Applicability of V2O5-WO3/TiO2 Catalysts for the SCR Denitrification of Alumina Calcining Flue Gas
Abstract
:1. Introduction
2. Results and Discussion
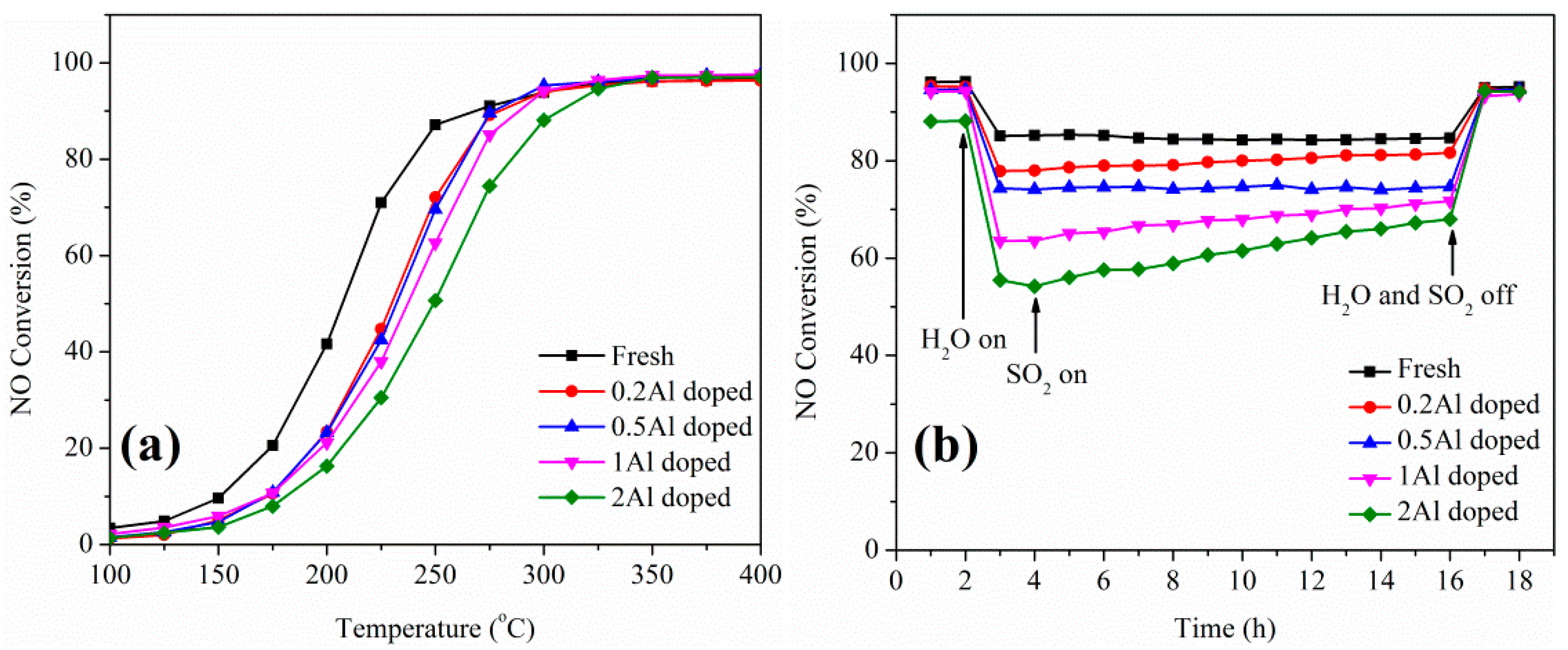
2.1. Catalytic Studies of V-W/Ti Catalysts
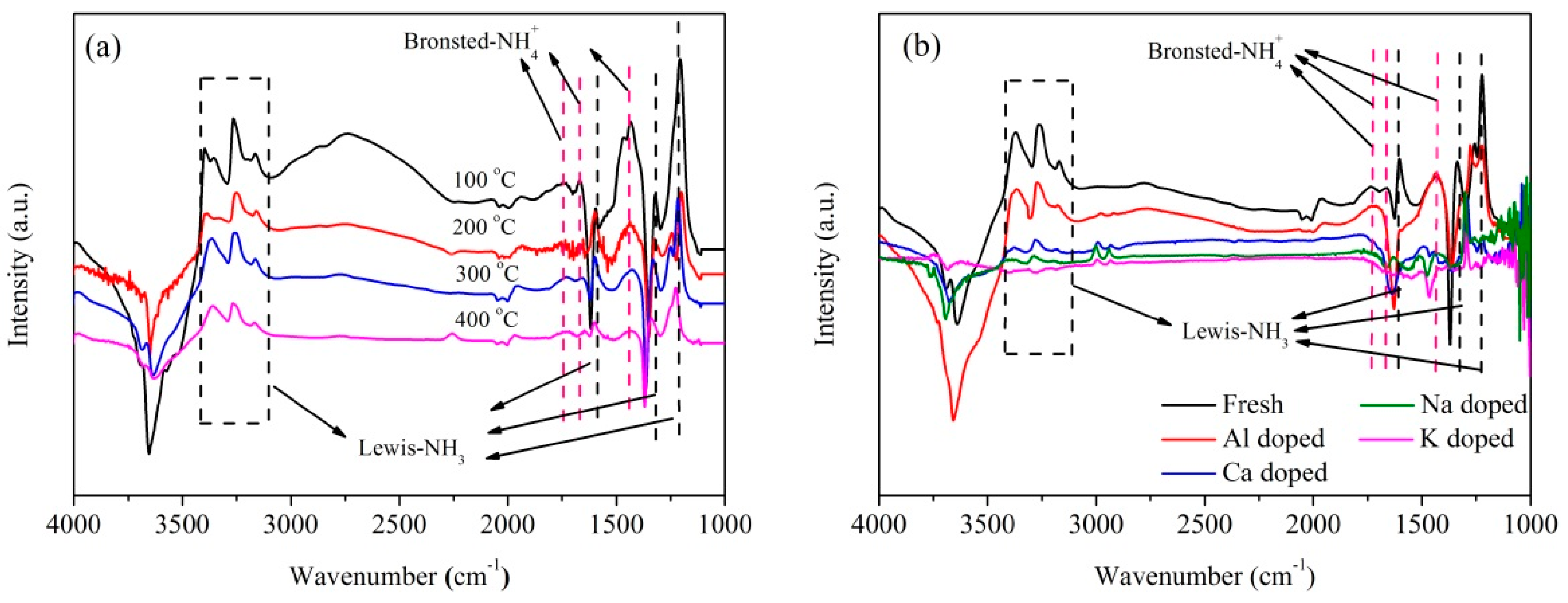
2.2. Influence of Basic Metals (Al, Ca, Na, and K) on Catalytic Performance
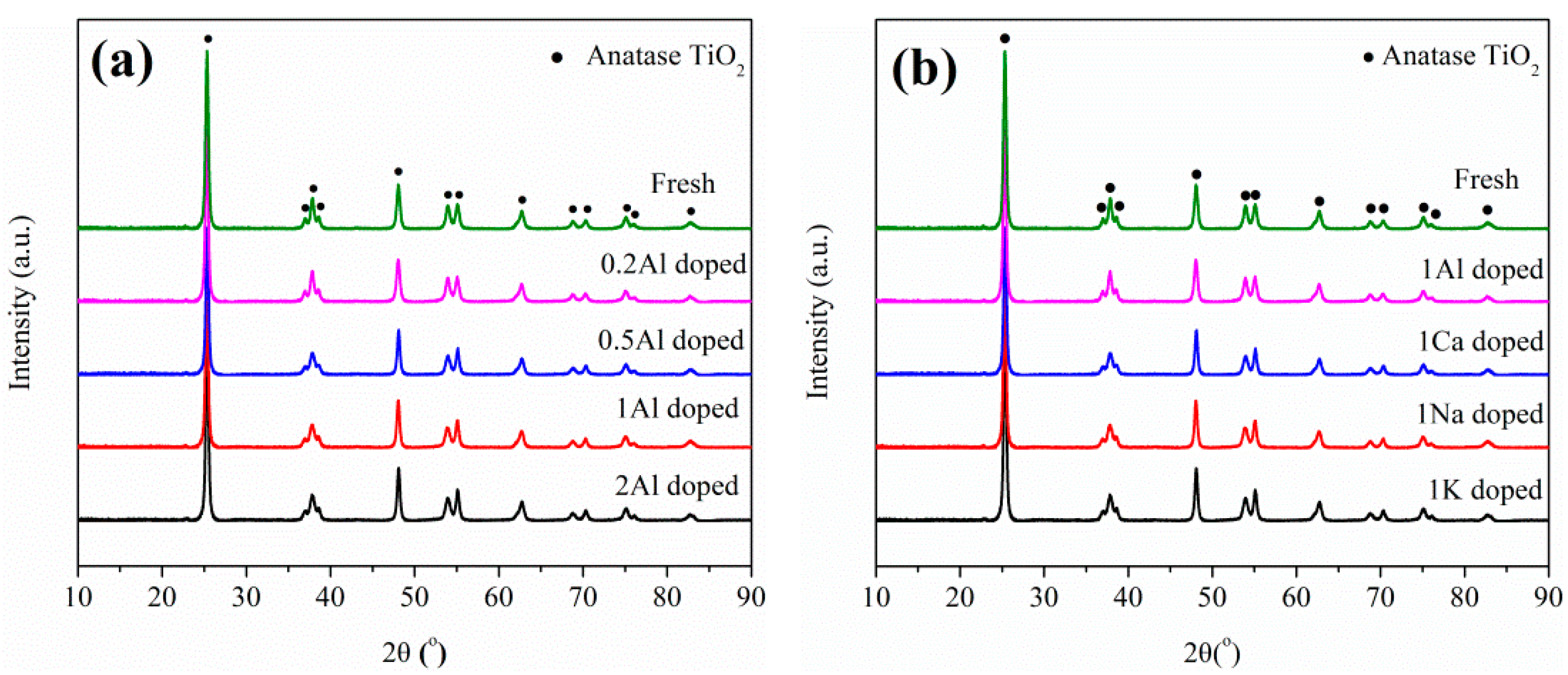
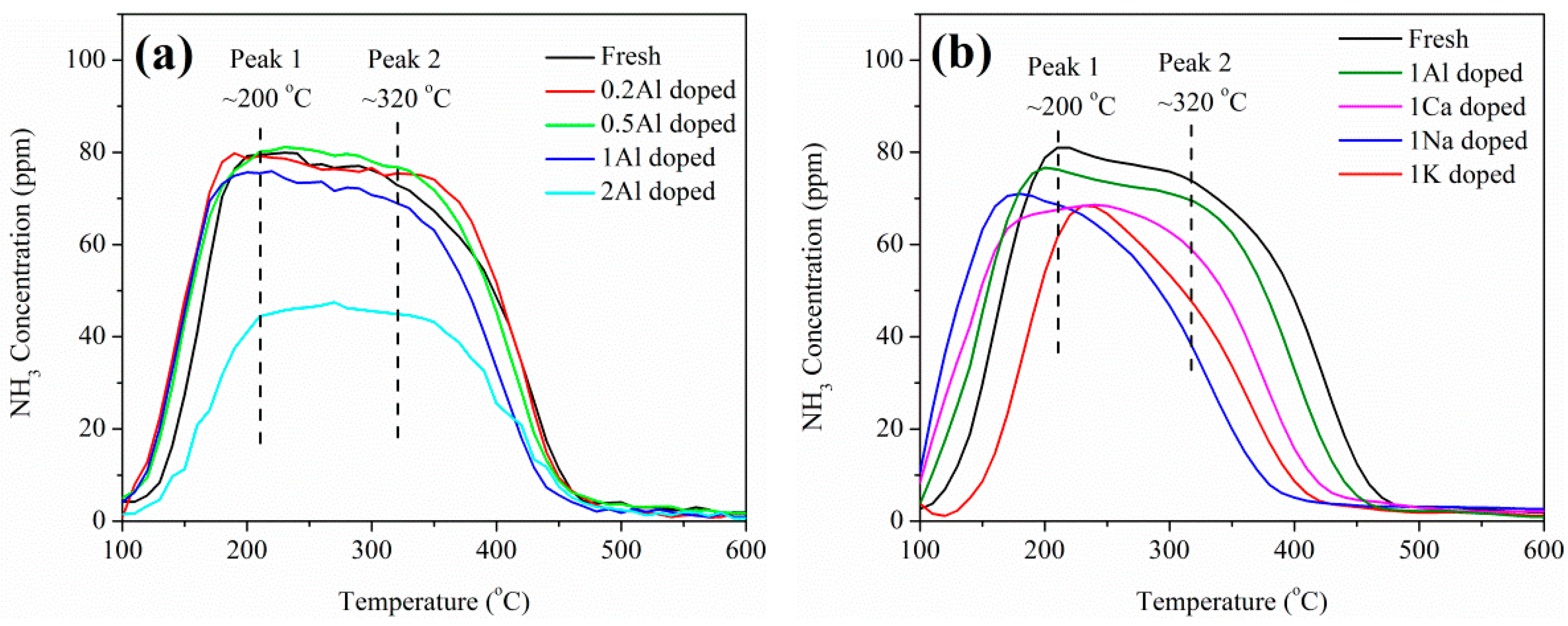
2.3. Catalyst Characterization
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterizations
3.3. Catalyst Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mori, H.; Asami, K.; Ohtsuka, Y. Role of iron catalyst in fate of fuel nitrogen during coal pyrolysis. Energy Fuels 1996, 10, 1022–1027. [Google Scholar] [CrossRef]
- Thomas, K.M. The release of nitrogen oxides during char combustion. Fuel 1997, 76, 457–473. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Baeyens, J.; Seville, J.P.K. NOx formation and selective non-catalytic reduction (SNCR) in a fluidized bed combustor of biomass. Biomass Bioenerg. 2010, 34, 1393–1409. [Google Scholar] [CrossRef]
- Bae, S.W.; Roh, S.A.; Kim, S.D. NO removal by reducing agents and additives in the selective non-catalytic reduction (SNCR) process. Chemosphere 2006, 65, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Li, Y.R.; Zhu, T.Y.; Ye, M. Effects of Concentration and Adsorption Product on the Adsorption of SO2 and NO on Activated Carbon. Energy Fuels 2013, 27, 360–366. [Google Scholar] [CrossRef]
