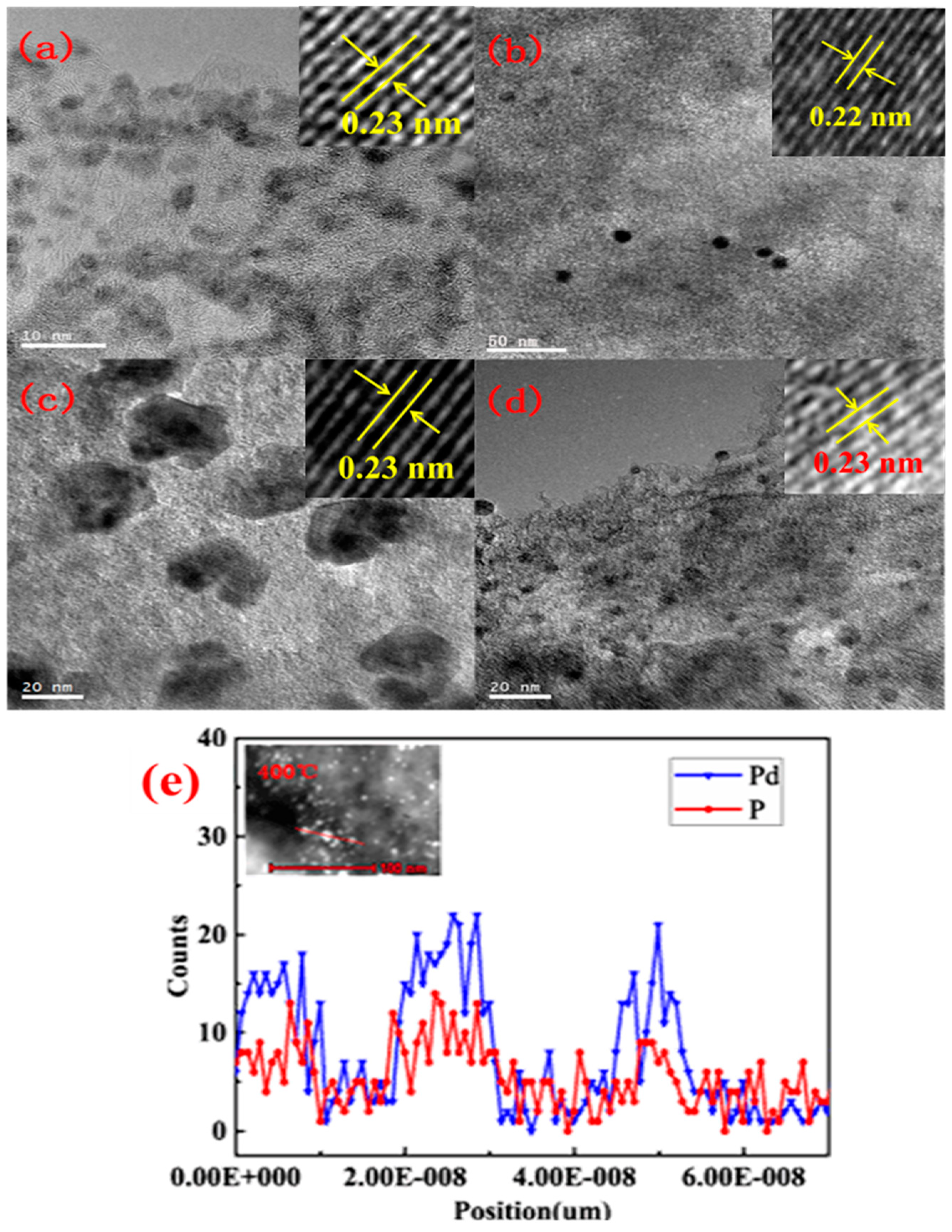
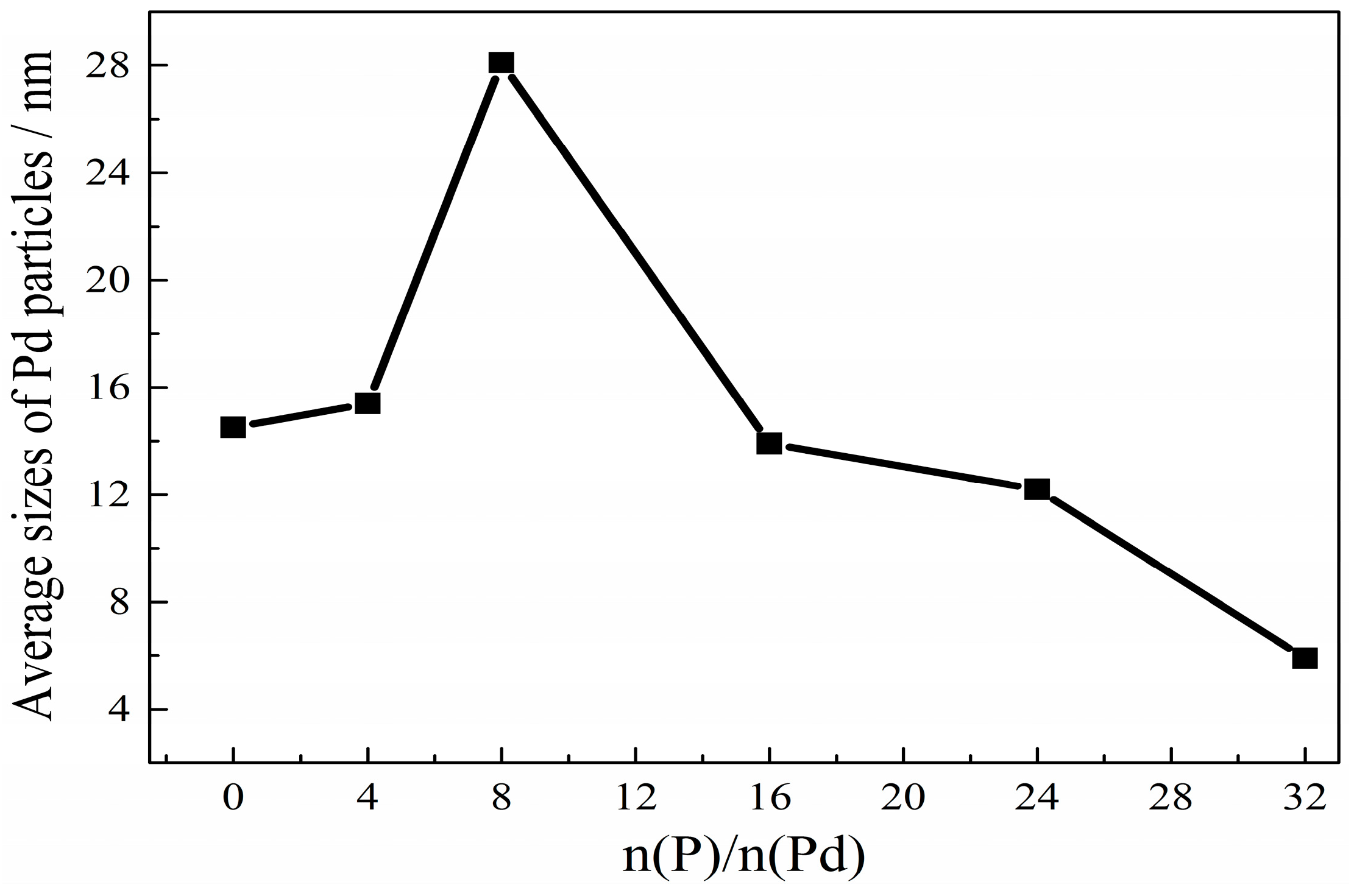
2.1. The Preparation of Palladium Phosphides Supported on Activated Carbon
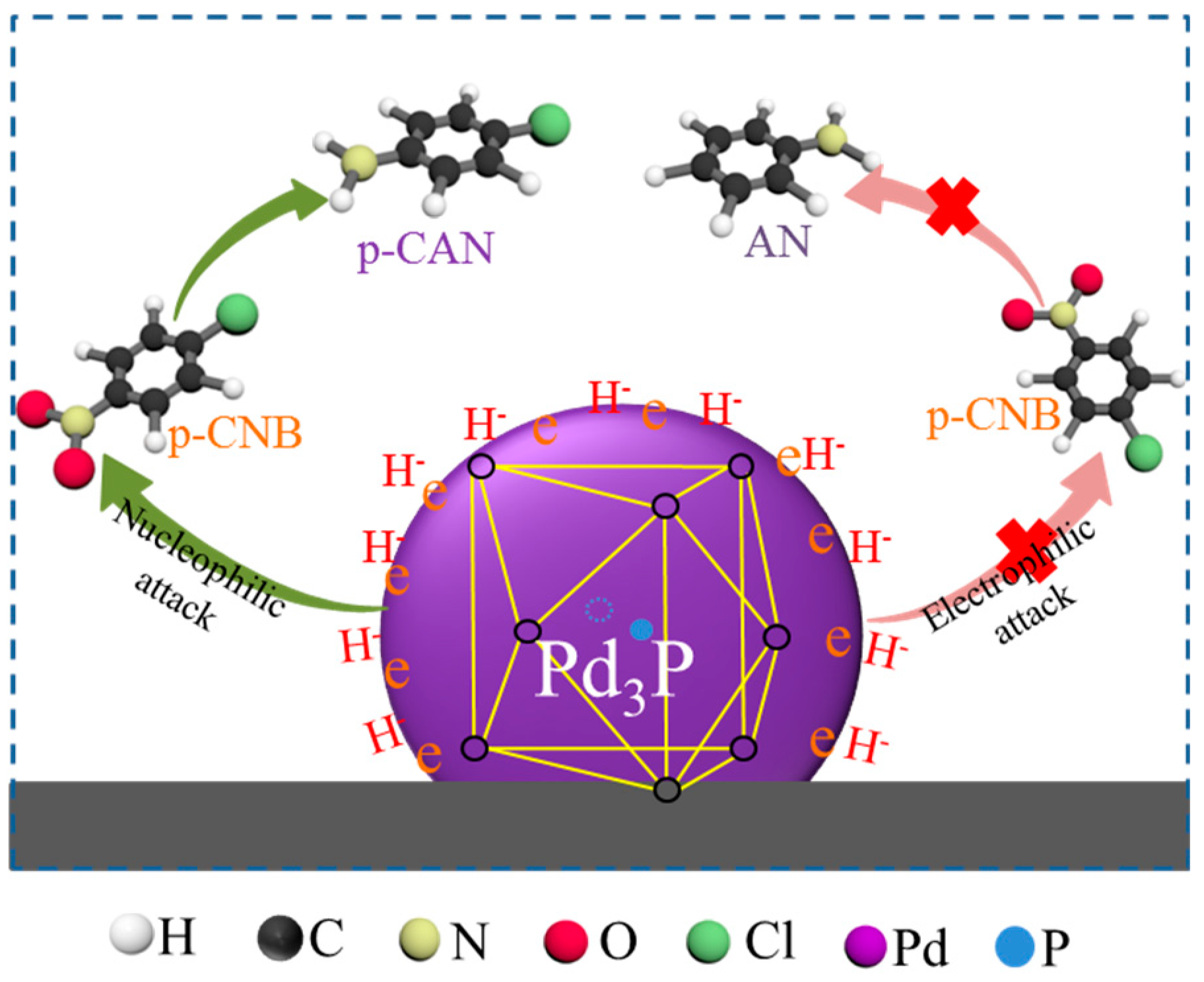
2.2. Generation Mechanism of Palladium Phosphide
2.3. Catalytic Performance of Pd3P/AC
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Hydrogenation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Song, T.; Ren, P.; Duan, Y.N.; Wang, Z.Z.; Chen, X.F.; Yang, Y. Cobalt nanocomposites on N-doped hierarchical porous carbon for highly selective formation of anilines and imines from nitroarenes. Green Chem. 2018, 20, 4629–4637. [Google Scholar] [CrossRef]
- Jiang, L.C.; Gu, H.Z.; Xu, X.Z.; Yan, X.H. Selective hydrogenation of o-chloronitrobenzene (o-CNB) over supported Pt and Pd catalysts obtained by laser vaporization deposition of bulk metals. J. Mol. Catal. A Chem. 2019, 310, 144–149. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Hao, Y.; Crespo-Quesada, M.; Yuranov, I.; Wang, X.; Keane, M.A.; Kiwi-Minsker, L. Selective gas phase hydrogenation of p-Chloronitrobenzene over Pd catalysts: Role of the support. ACS Catal. 2013, 3, 1386–1396. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Suo, Y.G.; He, J.P.; Li, G.N.; Hu, G.L.; Zheng, Y.Q. Selective hydrogenation of ortho-chloronitrobenzene over biosynthesized ruthenium-platinum bimetallic nanocatalysts. Ind. Eng. Chem. Res. 2016, 55, 7061–7068. [Google Scholar] [CrossRef]
- Chen, H.; He, D.; He, Q.; Jiang, P.; Zhou, G.; Fu, W. Selective hydrogenation of p-chloronitrobenzene over an Fe promoted Pt/AC catalyst. RSC Adv. 2017, 7, 29143–29148. [Google Scholar] [CrossRef]
- Iihama, S.; Furukawa, S.; Komatsu, T. Efficient catalytic system for chemoselective hydrogenation of halonitrobenzene to haloaniline using PtZn intermetallic compound. ACS Catal. 2015, 6, 742–746. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Hugon, A.; Delannoy, L.; Louis, C.; Keane, M.A. Pd-promoted selective gas phase hydrogenation of p-chloronitrobenzene over alumina supported Au. J. Catal. 2009, 262, 235–243. [Google Scholar] [CrossRef]
