Isolation of a Bacillus Aryabhattai Strain for the Resolution of (R, S)-Ethyl Indoline-2-Carboxylate to Produce (S)-Indoline-2-Carboxylic Acid
Abstract
:1. Introduction
2. Results
2.1. The Identification of Microorganisms Hydrolizing (R, S)-Ethyl Indoline-2-Carboxylate
2.2. Bacillus Aryabhattai’s Growth Curve
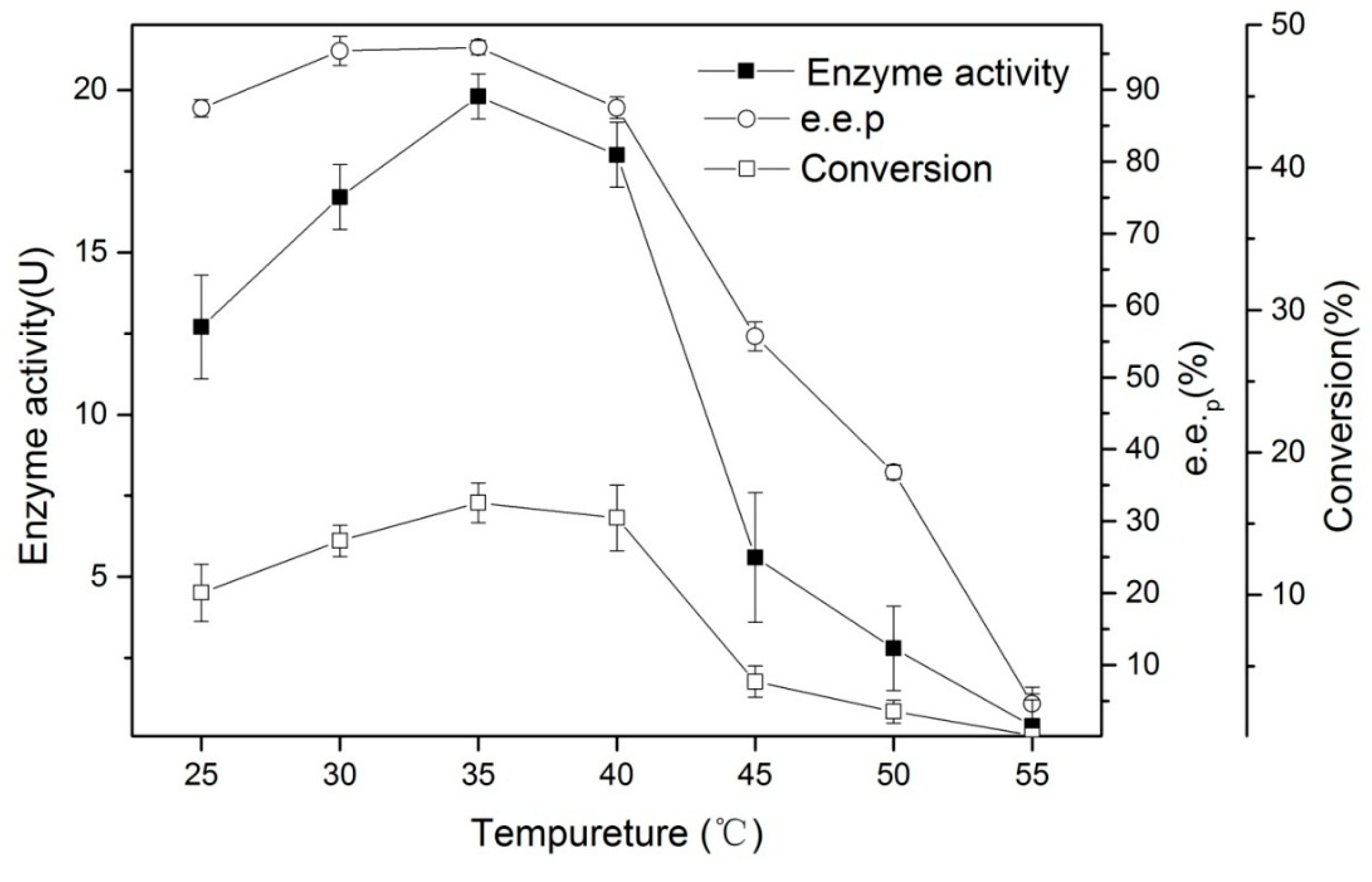
2.3. The Effects of pH, Temperature, Substrate Concentration, Co-Solvent, and Co-Solvent Addition on the Resolution of (R, S)-Ethyl Indoline-2-Carboxylate
2.4. The Time Course of the Hydrolysis Reaction
3. Discussion
4. Materials and Methods
4.1. Sources of Chemicals and Enzymes
4.2. The Detection of (R, S)-Ethyl Indoline-2-Carboxylate and Its Products
4.3. Activity Strains’ Enantioselective Hydrolysis Enrichment and Separation
4.4. Isolation and Identification of the Strains
4.5. The Production of Lipase in Bacillus Aryabhattai
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lebreton, L.; Dumas, C.; Massardier, C. Novel compounds of indoline. F.R. Patent 2,886,293, 12 January 2006. [Google Scholar]
- Kujala, T.; Loponen, J.; Pihlaja, K. Betalains and phenolics in red beetroot (beta vulgaris) peel extracts: Extraction and characterisation. Z. Naturforsch. Sect. C J. Biosci. 2001, 56, 343–348. [Google Scholar] [CrossRef]
- Thuillez, C.; Richard, C.; Loueslati, H.; Auzepy, P.; Giudicelli, J.F. Systemic and regional hemodynamic effects of perindopril in congestive heart failure. J. Cardiovasc. Pharmacol. 1990, 15, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F. Angiotensin-converting enzyme inhibitor and spironolactone combination therapy: New objectives in congestive heart failure treatment. Am. J. Cardiol. 1993, 71, A34–A39. [Google Scholar] [CrossRef]
- Vincent, M.; Baliarda, J.; Marchand, B.; Remond, G. Alpha-methyl Benzyl Amine Salt of Indoline-2-Carboxylic Acid. U.S. Patent 4,954,640, 4 September 1990. [Google Scholar]
- Viswanathan, R.; Prabhakaran, E.N.; Plotkin, M.A.; Johnston, J.N. Free radical-mediated aryl amination and its use in a convergent [3 + 2] strategy for enantioselective indoline alpha-amino acid synthesis. J. Am. Chem. Soc. 2003, 125, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Masanori, A.; Shigaki, H.; Hidetoshi, K.; Yoshio, N.; Hideyuki, T.; Kenji, T. Process for Preparing Optically Active Indoline-2-Carboxylic Acid. U.S. Patent 4,898,822, 6 February 1990. [Google Scholar]