- Ding, S.; Li, Y.R.; Zhu, T.Y.; Guo, Y.Y. Regeneration performance and carbon consumption of semi-coke and activated coke for SO2 and NO removal. J. Environ. Sci. 2015, 34, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, Z.H.; Lin, F.W.; Kuang, M.; Whiddon, R.; He, Y.; Liu, J.Z. Characteristics of O3 Oxidation for Simultaneous Desulfurization and Denitration with Limestone-Gypsum Wet Scrubbing: Application in a Carbon Black Drying Kiln Furnace. Energy Fuels 2016, 30, 2302–2308. [Google Scholar] [CrossRef]
- Ji, R.; Wang, J.; Xu, W.; Liu, X.; Zhu, T.; Yan, C.; Song, J. Study on the Key Factors of NO Oxidation Using O3: The Oxidation Product Composition and Oxidation Selectivity. Ind. Eng. Chem. Res. 2018, 57, 14440–14447. [Google Scholar] [CrossRef]
- Boningari, T.; Koirala, R.; Smirniotis, P.G. Low-temperature selective catalytic reduction of NO with NH3 over V/ZrO2 prepared by flame-assisted spray pyrolysis: Structural and catalytic properties. Appl. Catal. B Environ. 2012, 127, 255–264. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Smirniotis, P.G. Metal oxide-confined interweaved titania nanotubes M/TNT (M = Mn, Cu, Ce, Fe, V, Cr, and Co) for the selective catalytic reduction of NOx in the presence of excess oxygen. J. Catal. 2018, 365, 320–333. [Google Scholar] [CrossRef]
- Li, X.; Li, X.S.; Li, J.H. Hao High calcium resistance of CeO2-WO3 SCR catalysts: Structure. Chem. Eng. J. 2017, 317, 70–79. [Google Scholar] [CrossRef]