- Ning, J.B.; Xu, J.; Liu, J.; Miao, H.; Ma, H.; Chen, C.; Li, X.Q.; Zhou, L.P.; Yu, W.Q. A remarkable promoting effect of water addition on selective hydrogenation of p-chloronitrobenzene in ethanol. Catal. Commun. 2007, 8, 1763–1766. [Google Scholar] [CrossRef]
- Lyu, J.H.; Wang, J.G.; Lu, C.S.; Ma, L.; Zhang, Q.F.; He, X.B.; Li, X.N. Size-dependent halogenated nitrobenzene hydrogenation selectivity of Pd nanoparticles. J. Phys. Chem. C 2014, 118, 2594–2601. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Lu, C.S.; Zhang, Q.F.; Li, X.N. Highly selective hydrogenation of 3,4-dichloronitrobenzene over Pd/C catalysts without inhibitors. Catal. Today 2011, 173, 62–67. [Google Scholar] [CrossRef]
- Lu, C.S.; Lv, J.H.; Ma, L.; Zhang, Q.F.; Feng, F.; Li, X.N. Highly selective hydrogenation of halonitroaromatics to aromatic haloamines by ligand modified Ni-based catalysts. Chin. Chem. Lett. 2012, 23, 545–548. [Google Scholar] [CrossRef]
- Mori, A.; Mizusaki, T.; Kawase, M.; Maegawa, T.; Monguchi, Y.; Takao, S.; Takagi, Y.; Sajiki, H. Novel palladium-on-carbon/diphenyl sulfide complex for chemoselective hydrogenation: Preparation, characterization, and application. Adv. Synth. Catal. 2008, 350, 406–410. [Google Scholar] [CrossRef]
- Mori, A.; Mizusaki, T.; Miyakawa, Y.; Ohashi, E.; Haga, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Chemoselective hydrogenation method catalyzed by Pd/C using diphenylsulfide as a reasonable catalyst poison. Tetrahedron 2006, 62, 11925–11932. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Xu, W.; Li, X.N.; Jiang, D.H.; Xiang, Y.Z.; Wang, J.G.; Cen, J.; Romano, S.; Ni, J. Catalytic hydrogenation of sulfur-containing nitrobenzene over Pd/C catalysts: In situ sulfidation of Pd/C for the preparation of PdxSy catalysts. Appl. Catal. A 2015, 497, 17–21. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Iglesias-Juez, A.; Castillejos-López, E.; Guerrero-Ruiz, A.; Michiel, M.D.; Fernández-García, M.; Rodríguez-Ramos, I. Detecting the genesis of a high-performance carbon-supported Pd sulfide nanophase and its evolution in the hydrogenation of butadiene. ACS Catal. 2015, 5, 5235–5241. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Perret, N.; Kiwi-Minsker, L.; Keane, M.A. β-Molybdenum nitride: Synthesis mechanism and catalytic response in the gas phase hydrogenation of p-chloronitrobenzene. Catal. Sci. Technol. 2011, 1, 794. [Google Scholar] [CrossRef]
- Xu, X.S.; Li, X.Q.; Gu, H.Z.; Huang, Z.B.; Yan, X.H. A highly active and chemoselective assembled Pt/C(Fe) catalyst for hydrogenation of o-chloronitrobenzene. Appl. Catal. A 2012, 429–430, 17–23. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Amorim, C.; Keane, M.A. Gas phase hydrogenation of p-chloronitrobenzene over Pd–Ni/Al2O3. Appl. Catal. A 2014, 473, 41–50. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, B.S.; Lin, Y.M.; Wang, Q.; Zhang, Q.; Su, D.S. Enhanced chemoselective hydrogenation through tuning the interaction between Pt nanoparticles and carbon supports: Insights from identical location transmission electron microscopy and X-ray photoelectron spectroscopy. ACS Catal. 2016, 6, 7844–7854. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.W. Chemoselective hydrogenation of functionalized nitroarenes using MOF-derived co-based catalysts. J. Mol. Catal. A Chem. 2016, 420, 56–65. [Google Scholar] [CrossRef]
- Zhang, P.; Song, X.D.; Yu, C.; Gui, J.Z.; Qiu, J.S. Biomass-derived carbon nanospheres with turbostratic structure as metal-free catalysts for selective hydrogenation of o-chloronitrobenzene. ACS Sustain. Chem. Eng. 2018, 5, 7481–8485. [Google Scholar] [CrossRef]
- Sergio, N.; Amarajothi, D.; Mercedes, A.; Hermenegildo, G. Carbocatalysis by graphene-based materials. Chem. Rev. 2014, 12, 6179–6212. [Google Scholar]
- Park, B.J.; Jang, S.; Lee, J.H.; Chun, D.H.; Park, J.C.; Park, H.S. Hyperactive iron carbide@N-doped reduced graphene oxide/carbon nanotube hybrid architecture for rapid CO hydrogenation. J. Mater. Chem. A 2018, 6, 11134–11139. [Google Scholar] [CrossRef]