- De Lange, B.; Hyett, D.J.; Maas, P.J.D.; Mink, D.; Van Assema, F.B.J.; Sereinig, N. Asymmetric synthesis of (s)-2-indolinecarboxylic acid by combining biocatalysis and homogeneous catalysis. Chemcatchem 2011, 3, 289–292. [Google Scholar] [CrossRef]
- Huang, J.; Yan, R.; Shi, H.; Wang, P.; He, J. The characteristic and kinetic studies on the enantioselective hydrolysis of (r, s)-α-ethyl-2-oxo-1-pyrrolidine acetic acid ethyl ester catalyzed by tsukamurella tyrosinosolvens, e105-derived hydrolase. Asia-Pac. J. Chem. Eng. 2015, 9, 941–949. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. (Int. Ed.) 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Zaks, A.; Klibanov, A. Enzymatic catalysis in organic media at 100 degrees C. Science 1984, 224, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yun, Z.; Sun, A.; Hu, Y. Enantio-selective preparation of (S)-1-phenylethanol by a novel marine GDSL lipase MT6 with reverse stereo-selectivity. Chin. J. Catal. 2016, 37, 1966–1974. [Google Scholar] [CrossRef]
- Hudson, C.; Robertson, A. The synthesis and chemistry of DL-indoline-2-carboxylic acid. Aust. J. Chem. 1967, 20, 1935. [Google Scholar] [CrossRef]
- Youn, I.K.; Yon, G.H.; Pak, C.S. Magnesium-methanol as a simple convenient reducing agent for α,β-unsaturated esters. Cheminform 1986, 27, 2409–2410. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Sengupta, S. α-metallation of tetrahydroquinoline and indoline via their lithium carbamates: A versatile one-pot procedure. J. Chem. Soc. Perkin Trans. 1989, 1, 17–19. [Google Scholar] [CrossRef]
- Wang, Z.X.; Raheem, M.A.; Weeratunga, G. New Processes for the Preparation of Optically Pure Indoline-2-Carboxylic Acid and n-Acetyl-Indoline-2-Carboxylic Acid. Patent WO/2006/053440, 26 May 2006. [Google Scholar]
- Liu, J.Q.; Chen, X.Z.; Ji, B.; Zhao, B.T. Transformation of l-phenylalanine to (s)-indoline-2-carboxylic acid without group-protection. Res. Chem. Intermed. 2013, 39, 1143–1152. [Google Scholar] [CrossRef]
