K-Modulated Co Nanoparticles Trapped in La-Ga-O as Superior Catalysts for Higher Alcohols Synthesis from Syngas
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Powder Diffraction (XRPD)
2.2. Temperature-Programmed Reduction (TPR)
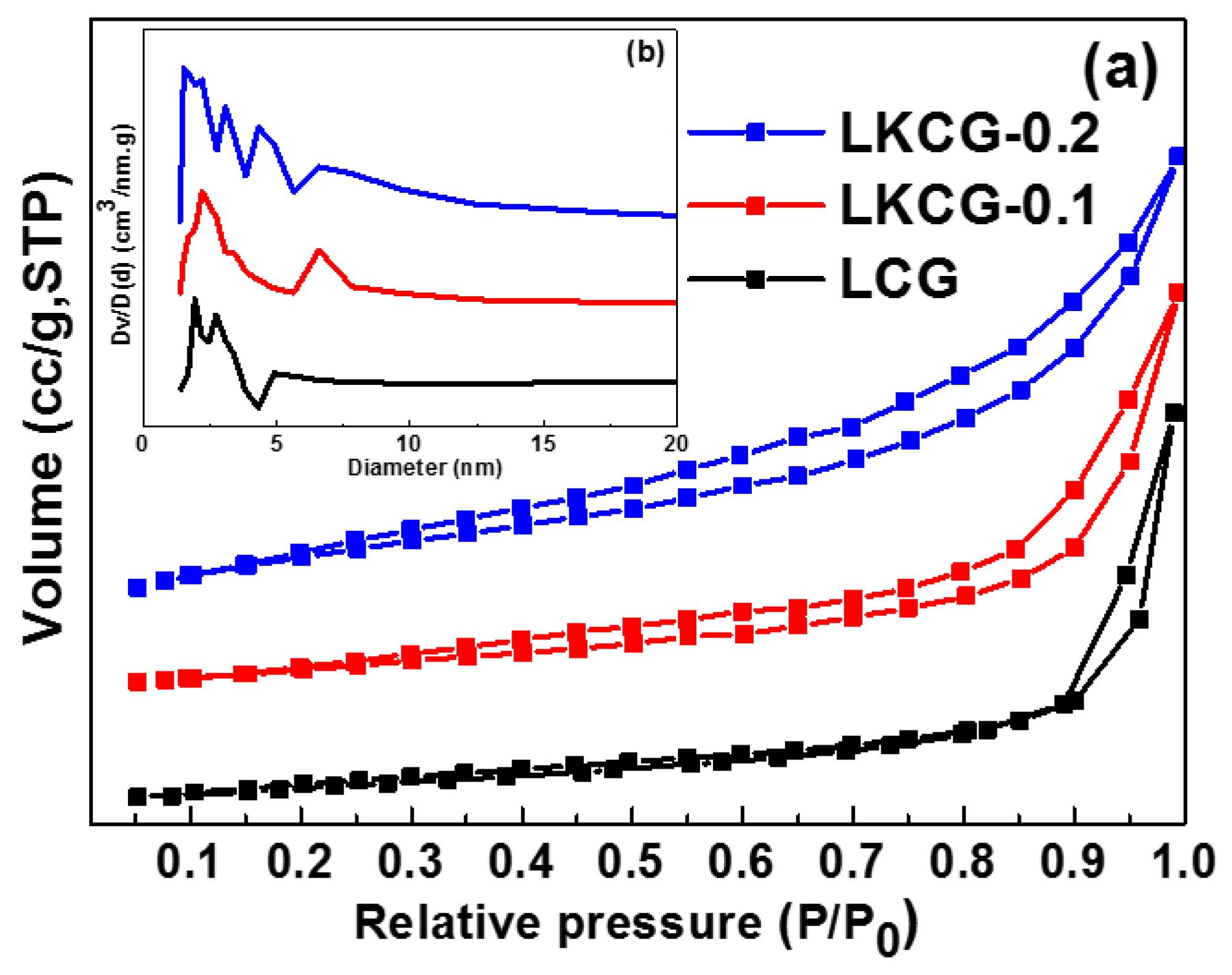
2.3. N2 Adsorption and Desorption Curves
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Transmission Electron Microscopy (TEM)
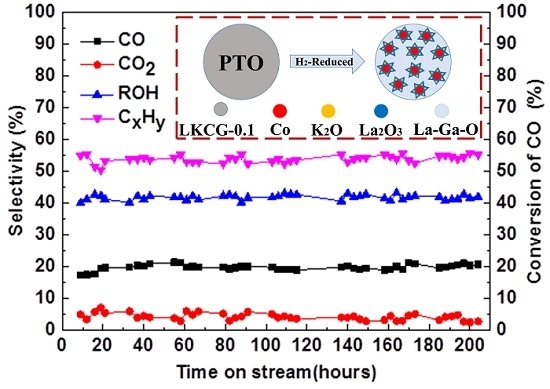
2.6. CO Hydrogenation Performance
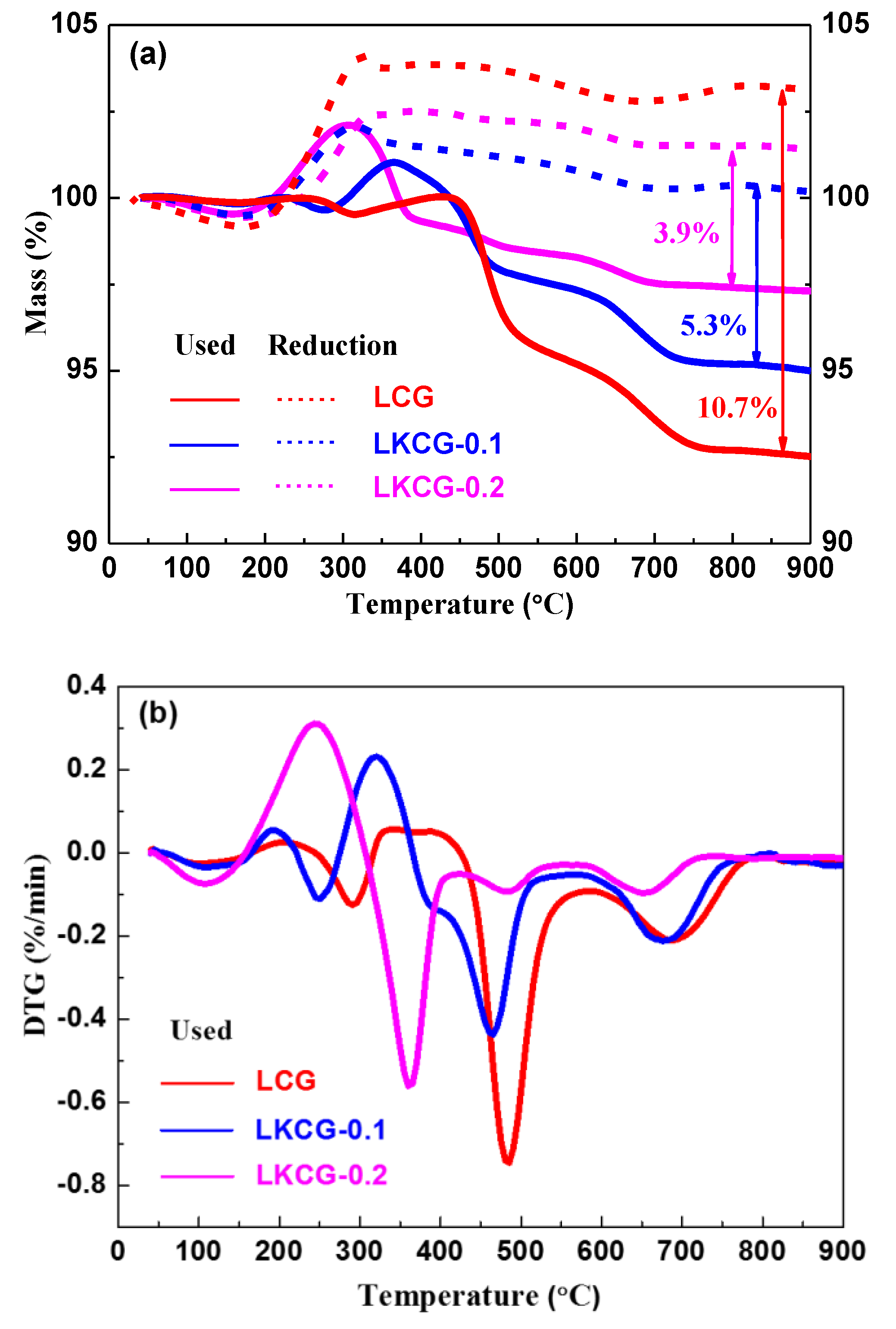
2.7. Thermo-Gravimetry (TG)
3. Materials and Methods
3.1. Material
3.2. Catalysts’ Synthesis
3.3. Catalysts’ Characterization
3.4. Catalysts’ Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, J.E.; Burch, R.; Golunski, S.E. Synthesis of higher alcohols over copper/cobalt catalysts. Influence of preparative procedures on the activity and selectivity of Cu/Co/Zn/Al mixed oxide catalysts. Appl. Catal. 1989, 53, 279–297. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Alcohols as alternative fuels: An overview. Appl. Catal. A-Gen. 2011, 404, 1–11. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.G. Advances in catalytic synthesis and utilization of higher alcohols. Catal. Today 2000, 55, 233–245. [Google Scholar] [CrossRef]
- Yu, S.; Tao, J. Economic, energy and environmental evaluations of biomass-based fuel ethanol projects based on life cycle assessment and simulation. Appl. Energy 2009, 86, S178–S188. [Google Scholar] [CrossRef]
- Subramanian, N.D.; Gao, J.; Mo, X.; Goodwin, J.G., Jr.; Torres, W.; Spivey, J.J. La and/or V oxide promoted Rh/SiO2 catalysts: Effect of temperature, H2/CO ratio, space velocity, and pressure on ethanol selectivity from syngas. J. Catal. 2010, 272, 204–209. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Shekhawat, D.; Poston, J.A.; Spivey, J.J. Synthesis, characterization, and catalytic activity of Rh-based lanthanum zirconate pyrochlores for higher alcohol synthesis. Catal. Today 2013, 207, 65–73. [Google Scholar] [CrossRef]
- Surisetty, V.; Eswaramoorthi, I.; Dalai, A. Comparative study of higher alcohols synthesis over alumina and activated carbon-supported alkali-modified MoS2 catalysts promoted with group VIII metals. Fuel 2012, 96, 77–84. [Google Scholar] [CrossRef]
