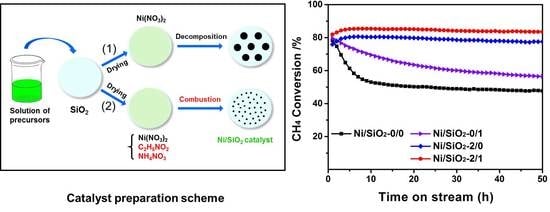
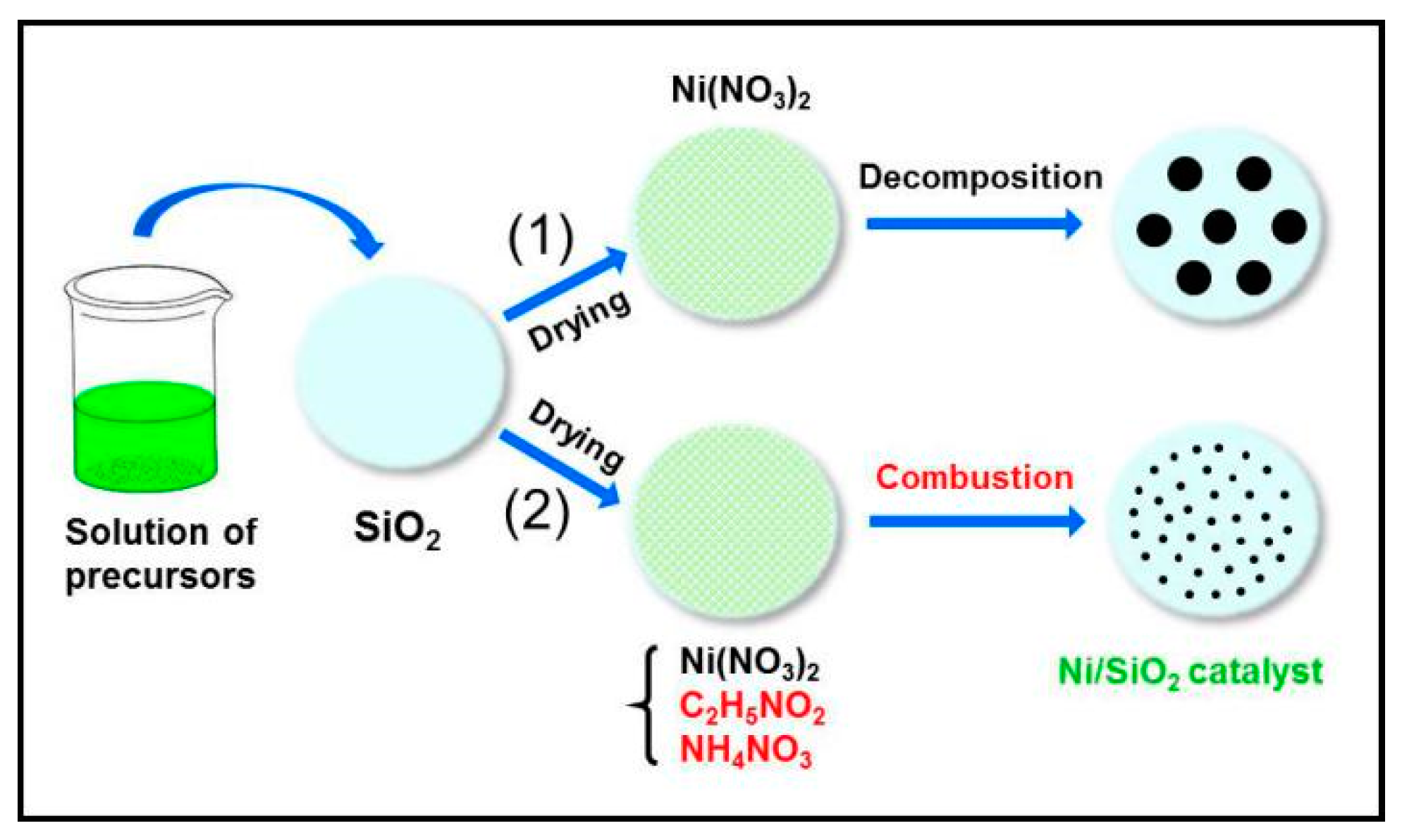
A Facile Fabrication of Supported Ni/SiO2 Catalysts for Dry Reforming of Methane with Remarkably Enhanced Catalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalyst Sample
2.2. Activity Evaluation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Caballero, A.; Pérez, P.J. Methane as Raw Material in Synthetic Chemistry: The Final Frontier. Chem. Soc. Rev. 2013, 42, 8809–8820. [Google Scholar] [CrossRef] [PubMed]
- Schwach, P.; Pan, X.L.; Bao, X.H. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef] [PubMed]
- Pruett, R.L. Synthesis Gas: A Raw Material for Industrial Chemicals. Science 1981, 211, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Reubroycharoen, P.; Yamagami, T.; Vitidsant, T.; Yoneyama, Y.; Ito, M.; Tsubaki, N. Continuous Low-Temperature Methanol Synthesis from Syngas Using Alcohol Promoters. Energy Fuels 2003, 17, 817–821. [Google Scholar] [CrossRef]
- Xiong, H.F.; Jewell, L.L.; Coville, N.J. Shaped Carbons as Supports for the Catalytic Conversion of Syngas to Clean Fuels. ACS Catal. 2015, 5, 2640–2658. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Kang, J.C.; Wang, Y. Development of Novel Catalysts for Fischer-Tropsch Synthesis: Tuning the Product Selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, B.; Li, W.P.; Zhang, M.; Li, Z.J.; Liu, X.H. Two-Dimensional Graphene-Directed Formation of Cylindrical Iron Carbide Nanocapsules for Fischer–Tropsch Synthesis. Catal. Sci. Technol. 2017, 7, 4609–4621. [Google Scholar] [CrossRef]
- Zheng, J.; Cai, J.; Jiang, F.; Xu, Y.B.; Liu, X.H. Investigation of the Highly Tunable Selectivity to Linear α-Olefins in Fischer-Tropsch Synthesis over Silica-Supported Co and CoMn Catalysts by Carburization–Reduction Pretreatment. Catal. Sci. Technol. 2017, 7, 4736–4755. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, M.; Liu, B.; Xu, Y.B.; Liu, X.H. Insights into the Influence of Support and Potassium or Sulfur Promoter on Iron-based Fischer-Tropsch Synthesis: Understanding the Control of Catalytic Activity, Selectivity to Lower Olefins, and Catalyst Deactivation. Catal. Sci. Technol. 2017, 7, 1245–1265. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, F.; Liu, X.H. Exploring Pretreatment Effects in Co/SiO2 Fischer-Tropsch Catalysts: Different Oxidizing Gases Applied to Oxidation-Reduction Process. Appl. Catal. B Environ. 2017, 210, 1–13. [Google Scholar] [CrossRef]