- Cho, N.R.; Lim, J.H.; Kim, J.K. Method for Preparing (s)-Indoline-2-Carboxylic Acid and (s)-Indoline-2-Carboxylic Acid Methyl Ester Using Hydrolytic Enzyme. U.S. Patent US20,070,077,632A1, 5 April 2007. [Google Scholar]
- Hsiao, H.T.; Lin, S.Y.; Tsai, S.W. Quantitative insights and improvements of enzyme activity and stereoselectivity for calb-catalyzed alcoholysis in two-step desymmetrization. J. Mol. Catal. B Enzym. 2016, 127, 82–88. [Google Scholar] [CrossRef]
- Wilson, K.H.; Blitchington, R.B. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 1996, 62, 2273–2278. [Google Scholar] [PubMed]








| Number | e.e.p (%) |
|---|---|
| C26-1 | 92 |
| CM6 | 85 |
| 206 O | 80 |
| S26 | 77 |
| Pt6 | 72 |
| X210 | 43 |
| 208 O | 10 |
| 155-2 | 1 |
| 198-2 | 15(R) |
| X195 | 24(R) |
| C046 | 28(R) |
| X055 | 39(R) |
| Name of Enzyme | e.e.p (%) |
|---|---|
| Novozym 435 | 2(R) |
| TLIM | 3(R) |
| SKH | 3 |
| YM | 34 |
| LBK | 45 |
| XS | 34 |
| Organic Solvent | e.e.p (%) | Enzyme Activity (U) |
|---|---|---|
| Dichloromethane | 0 | 0 |
| Acetone | 88 | 7 |
| Ethyl acetate | 0 | 0 |
| Methanol | 88 | 18 |
| Acetonitrile | 89 | 9 |
| Tetrahydrofuran | 96(R) | 2 |
| Hexane | 97 | 27 |
| Control | 94 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, J.; Chen, C.; Wu, S. Isolation of a Bacillus Aryabhattai Strain for the Resolution of (R, S)-Ethyl Indoline-2-Carboxylate to Produce (S)-Indoline-2-Carboxylic Acid. Catalysts 2019, 9, 206. https://doi.org/10.3390/catal9020206
Zhang Y, Chen J, Chen C, Wu S. Isolation of a Bacillus Aryabhattai Strain for the Resolution of (R, S)-Ethyl Indoline-2-Carboxylate to Produce (S)-Indoline-2-Carboxylic Acid. Catalysts. 2019; 9(2):206. https://doi.org/10.3390/catal9020206
Chicago/Turabian StyleZhang, Yinjun, Jialin Chen, Changsheng Chen, and Shijin Wu. 2019. "Isolation of a Bacillus Aryabhattai Strain for the Resolution of (R, S)-Ethyl Indoline-2-Carboxylate to Produce (S)-Indoline-2-Carboxylic Acid" Catalysts 9, no. 2: 206. https://doi.org/10.3390/catal9020206
APA StyleZhang, Y., Chen, J., Chen, C., & Wu, S. (2019). Isolation of a Bacillus Aryabhattai Strain for the Resolution of (R, S)-Ethyl Indoline-2-Carboxylate to Produce (S)-Indoline-2-Carboxylic Acid. Catalysts, 9(2), 206. https://doi.org/10.3390/catal9020206