- Phil, H.H.; Reddy, M.P.; Kumar, P.A.; Ju, L.K.; Hyo, J.S. SO2 resistant antimony promoted V2O5/TiO2 catalyst for NH3-SCR of NOx at low temperatures. Appl. Catal. B Environ. 2008, 78, 301–308. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A comparative study of the NH3-SCR reactions over a Cu-zeolite and a Fe-zeolite catalyst. Catal. Today 2010, 151, 223–230. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Tian, Y.; Kong, B.; Liu, Q.; Ren, S.; Li, J.; Kong, M. Effect of Ce doping on the resistance of Na over V2O5-WO3/TiO2 SCR catalysts. Mater. Res. Bull. 2018, 104, 112–118. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. J. Catal. 2012, 288, 74–83. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Sreekanth, P.M.; Pena, D.A.; Jenkins, R.G. Manganese oxide catalysts supported on TiO2, Al2O3, and SiO2: A comparison for low-temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 2006, 45, 6436–6443. [Google Scholar] [CrossRef]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. Influence of tungsten on the activity of a Mn/Ce/W/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. A Gen. 2015, 497, 160–166. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Huang, X.; Li, X.; Su, W.; Sun, X.; Wang, D.; Hao, J. Deactivation Mechanism of Potassium on the V2O5/CeO2 Catalysts for SCR Reaction: Acidity, Reducibility and Adsorbed-NOx. Environ. Sci. Technol. 2014, 48, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yu, W.; Si, Z.; Weng, D. Chemical deactivation of V2O5-WO3/TiO2 SCR catalyst by combined effect of potassium and chloride. Front. Env. Sci. Eng. 2013, 7, 420–427. [Google Scholar] [CrossRef]