- Liu, X.H.; Tokunaga, M. Controllable Fischer-Tropsch Synthesis by in Situ-Produced 1-Olefins. ChemCatChem 2010, 2, 1569–1572. [Google Scholar] [CrossRef]
- Liu, X.H.; Linghu, W.S.; Li, X.H.; Asami, K.; Fujimoto, K. Effects of Solvent on Fischer-Tropsch Synthesis. Appl. Catal. A Gen. 2006, 303, 251–257. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.R.H. Natural Gas Reforming and CO2 Mitigation. Catal. Today 2005, 100, 151–158. [Google Scholar] [CrossRef]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. On the Contribution of X-ray Absorption Spectroscopy to Explore Structure and Activity Relations of Pt/ZrO2 Catalysts for CO2/CH4 Reforming. Top. Catal. 2000, 10, 295–305. [Google Scholar] [CrossRef]
- Galuszka, J.; Pandey, R.N.; Ahmed, S. Methane Conversion to Syngas in a Palladium Membrane Reactor. Catal. Today 1998, 46, 83–89. [Google Scholar] [CrossRef]
- Vooradi, R.; Bertran, M.O.; Frauzem, R.; Anne, S.B.; Gani, R. Sustainable Chemical Processing and Energy-Carbon Dioxide Management: Review of Challenges and Opportunities. Chem. Eng. Res. Des. 2018, 131, 440–464. [Google Scholar] [CrossRef]
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Ha, K.S.; Bae, J.W.; Woo, K.J.; Jun, K.W. Efficient Utilization of Greenhouse Gas in a Gas-to-Liquids Process Combined with Carbon Dioxide Reforming of Methane. Environ. Sci. Technol. 2010, 44, 1412–1417. [Google Scholar] [CrossRef]
- De Vasconcelos, B.R.; Minh, D.P.; Lyczko, N.T.; Phan, S.; Sharrock, P.; Nzihou, A. Upgrading Greenhouse Gases (Methane and Carbon Dioxide) into Syngas using Nickel-Based Catalysts. Fuel 2018, 226, 195–203. [Google Scholar] [CrossRef]
- Ginsburg, J.M.; Pina, J.; El Solh, T.; De Lasa, H.I. Coke Formation over a Nickel Catalyst under Methane Dry Reforming Conditions: Thermodynamic and Kinetic Models. Ind. Eng. Chem. Res. 2005, 44, 4846–4854. [Google Scholar] [CrossRef]
- Schulz, L.A.; Kahle, L.C.S.; Delgado, K.H.; Schunk, S.A.; Jentys, A.; Deutschmann, O.; Lercher, J.A. On the Coke Deposition in Dry Reforming of Methane at Elevated Pressures. Appl. Catal. A Gen. 2015, 504, 599–607. [Google Scholar] [CrossRef]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. Deactivation and Coke Accumulation during CO2/CH4 Reforming over Pt Catalysts. J. Catal. 1999, 183, 336–343. [Google Scholar] [CrossRef]
- Mette, K.; Kühl, S.; Tarasov, A.; Willinger, M.G.; Kröhnert, J.; Wrabetz, S.; Trunschke, A.; Scherzer, M.; Girgsdies, F.; Düdder, H.; et al. High-Temperature Stable Ni Nanoparticles for the Dry Reforming of Methane. ACS Catal. 2016, 6, 7238–7248. [Google Scholar] [CrossRef]