- Bodin, A.; Christoffersen, A.N.; Elkjaer, C.F.; Brorson, M.; Kibsgaard, J.; Helveg, S.; Chorkendorff, I. Engineering Ni-Mo-S nanoparticles for hydrodesulfurization. Nano Lett. 2018, 18, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, X.; Wang, A.J.; Chen, Y.Y.; Li, H.T.; Hu, Y.K. Hydrodenitrogenation of quinoline and decahydroquinoline over a surface nickel phosphosulfide phase. Catal. Lett. 2018, 148, 1579–1588. [Google Scholar] [CrossRef]
- Kojima, R.; Aika, K.I. Molybdenum nitride and carbide catalysts for ammonia synthesis. Appl. Catal. A 2001, 219, 141–147. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Cai, Q. Single transition metal atom embedded into a MoS2 nanosheet as a promising catalyst for electrochemical ammonia synthesis. Phys. Chem. Chem. Phys. 2018, 20, 9248–9255. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, Y.J.; Tian, C.G.; Wang, L.; Zhou, W.; Dong, Y.L.; Han, Q.; Liu, Y.F.; Yuan, F.L.; Fu, H.G. Synergistic effect of Mo2N and Pt for promoted selective hydrogenation of cinnamaldehyde over Pt-Mo2N/SBA-15. Catal. Sci. Technol. 2016, 6, 2403–2412. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Lamey, D.; Perret, N.; Gómez-Quero, S.; Kiwi-Minsker, L.; Keane, M.A. Au/Mo2N as a new catalyst formulation for the hydrogenation of p-chloronitrobenzene in both liquid and gas phases. Catal. Commun. 2012, 21, 46–51. [Google Scholar] [CrossRef]
- Clark, P.; Wang, X.; Oyama, S.T. Characterization of silica-supported molybdenum and tungsten phosphide hydroprocessing catalysts by 31P nuclear magnetic resonance spectroscopy. J. Catal. 2002, 207, 256–265. [Google Scholar] [CrossRef]
- Yang, P.F.; Jiang, Z.X.; Ying, P.L.; Li, C. Effect of surface composition on the catalytic performance of molybdenum phosphide catalysts in the hydrogenation of acetonitrile. J. Catal. 2008, 253, 66–73. [Google Scholar] [CrossRef]
- Clark, P.; Li, W.; Oyama, S.T. Synthesis and activity of a new catalyst for hydroprocessing: Tungsten phosphide. J. Catal. 2001, 200, 140–147. [Google Scholar] [CrossRef]