- Liu, Z.M.; Liu, Y.X.; Chen, B.H.; Zhu, T.L.; Ma, L.L. Novel Fe-Ce-Ti catalyst with remarkable performance for the selective catalytic reduction of NOx by NH3. Catal. Sci. Technol. 2016, 6, 6688–6696. [Google Scholar] [CrossRef]
- Yang, S.; Liu, C.; Chang, H.; Ma, L.; Qu, Z.; Yan, N.; Wang, C.; Li, J. Improvement of the Activity of gamma-Fe2O3 for the Selective Catalytic Reduction of NO with NH3 at High Temperatures: NO Reduction. Ind. Eng. Chem. Res. 2013, 52, 5601–5610. [Google Scholar] [CrossRef]
- Forzatti, P.; Nova, I.; Tronconi, E.; Kustov, A.; Thogersen, J.R. Effect of operating variables on the enhanced SCR reaction over a commercial V2O5-WO3/TiO2 catalyst for stationary applications. Catal. Today 2012, 184, 153–159. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Weibel, M. Unifying Redox Kinetics for Standard and Fast NH3-SCR over a V2O5-WO3/TiO2 Catalyst. Aiche J. 2009, 55, 1514–1529. [Google Scholar] [CrossRef]
- Choo, S.T.; Yim, S.D.; Nam, I.S.; Ham, S.W.; Lee, J.B. Effect of promoters includingWO3 and BaO on the activity and durability of V2O5/sulfated TiO2 catalyst for NO reduction by NH3. Appl. Catal. B Environ. 2003, 44, 237–252. [Google Scholar] [CrossRef]
- Xu, L.W.; Wang, C.Z.; Chang, H.Z.; Wu, Q.R.; Zhang, T.; Li, J.H. New Insight into SO2 Poisoning and Regeneration of CeO2-WO3/TiO2 and V2O5-WO3/TiO2 Catalysts for Low-Temperature NH3-SCR. Environ. Sci. Technol. 2018, 52, 7064–7071. [Google Scholar] [CrossRef] [PubMed]