- Christensen, J.M.; Duchstein, L.D.L.; Wagner, J.B.; Jensen, P.A.; Temel, B.; Jensen, A.D. Catalytic Conversion of Syngas into Higher Alcohols over Carbide Catalysts. Ind. Eng. Chem. Res. 2012, 51, 4161–4172. [Google Scholar] [CrossRef]
- Bai, Y.; He, D.; Ge, S.; Liu, H.; Liu, J.; Huang, W. Influences of preparation methods of ZrO2 support and treatment conditions of Cu/ZrO2 catalysts on synthesis of methanol via CO hydrogenation. Catal. Today 2010, 149, 111–116. [Google Scholar] [CrossRef]
- Nunan, J.G.; Bogdan, C.E.; Klier, K.; Smith, K.J.; Young, C.W.; Herman, R.G. Methanol and C2 oxygenate synthesis over cesium doped CuZnO and Cu/ZnO/Al2O3 catalysts: A study of selectivity and 13C incorporation patterns. J. Catal. 1988, 113, 410–433. [Google Scholar] [CrossRef]
- Fang, K.; Li, D.; Lin, M.; Xiang, M.; Wei, W.; Sun, Y. A short review of heterogeneous catalytic process for mixed alcohols synthesis via syngas. Catal. Today 2009, 147, 133–138. [Google Scholar] [CrossRef]
- Xiao, K.; Bao, Z.; Qi, X.; Wang, X.; Zhong, L.; Fang, K.; Lin, M.; Sun, Y. Structural evolution of CuFe bimetallic nanoparticles for higher alcohol synthesis. J. Mol. Catal. A-Chem. 2013, 378, 319–325. [Google Scholar] [CrossRef]
- Xiao, K.; Qi, X.; Bao, Z.; Wang, X.; Zhong, L.; Fang, K.; Lin, M.; Sun, Y. CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas: A comparative study. Catal. Sci. Technol. 2013, 3, 1591–1602. [Google Scholar] [CrossRef]
- An, Z.; Ning, X.; He, J. Ga-promoted CO insertion and C–C coupling on Co catalysts for the synthesis of ethanol and higher alcohols from syngas. J. Catal. 2017, 356, 157–164. [Google Scholar] [CrossRef]
- Ning, X.; An, Z.; He, J. Remarkably efficient CoGa catalyst with uniformly dispersed and trapped structure for ethanol and higher alcohol synthesis from syngas. J. Catal. 2016, 340, 236–247. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Li, Y.; Yu, H.; Zhang, F.; Sun, Y.; Fang, H.; Zhang, X.; Liang, X.; Yuan, Y. Effects of gallium as an additive on activated carbon-supported cobalt catalysts for the synthesis of higher alcohols from syngas. Fuel 2018, 230, 194–201. [Google Scholar] [CrossRef]