- Ni, Y.H.; Li, J.; Zhang, L.; Yang, S.; Wei, X.W. Urchin-like Co2P nanocrystals: Synthesis, characterization, influencing factors and photocatalytic degradation property. Mater. Res. Bull. 2009, 44, 1166–1172. [Google Scholar] [CrossRef]
- Bui, P.; Cecilia, J.A.; Oyama, S.T.; Takagaki, A.; Infantes-Molina, A.; Zhao, H.; Li, D.; Rodríguez-Castellón, E.; Jiménez López, A. Studies of the synthesis of transition metal phosphides and their activity in the hydrodeoxygenation of a biofuel model compound. J. Catal. 2012, 294, 184–198. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gott, T.; Zhao, H.Y.; Lee, Y.K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Chen, P.; Zhong, W.B.; Liu, Q.Z.; Li, M.F.; Wang, Y.D.; Wang, W.W.; Lu, Z.T.; Wang, D. Noncrystalline nickel phosphide decorated poly(vinyl alcohol-co-ethylene) nanofibrous membrane for catalytic hydrogenation of p-nitrophenol. Appl. Catal. B 2016, 196, 223–231. [Google Scholar] [CrossRef]
- Xin, H.; Guo, K.; Li, D.; Yang, H.Q.; Hu, C.W. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts. Appl. Catal. B 2016, 187, 375–385. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Jiménez-Morales, I.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Jiménez-López, A. Influence of the silica support on the activity of Ni and Ni2P based catalysts in the hydrodechlorination of chlorobenzene. Study of factors governing catalyst deactivation. J. Mol. Catal. A Chem. 2013, 368–369, 78–87. [Google Scholar] [CrossRef]
- Lu, C.S.; Wang, M.J.; Feng, Z.L.; Qi, Y.N.; Feng, F.; Ma, L.; Zhang, Q.F.; Li, X.N. A phosphorus–carbon framework over activated carbon supported palladium nanoparticles for the chemoselective hydrogenation of para-chloronitrobenzene. Catal. Sci. Technol. 2017, 7, 1581–1589. [Google Scholar] [CrossRef]
- Zhang, J.F.; Xu, Y.; Zhang, B. Facile synthesis of 3D Pd-P nanoparticle networks with enhanced electrocatalytic performance towards formic acid electrooxidation. Chem. Commun. 2014, 50, 13451–13453. [Google Scholar] [CrossRef]