- Amiridis, M.D.; Wachs, I.E.; Deo, G.; Jehng, J.M.; Kim, D.S. Reactivity of V2O5 catalysts for the selective catalytic reduction of NO by NH3: Influence of vanadia loading, H2O, and SO2. J. Catal. 1996, 161, 247–253. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, T.; Li, Y.; Ma, W.; Qu, H. NO (or NH3) + O2 adsorption on fluorine-doped vanadia/titania and its role in the mechanism of a two-step process characterized by EPR. Chem. Eng. J. 2011, 174, 390–395. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, H.; Du, T.; Ma, W.; Zhong, Q. H2O and SO2 tolerance, activity and reaction mechanism of sulfated Ni-Ce-La composite oxide nanocrystals in NH3-SCR. Chem. Eng. J. 2016, 296, 122–131. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, N.; Zou, W.; Li, L.; Tang, C.; Dong, L. A general and inherent strategy to improve the water tolerance of low temperature NH3-SCR catalysts via trace SiO2 deposition. Catal. Commun. 2016, 84, 75–79. [Google Scholar] [CrossRef]
- Klimczak, M.; Kern, P.; Heinzelmann, T.; Lucas, M.; Claus, P. High-throughput study of the effects of inorganic additives and poisons on NH3-SCR catalysts Part I: V2O5-WO3/TiO2 catalysts. Appl. Catal. B Environ. 2010, 95, 39–47. [Google Scholar] [CrossRef]
- Yuan, L.; Zheng, X.; Duan, K.; Hu, H.; Wang, J.; Woo, S.I.; Liu, Z. The effect of preparation conditions of Pt/Al2O3 on its catalytic performance for the H2-SCR in the presence of oxygen. Front. Env. Sci. Eng. 2013, 7, 457–463. [Google Scholar] [CrossRef]
