Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural/Structural Analysis (BET and XRD)
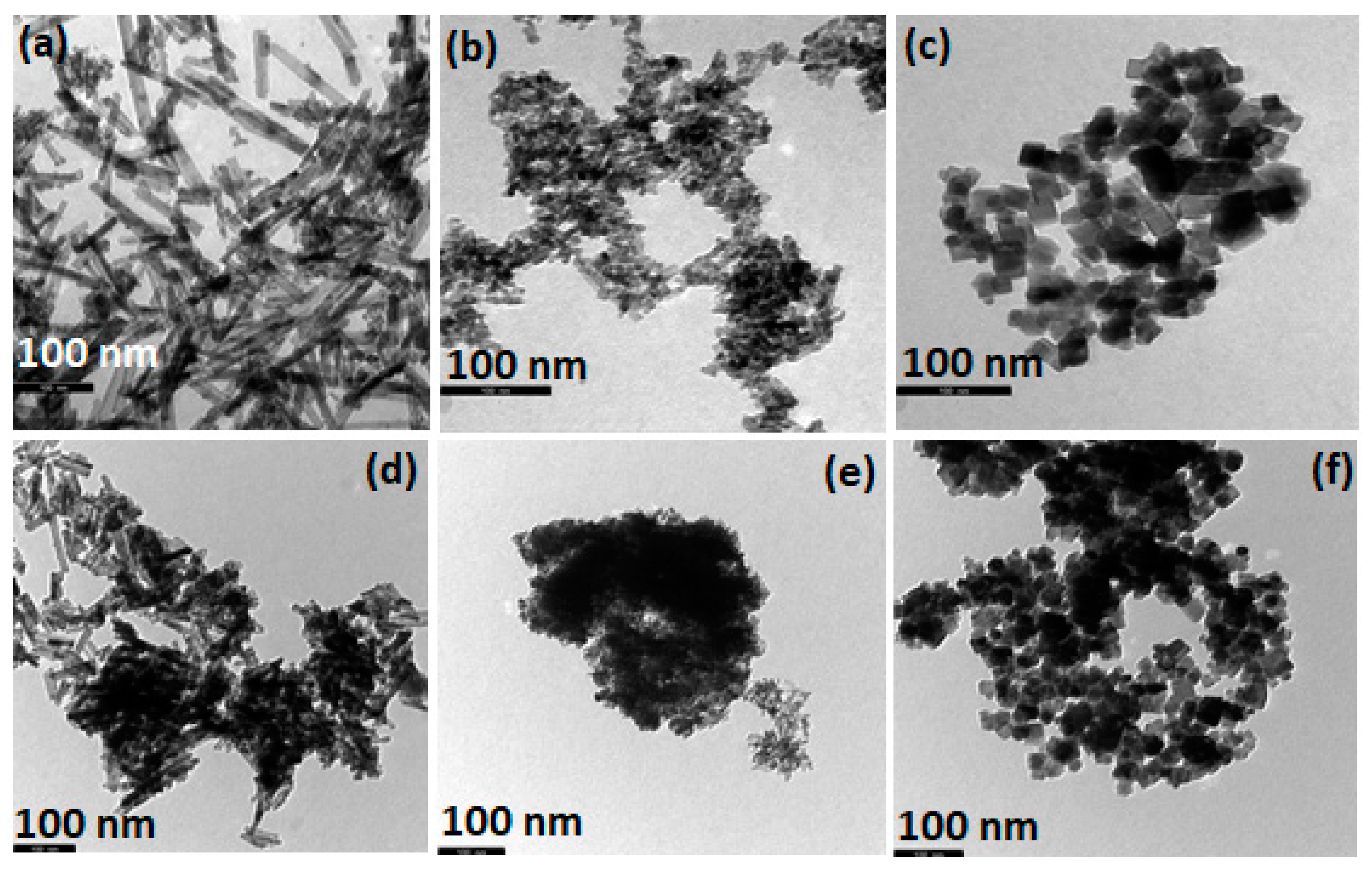
2.2. Morphological Characterization (TEM)
2.3. Redox Properties (H2-Temperature Programmed Reduction (TPR))
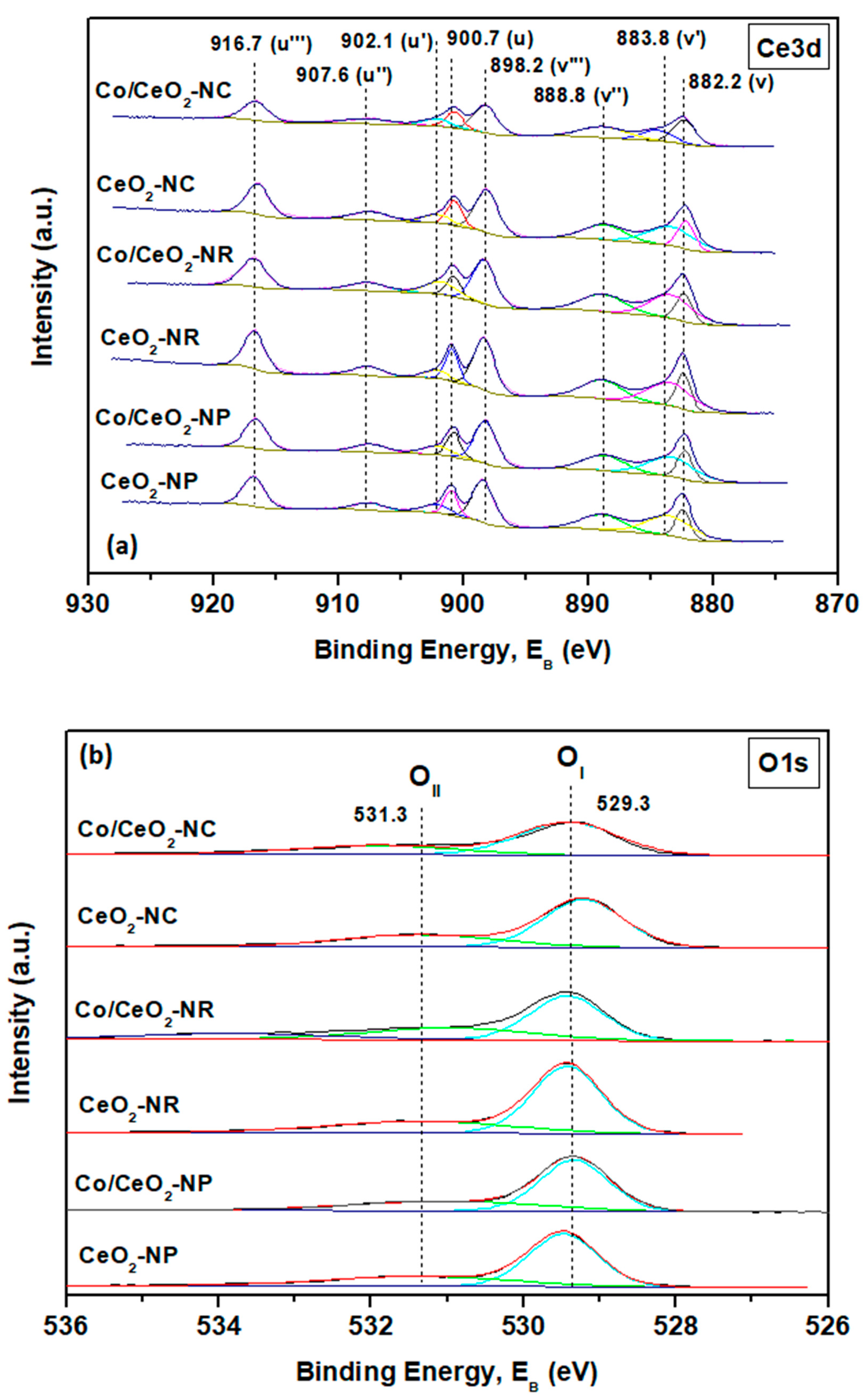
2.4. Surface Analysis (X-ray Photoelectron Spectroscopy (XPS))