- Cheng, L.F.; Zhang, Z.H.; Niu, W.X.; Xu, G.B.; Zhu, L.D. Carbon-supported Pd nanocatalyst modified by non-metal phosphorus for the oxygen reduction reaction. J. Power Sources 2008, 182, 91–94. [Google Scholar] [CrossRef]
- Habas, S.E.; Baddour, F.G.; Ruddy, D.A.; Nash, C.P.; Wang, J.; Pan, M.; Hensley, J.E.; Schaidle, J.A. A facile molecular precursor route to metal phosphide nanoparticles and their evaluation as hydrodeoxygenation catalysts. Chem. Mater. 2015, 27, 7580–7592. [Google Scholar] [CrossRef]
- Liu, Y.N.; McCue, A.J.; Miao, C.L.; Feng, J.T.; Li, D.Q.; Anderson, J.A. Palladium phosphide nanoparticles as highly selective catalysts for the selective hydrogenation of acetylene. J. Catal. 2018, 364, 406–414. [Google Scholar] [CrossRef]
- Zhang, L.L.; Tang, Y.W.; Bao, J.C.; Lu, T.H.; Li, C. A carbon-supported Pd-P catalyst as the anodic catalyst in a direct formic acid fuel cell. J. Power Sources 2006, 162, 177–179. [Google Scholar] [CrossRef]
- Yang, G.X.; Chen, Y.; Zhou, Y.M.; Tang, Y.W.; Lu, T.H. Preparation of carbon supported Pd–P catalyst with high content of element phosphorus and its electrocatalytic performance for formic acid oxidation. Electrochem. Commun. 2010, 12, 492–495. [Google Scholar] [CrossRef]
- Belykh, L.B.; Skripov, N.I.; Stepanova, T.P.; Akimov, V.V.; Tauson, V.L.; Schmidt, F.K. The catalytic properties of Pd nanoparticles modified by phosphorus in liquid-phase hydrogenation of o-chloronitrobenzene. Curr. Nanosci. 2015, 11, 175–185. [Google Scholar] [CrossRef]
- Yao, N.; Chen, J.X.; Zhang, J.X.; Zhang, J.Y. Influence of support calcination temperature on properties of Ni/TiO2 for catalytic hydrogenation of o-chloronitrobenzene to o-chloroaniline. Catal. Commun. 2008, 9, 1510–1516. [Google Scholar] [CrossRef]
- Bertero, N.M.; Trasarti, A.F.; Apesteguía, C.R.; Marchi, A.J. Solvent effect in the liquid-phase hydrogenation of acetophenone over Ni/SiO2: A comprehensive study of the phenomenon. Appl. Catal. A 2011, 394, 228–238. [Google Scholar] [CrossRef]