- Zhao, L.; Wei, Y.; Huang, Y.; Liu, Y. La1−xKxFe0.7Ni0.3O3 catalyst for ethanol steam reforming—The effect of K-doping. Catal. Today 2016, 259, 430–437. [Google Scholar] [CrossRef]
- Morales, M.; Segarra, M. Steam reforming and oxidative steam reforming of ethanol over La0.6Sr0.4CoO3−δ perovskite as catalyst precursor for hydrogen production. Appl. Catal. A-Gen. 2015, 502, 305–311. [Google Scholar] [CrossRef]
- Lee, M.; Jung, W. Hydrothermal Synthesis of LaCO3OH and Ln3+-doped LaCO3OH Powders under Ambient Pressure and Their Transformation to La2O2CO3 and La2O3. Bull. Korean Chem. Soc. 2013, 34, 3609–3614. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Yang, N.-T.; Zahid, S.; Kawi, S. Oxygen permeation and stability study of La0.6Sr0.4Co0.8Ga0.2O3−δ (LSCG) hollow fiber membrane with exposure to CO2, CH4 and He. J. Membr. Sci. 2013, 427, 240–249. [Google Scholar] [CrossRef]
- Levasseur, B.; Kaliaguine, S. Methanol oxidation on LaBO3 (B=Co, Mn, Fe) perovskite-type catalysts prepared by reactive grinding. Appl. Catal. A-Gen. 2008, 343, 29–38. [Google Scholar] [CrossRef]
- Royer, S.; Bérubé, F.; Kaliaguine, S. Effect of the synthesis conditions on the redox and catalytic properties in oxidation reactions of LaCo1−xFexO3. Appl. Catal. A-Gen. 2005, 282, 273–284. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Deng, W.; Liu, J. Cu-Co bi-metal catalyst prepared by perovskite CuO/LaCoO3 used for higher alcohol synthesis from syngas. J. Energy Chem. 2014, 23, 527–534. [Google Scholar] [CrossRef]
- Chagas, C.A.; Toniolo, F.S.; Magalhães, R.N.; Schmal, M. Alumina-supported LaCoO3 perovskite for selective CO oxidation (SELOX). Int. J. Hydrogen Energy 2012, 37, 5022–5031. [Google Scholar] [CrossRef]
- Song, Z.; Shi, X.; Ning, H.; Liu, G.; Zhong, H.; Liu, Y. Loading clusters composed of nanoparticles on ZrO2 support via a perovskite-type oxide of La0.95Ce0.05Co0.7Cu0.3O3 for ethanol synthesis from syngas and its structure variation with reaction time. Appl. Surf. Sci. 2017, 405, 1–12. [Google Scholar] [CrossRef]
- Zhan, H.; Li, F.; Gao, P.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Methanol synthesis from CO2 hydrogenation over La–M–Cu–Zn–O (M = Y, Ce, Mg, Zr) catalysts derived from perovskite-type precursors. J. Power Sources 2014, 251, 113–121. [Google Scholar] [CrossRef]