- Pawar, V.; Ray, D.; Subrahmanyam, C.; Janardhanan, V.M. Study of Short-Term Catalyst Deactivation due to Carbon Deposition during Biogas Dry Reforming on Supported Ni Catalyst. Energy Fuels 2015, 29, 8047–8052. [Google Scholar] [CrossRef]
- Li, S.R.; Gong, J.L. Strategies for Improving the Performance and Stability of Ni-Based Catalysts for Reforming Reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, H.A.; Pegios, N.; Palkovits, R.; Simeonov, K.; Vayssilov, G.N. Elucidation of the Higher Coking Resistance of Small Versus Large Nickel Nanoparticles in Methane Dry Reforming via Computational Modeling. Catal. Sci. Technol. 2017, 7, 3339–3347. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of Supports and Ni Crystal Size on Carbon Formation and Sintering during Steam Methane Reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Gonzalez-Delacruz, V.M.; Pereniguez, R.; Ternero, F.; Holgado, J.P.; Caballero, A. Modifying the Size of Nickel Metallic Particles by H2/CO Treatment in Ni/ZrO2 Methane Dry Reforming Catalysts. ACS Catal. 2011, 1, 82–88. [Google Scholar] [CrossRef]
- Baudouin, D.; Rodemerck, U.; Krumeich, F.; de Mallmann, A.; Szeto, K.C.; Ménard, H.; Veyre, L.; Candy, J.P.; Webb, P.B.; Thieuleux, C.; et al. Particle size Effect in the Low Temperature Reforming of Methane by Carbon Dioxide on Silica-Supported Ni Nanoparticles. J. Catal. 2013, 297, 27–34. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Fedorov, A.; Chen, P.; Müller, C.R.; Copéret, C. Molecularly-Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Utilization. J. Am. Chem. Soc. 2017, 139, 6919–6927. [Google Scholar] [CrossRef] [PubMed]
- Abba, M.O.; Gonzalez-DelaCruz, V.M.; Colón, G.; Sebti, S.; Caballero, A. In Situ XAS Study of an Improved Natural Phosphate Catalyst for Hydrogen Production by Reforming of Methane. Appl. Catal. B Environ. 2014, 150–151, 459–465. [Google Scholar] [CrossRef]
- Tian, H.; Li, X.Y.; Zeng, L.; Gong, J.L. Recent Advances on the Design of Group VIII Base-Metal Catalysts with Encapsulated Structures. ACS Catal. 2015, 5, 4959–4977. [Google Scholar] [CrossRef]
- Gould, T.D.; Izar, A.; Weimer, A.W.; Falconer, J.L.; Medlin, J.W. Stabilizing Ni Catalysts by Molecular Layer Deposition for Harsh, Dry Reforming Conditions. ACS Catal. 2014, 4, 2714–2717. [Google Scholar] [CrossRef]
- Tomishige, K.; Yamazaki, O.; Chen, Y.G.; Yokoyama, K.; Li, X.H.; Fujimoto, K. Development of Ultra-Stable Ni Catalysts for CO2 Reforming of Methane. Catal. Today 1998, 45, 35–39. [Google Scholar] [CrossRef]