- Han, X.X.; Zhou, R.X.; Lai, G.H.; Zheng, X.M. Influence of support and transition metal (Cr, Mn, Fe, Co, Ni and Cu) on the hydrogenation of p-chloronitrobenzene over supported platinum catalysts. Catal. Today 2004, 93–95, 433–437. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Jayasri, V.; Basha, P.M. Vapor phase hydrogenation of o-chloronitrobenzene (o-CNB) over alumina supported palladium catalyst—A kinetic study. React. Kinet. Catal. Lett. 2007, 91, 291–298. [Google Scholar] [CrossRef]
- Tamura, M.; Tokonami, K.; Nakagawa, Y.; Tomishige, K. Selective hydrogenation of crotonaldehyde to crotyl alcohol over metal oxide modified Ir catalysts and mechanistic insight. ACS Catal. 2016, 6, 3600–3609. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Devassy, B.M.; Lefebvre, F.; Halligudi, S.B. Zirconia-supported 12-tungstophosphoric acid as a solid catalyst for the synthesis of linear alkyl benzenes. J. Catal. 2005, 231, 1–10. [Google Scholar] [CrossRef]



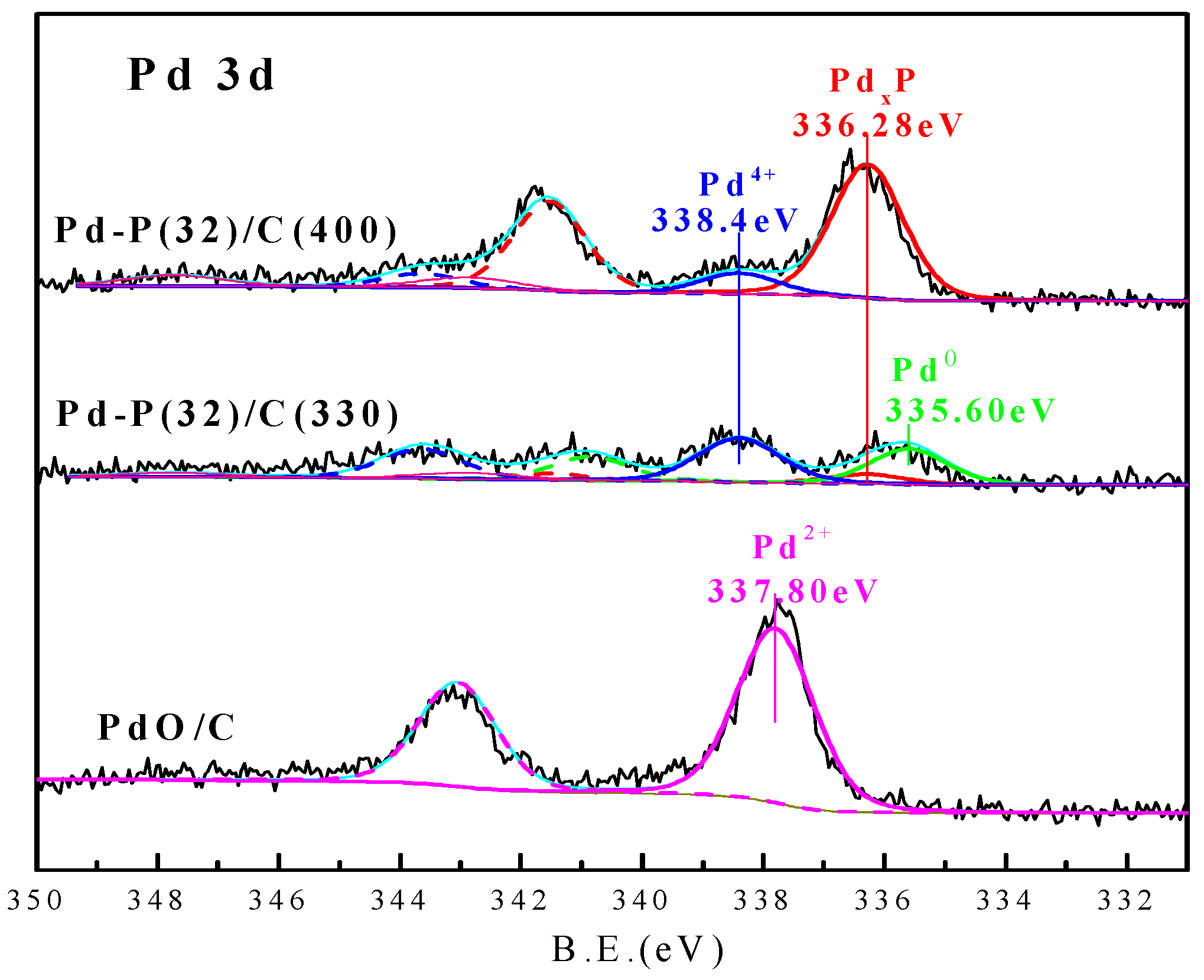


| Catalyst | Pd Species | Pd 3d5/2 (eV) | Percentage (%) | P Species | P 2p3/2 (eV) | Percentage (%) |
|---|---|---|---|---|---|---|
| Pd-P(32)/C(400) | PdxP | 336.28 | 85.71 | PdxP | 130.1 | 10.27 |
| Pd4+ | 338.40 | 14.29 | PO43− | 133.6 | 89.73 | |
| Pd-P(32)/C(330) | Pd0 | 335.60 | 38.64 | / | / | / |
| PdxP | 336.28 | 10.23 | PdxP | 130.1 | 10.20 | |
| Pd4+ | 338.40 | 51.13 | PO43− | 133.9 | 89.80 | |
| PdO/C | Pd2+ | 337.80 | 100 | / | / | / |
| Catalyst | Solvents | Temperature °C | Pressure MPa | Reaction Time (min) | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|---|---|---|
| p-CAN | AN | ||||||
| Pd-P(0)/C(400) | / | 90 | 1.6 | 186 | 100 | 82.38 | 17.62 |
| Pd-P(4)/C(400) | / | 90 | 1.6 | 168 | 100 | 97.79 | 2.21 |
| Pd-P(8)/C(400) | / | 90 | 1.6 | 318 | 100 | 99.35 | 0.65 |
| Pd-P(16)/C(400) | / | 90 | 1.6 | 158 | 100 | 97.63 | 2.37 |
| Pd-P(24)/C(400) | / | 90 | 1.6 | 156 | 100 | 97.51 | 2.49 |
| Pd-P(32)/C(400) | / | 90 | 1.6 | 150 | 100 | 97.96 | 2.04 |