- Jena, H.; Govindan, K.; Kutty, T. Novel wet chemical synthesis and ionic transport properties of LaGaO3 and selected doped compositions at elevated temperatures. Mater. Sci. Eng. B 2004, 113, 30–41. [Google Scholar] [CrossRef]
- Majima, T.; Kono, E.; Ogo, S.; Sekine, Y. Pre-reduction and K loading effects on noble metal free Co-system catalyst for water gas shift reaction. Appl. Catal. A-Gen. 2016, 523, 92–96. [Google Scholar] [CrossRef]
- Yang, Q.; Cao, A.; Kang, N.; An, K.; Liu, Z.; Liu, Y. A new catalyst of Co/La2O3-doped La4Ga2O9 for direct ethanol synthesis from syngas. Fuel Process. Technol. 2018, 179, 42–52. [Google Scholar] [CrossRef]
- Boz, I. Higher alcohol synthesis over a K-promoted Co2O3-CuO-ZnO-Al2O3. Catal. Lett. 2003, 87, 187–194. [Google Scholar] [CrossRef]
- Courty, P.; Durand, D.; Freund, E.; Sugeer, A. C1-C6 alcohols from synthesis gas on copper-cobalt catalysts. J. Mol. Catal. 1982, 17, 241–254. [Google Scholar] [CrossRef]
- Sheffer, G.R.; Jacobson, R.A.; King, T.S. Chemical nature of alkali-promoted copper-cobalt-chromium oxide higher alcohol catalysts. J. Catal. 1989, 116, 95–107. [Google Scholar] [CrossRef]
- Sheffer, G.R.; King, T.S. Effect of preparation parameters on the catalytic nature of potassium promoted CuCoCr higher alcohol catalysts. Appl. Catal. 1988, 44, 153–164. [Google Scholar] [CrossRef]
- Wang, J.; Chernavskii, P.A.; Khodakov, A.Y.; Wang, Y. Structure and catalytic performance of alumina-supported copper–cobalt catalysts for carbon monoxide hydrogenation. J. Catal. 2012, 286, 51–61. [Google Scholar] [CrossRef]
- Liu, G.; Niu, T.; Pan, D.; Liu, F.; Liu, Y. Preparation of bimetal Cu–Co nanoparticles supported on meso–macroporous SiO2 and their application to higher alcohols synthesis from syngas. Appl. Catal. A-Gen. 2014, 483, 10–18. [Google Scholar] [CrossRef]
- Tien-Thao, N.; Zahedi-Niaki, M.H.; Alamdari, H.; Kaliaguine, S. Conversion of syngas to higher alcohols over nanosized LaCo0.7Cu0.3O3 perovskite precursors. Appl. Catal. A-Gen. 2007, 326, 152–163. [Google Scholar] [CrossRef]