- Takamitsu, Y.; Ito, Y.; Ogawa, H.; Sano, T. Effect of Oxygen Concentration in NH3-SCR Reaction over Fe- and Cu-loaded Beta Zeolites. J. Jpn. Pet. Inst. 2012, 55, 57–66. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of tungsten oxide on SCR performance of V2O5-WO3/TiO2: Acidity, surface species and catalytic activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Ana, S.N.; Jennifer, W. Role of WO3 in the Hg Oxidation across the V2O5−WO3−TiO2 SCR Catalyst: A DFT Study. J. Phys. Chem. C 2013, 117, 24397–24406. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5-WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Fang, P.; Ren, T.; Liu, Y.; Cen, C.; Wang, H.; Wu, Z. Tuning the property of Mn-Ce composite oxides by titanate nanotubes to improve the activity, selectivity and SO2/H2O tolerance in middle temperature NH3-SCR reaction. Fuel Process. Technol. 2017, 167, 221–228. [Google Scholar] [CrossRef]
- Yu, Y.; Miao, J.; He, C.; Chen, J.; Li, C.; Douthwaite, M. The remarkable promotional effect of SO2 on Pb-poisoned V2O5-WO3/TiO2 catalysts: An in-depth experimental and theoretical study. Chem. Eng. J. 2018, 338, 191–201. [Google Scholar] [CrossRef]
- Huang, Q.; Song, L.; He, H.; Qiu, W.; Su, Y. Effects of SO2 Treatment of Commercial Catalysts on Selective Catalytic Reduction of NOx by NH3. Chem. Res. Chin. Univ. 2016, 32, 414–417. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. The poisoning effect of alkali metals doping over nano V2O5-WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.; Zhou, J.; Jiang, L.; Tian, Y.; Yang, J.; Ren, S.; Li, J. Effect of different potassium species on the deactivation of V2O5-WO3/TiO2 SCR catalyst: Comparison of K2SO4, KCl and K2O. Chem. Eng. J. 2018, 348, 637–643. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Chen, J.; Peng, Y.; Ma, L.; Chang, H.; Chen, L.; Liu, C.; Xu, J.; Li, J.; et al. A novel magnetic Fe-Ti-V spinel catalyst for the selective catalytic reduction of NO with NH3 in a broad temperature range. Catal. Sci. Technol. 2012, 2, 915–917. [Google Scholar] [CrossRef]
- Jing, L.Q.; Xu, Z.L.; Sun, X.J.; Shang, J.; Cai, W.M. The surface properties and photocatalytic activities of ZnO ultrafine particles. Appl. Surf. Sci. 2001, 180, 308–314. [Google Scholar] [CrossRef]
- Wan, Q.; Duan, L.; Li, J.; Chen, L.; He, K.; Hao, J. Deactivation performance and mechanism of alkali (earth) metals on V2O5-WO3/TiO2 catalyst for oxidation of gaseous elemental mercury in simulated coal-fired flue gas. Catal. Today 2011, 175, 189–195. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Q.; Pan, Y.; Zhang, R. Systematic effects of S-doping on the activity of V2O5/TiO2 catalyst for low-temperature NH3-SCR. Chem. Eng. J. 2013, 228, 815–823. [Google Scholar] [CrossRef]
- Topsøe, N.Y.; Topsøe, H.; Dumesic, J.A. Vanadia/Titania Catalysts for Selective Catalytic Reduction (SCR) of Nitric Oxide by Ammonia: I. Combined Temperature Programmed in Situ FTIR and On-line Mass Spectroscopy Studies. J. Catal. 1995, 151, 226–240. [Google Scholar] [CrossRef]