| Pd-P(32)/C(400) | / | 85 | 1.5 | 145 | 100 | 99.69 | 0.31 |
| Pd-P(32)/C(400) | / | 100 | 1.5 | 136 | 100 | 96.88 | 3.12 |
| Pd-P(32)/C(400) | / | 85 | 1.0 | 178 | 100 | 99.78 | 0.22 |
| Pd-P(32)/C(400) | / | 85 | 2.0 | 119 | 100 | 99.37 | 0.63 |
| Pd-P(32)/C(400) | 150 mL of methanol | 85 | 1.5 | 140 | 100 | 95.12 | 4.82 |
| Pd-P(0)/C(400) + Na3PO4 | / | 85 | 1.5 | 267 | 100 | 83.69 | 16.31 |
| Recycling Time | Catalyst Addition | Reaction Time | Conversion | Selectivity (%) | |
|---|---|---|---|---|---|
| (g) | (min) | (%) | p-CAN | AN | |
| 1 | 0.5 | 148 | 100 | 99.70 | 0.30 |
| 2 | 0.025 | 150 | 100 | 99.66 | 0.34 |
| 3 | 0 | 146 | 100 | 99.68 | 0.32 |
| 4 | 0 | 152 | 100 | 99.70 | 0.30 |
| 5 | 0 | 145 | 100 | 99.71 | 0.29 |
| 6 | 0 | 146 | 100 | 99.69 | 0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Zhu, Q.; Zhang, X.; Liu, Q.; Nie, J.; Feng, F.; Zhang, Q.; Ma, L.; Han, W.; Li, X. Preparation and Catalytic Performance of Metal-Rich Pd Phosphides for the Solvent-Free Selective Hydrogenation of Chloronitrobenzene. Catalysts 2019, 9, 177. https://doi.org/10.3390/catal9020177
Lu C, Zhu Q, Zhang X, Liu Q, Nie J, Feng F, Zhang Q, Ma L, Han W, Li X. Preparation and Catalytic Performance of Metal-Rich Pd Phosphides for the Solvent-Free Selective Hydrogenation of Chloronitrobenzene. Catalysts. 2019; 9(2):177. https://doi.org/10.3390/catal9020177
Chicago/Turabian StyleLu, Chunshan, Qianwen Zhu, Xuejie Zhang, Qiangqiang Liu, Juanjuan Nie, Feng Feng, Qunfeng Zhang, Lei Ma, Wenfeng Han, and Xiaonian Li. 2019. "Preparation and Catalytic Performance of Metal-Rich Pd Phosphides for the Solvent-Free Selective Hydrogenation of Chloronitrobenzene" Catalysts 9, no. 2: 177. https://doi.org/10.3390/catal9020177
APA StyleLu, C., Zhu, Q., Zhang, X., Liu, Q., Nie, J., Feng, F., Zhang, Q., Ma, L., Han, W., & Li, X. (2019). Preparation and Catalytic Performance of Metal-Rich Pd Phosphides for the Solvent-Free Selective Hydrogenation of Chloronitrobenzene. Catalysts, 9(2), 177. https://doi.org/10.3390/catal9020177