- Dong, X.; Liang, X.; Li, H.; Lin, G.; Zhang, P.; Zhan, H. Preparation and characterization of carbon nanotube-promoted Co–Cu catalyst for higher alcohol synthesis from syngas. Catal. Today 2009, 147, 158–165. [Google Scholar] [CrossRef]
- Liu, G.; Cui, J.; Luo, R.; Liu, Y.; Huang, X.; Wu, N.; Jin, X.; Chen, H.; Tang, S.; Kim, J.; et al. 2D MoS2 grown on biomass-based hollow carbon fibers for energy storage. Appl. Surf. Sci. 2019, 469, 854–863. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Verykios, X.E. Carbon and Oxygen Reaction Pathways of CO2 Reforming of Methane over Ni-La2O3 and Ni-Al2O3 Catalysts Studied by Isotopic Tracing Techniques. J. Catal. 1999, 187, 85–94. [Google Scholar] [CrossRef]
- Verykios, X.E. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Int. J. Hydrogen Energy 2003, 106, 1045–1063. [Google Scholar] [CrossRef]
- Li, S.; Tang, H.; Gong, D.; Ma, Z.; Liu, Y. Loading Ni/La2O3 on SiO2 for CO methanation from syngas. Catal. Today 2017, 297, 298–307. [Google Scholar] [CrossRef]
- Liu, G.; Geng, Y.; Pan, D.; Zhang, Y.; Niu, T.; Liu, Y. Bi-metal Cu–Co from LaCo1−xCuxO3 perovskite supported on zirconia for the synthesis of higher alcohols. Fuel Process. Technol. 2014, 128, 289–296. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal. Sci. Technol. 2013, 3, 2627–2633. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Z.; Gao, J.; Zheng, X. Production of Syngas via Partial Oxidation and CO2 Reforming of Coke Oven Gas over a Ni Catalyst. Energy Fuels 2008, 22, 1444–1448. [Google Scholar] [CrossRef]






| Catalysts | Experimental Measure a,b | Theoretical Measure | ||
|---|---|---|---|---|
| TL | TH | Co3+ → Co2+ | Co2+ → Co | |
| LCG | 0.064 | 0.132 | 0.065 | 0.130 |
| LKCG-0.1 | 0.067 | 0.133 | 0.068 | 0.135 |
| LKCG-0.2 | 0.069 | 0.136 | 0.070 | 0.140 |
| Catalysts | SBET (m2 g−1) | Pore Size (nm) | VBJH (cm3 g−1) | Crystal Size (nm) a | D Co (%) e | d Co (nm) f | |
|---|---|---|---|---|---|---|---|
| PTO | Co b | ||||||
| LCG | 8.6 | 14.9 | 0.03 | 17.4 | 15.9 | 7.3 b (6.1) c | 13.2 b (15.7) c |
| LKCG-0.1 | 11.7 | 12.6 | 0.04 | 16.2 | 8.3 | 13.7 b (11.4) c (9.9) d | 7.0 b (8.4) c (9.7) d |
| LKCG-0.2 | 15.0 | 9.8 | 0.04 | 13.4 | 7.4 | 15.5 b (12.6) c | 6.2 b (7.6) c |