- Topsøe, N.Y.; Dumesic, J.A.; Topsøe, H. Vanadia/Titania Catalysts for Selective Catalytic Reduction of Nitric Oxide by Ammonia: II. Studies of Active Sites and Formulation of Catalytic Cycles. J. Catal. 1995, 151, 241–252. [Google Scholar] [CrossRef]




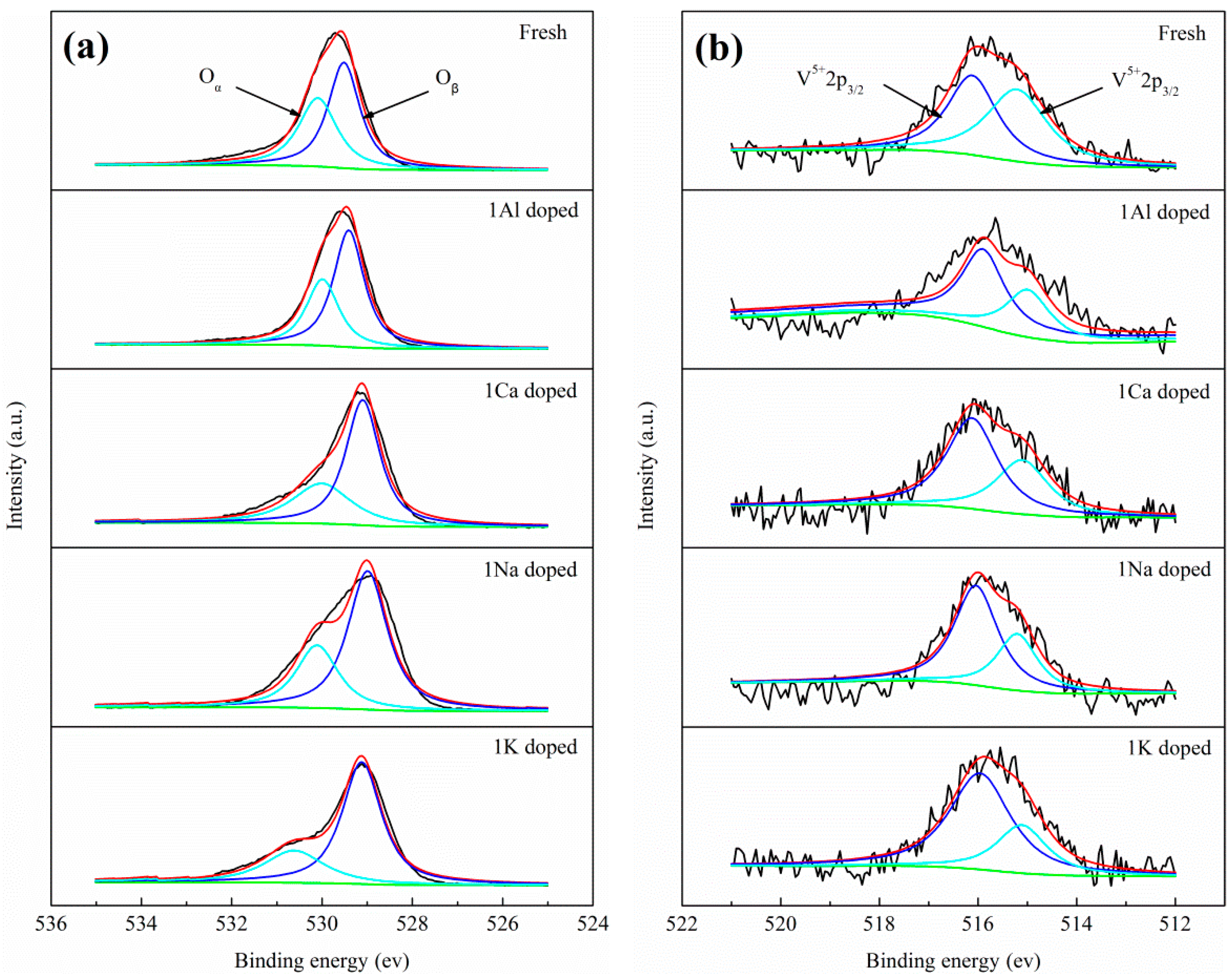
| Sample | Oα/(Oα + Oβ)% | V5+/(V5+ + V4+) % |
|---|---|---|
| Fresh | 43.9 | 47.5 |
| 1Al doped | 37.4 | 60.7 |
| 1Ca doped | 35.7 | 61.9 |
| 1Na doped | 32.4 | 65.6 |
| 1K doped | 28.6 | 70.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, R.; Chen, L.; Li, E.; Liu, X.; Zhu, T. Applicability of V2O5-WO3/TiO2 Catalysts for the SCR Denitrification of Alumina Calcining Flue Gas. Catalysts 2019, 9, 220. https://doi.org/10.3390/catal9030220
Ning R, Chen L, Li E, Liu X, Zhu T. Applicability of V2O5-WO3/TiO2 Catalysts for the SCR Denitrification of Alumina Calcining Flue Gas. Catalysts. 2019; 9(3):220. https://doi.org/10.3390/catal9030220
Chicago/Turabian StyleNing, Ruliang, Li Chen, Erwei Li, Xiaolong Liu, and Tingyu Zhu. 2019. "Applicability of V2O5-WO3/TiO2 Catalysts for the SCR Denitrification of Alumina Calcining Flue Gas" Catalysts 9, no. 3: 220. https://doi.org/10.3390/catal9030220
APA StyleNing, R., Chen, L., Li, E., Liu, X., & Zhu, T. (2019). Applicability of V2O5-WO3/TiO2 Catalysts for the SCR Denitrification of Alumina Calcining Flue Gas. Catalysts, 9(3), 220. https://doi.org/10.3390/catal9030220