- Chen, Y.G.; Tomishige, K.; Yokoyama, K.; Fujimoto, K. Promoting Effect of Pt, Pd and Rh Noble Metals to the Ni0.03Mg0.97O Solid Solution Catalysts for the Reforming of CH4 with CO2. Appl. Catal. A Gen. 1997, 165, 335–347. [Google Scholar] [CrossRef]
- Li, Z.W.; Mo, L.Y.; Kathiraser, Y.; Kawi, S. Yolk–Satellite–Shell Structured Ni–Yolk@Ni@SiO2 Nanocomposite: Superb Catalyst toward Methane CO2 Reforming Reaction. ACS Catal. 2014, 4, 1526–1536. [Google Scholar] [CrossRef]
- Huang, Q.; Fang, X.Z.; Cheng, Q.Z.; Li, Q.; Xu, L.J.; Xu, X.L.; Liu, W.M.; Gao, Z.X.; Zhou, W.F.; Wang, X. Synthesis of Highly Active and Stable Ni@Al2O3 Embedded Catalyst for Methane Dry Reforming: On the Confinement Effects of Al2O3 Shells for Ni Nanoparticles. ChemCatChem 2017, 9, 3563–3571. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Chen, Y.S.; Rouvimov, S.; Li, P.; Li, X.; Dong, S.; Liu, X.Y.; Furdyna, J.K.; Orlov, A.; Bernstein, G.H.; et al. Ultrasmall α-Fe2O3 Superparamagnetic Nanoparticles with High Magnetization Prepared by Template-Assisted Combustion Process. J. Phys. Chem. C 2014, 118, 16264–16271. [Google Scholar] [CrossRef]
- Gao, X.Y.; Hidajat, K.; Sawi, S. Facile synthesis of Ni/SiO2 catalyst by sequential hydrogen/air treatment: A superior anti-coking catalyst for dry reforming of methane. J. CO2 Util. 2016, 15, 146–153. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Lin, Q.; Liu, B.; Jiang, F.; Xu, Y.; Liu, X. A Facile Fabrication of Supported Ni/SiO2 Catalysts for Dry Reforming of Methane with Remarkably Enhanced Catalytic Performance. Catalysts 2019, 9, 183. https://doi.org/10.3390/catal9020183
Xu Y, Lin Q, Liu B, Jiang F, Xu Y, Liu X. A Facile Fabrication of Supported Ni/SiO2 Catalysts for Dry Reforming of Methane with Remarkably Enhanced Catalytic Performance. Catalysts. 2019; 9(2):183. https://doi.org/10.3390/catal9020183
Chicago/Turabian StyleXu, Yan, Qiang Lin, Bing Liu, Feng Jiang, Yuebing Xu, and Xiaohao Liu. 2019. "A Facile Fabrication of Supported Ni/SiO2 Catalysts for Dry Reforming of Methane with Remarkably Enhanced Catalytic Performance" Catalysts 9, no. 2: 183. https://doi.org/10.3390/catal9020183
APA StyleXu, Y., Lin, Q., Liu, B., Jiang, F., Xu, Y., & Liu, X. (2019). A Facile Fabrication of Supported Ni/SiO2 Catalysts for Dry Reforming of Methane with Remarkably Enhanced Catalytic Performance. Catalysts, 9(2), 183. https://doi.org/10.3390/catal9020183