| Catalysts | La 3d5/2 | Co 2p3/2 | Ga 3d5/2 | ||
|---|---|---|---|---|---|
| LCG | 835.2 | 838.6 | 778.3 | 17.6 | 19.7 |
| LKCG-0.1 | 834.9 | 838.4 | 778.1 | 17.7 | 19.8 |
| Catalysts | Xco a (%) | Sco2 b (%) | SROH c (%) | Selectivity to Hydrocarbon (%) | Distribution of Alcohols (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4+ | C1 | C2 | C3 | C4+ | ||||
| LCG | 4.1 | 1.6 | 27.2 | 45.1 | 10.5 | 10.8 | 4.9 | 36.3 | 45.2 | 1.6 | 16.9 |
| LKCG-0.1 | 13.2 | 5.8 | 43.6 | 24.7 | 9.2 | 11.9 | 4.8 | 30.9 | 58.1 | 3.2 | 7.8 |
| LKCG-0.2 | 11.7 | 6.6 | 40.0 | 26.3 | 11.5 | 11.7 | 3.9 | 27.8 | 59.1 | 3.9 | 9.2 |
| Catalysts | Temperature (°C) | H2/CO a | Pressure (MPa) | GHSV (h−1) | XCO (%) | SROH (%) | EtOH (%) b | C2+OH c (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| LaCo0.7Cu0.3O3 | 300 | 2 | 6.9 | 15000 | 16.0 | 38.1 | 37.0 | 53.8 | [37] |
| Cu-Co/La2O3-SiO2 | 330 | 2 | 3 | 3900 | 32.1 | 39.5 | 47.5 | 66.1 | [36] |
| Cu-Co/Al2O3 | 250 | 2 | 2 | 1800 | 23.2 | 23.3 | - | 79.3 | [35] |
| Co3Cu1-11%CNT | 300 | 2 | 5 | 7000 | 26.5 | 49.8 | - | 69.9 | [38] |
| 15Co-2.5Ga/AC | 220 | 2 | 3 | 4000 | 13.1 | 30.3 | - | 24.5 | [17] |
| LKCG-0.1 | 290 | 2 | 4 | 6000 | 13.2 | 43.6 | 58.1 | 69.1 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Liu, G.; Han, T.; Zhang, Z.; Liu, Y. K-Modulated Co Nanoparticles Trapped in La-Ga-O as Superior Catalysts for Higher Alcohols Synthesis from Syngas. Catalysts 2019, 9, 218. https://doi.org/10.3390/catal9030218
Guo S, Liu G, Han T, Zhang Z, Liu Y. K-Modulated Co Nanoparticles Trapped in La-Ga-O as Superior Catalysts for Higher Alcohols Synthesis from Syngas. Catalysts. 2019; 9(3):218. https://doi.org/10.3390/catal9030218
Chicago/Turabian StyleGuo, Shaoxia, Guilong Liu, Tong Han, Ziyang Zhang, and Yuan Liu. 2019. "K-Modulated Co Nanoparticles Trapped in La-Ga-O as Superior Catalysts for Higher Alcohols Synthesis from Syngas" Catalysts 9, no. 3: 218. https://doi.org/10.3390/catal9030218
APA StyleGuo, S., Liu, G., Han, T., Zhang, Z., & Liu, Y. (2019). K-Modulated Co Nanoparticles Trapped in La-Ga-O as Superior Catalysts for Higher Alcohols Synthesis from Syngas. Catalysts, 9(3), 218. https://doi.org/10.3390/catal9030218