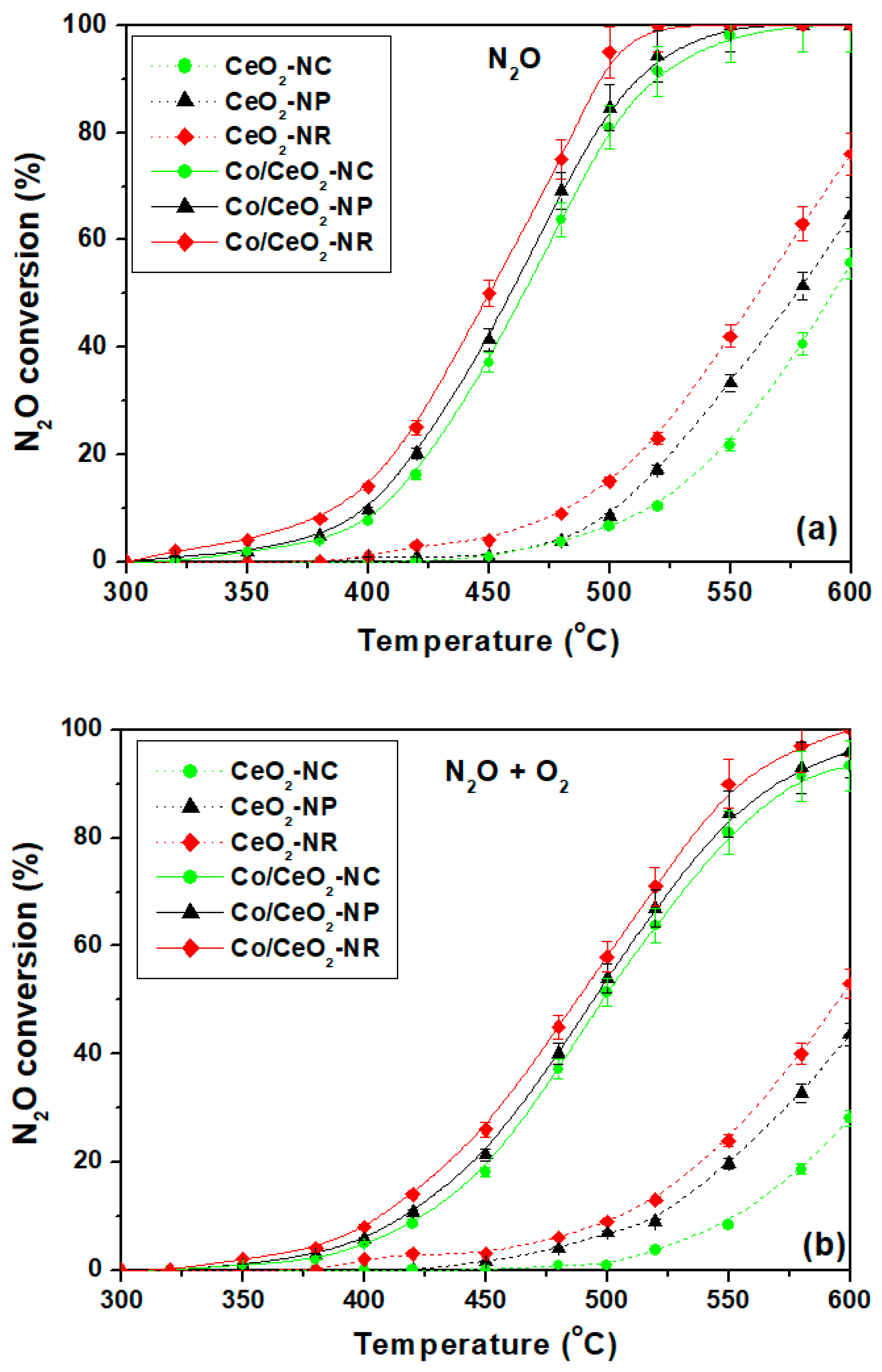
2.5. Catalytic Evaluation Studies
3. Materials and Methods
3.1. Materials Synthesis
3.2. Materials Characterization
3.3. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Konsolakis, M. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations and Surface Chemistry Aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Ma, L.; Peng, S. Recent Advances in Catalytic Decomposition of N2O on Noble Metal and Metal Oxide Catalysts. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.J.; Ryu, I.S.; Jeon, M.W.; Moon, S.H.; Roh, H.S.; Jeon, S.G. Catalytic decomposition of N2O over cobalt based spinel oxides: The role of additives. Mol. Catal. 2017, 442, 202–207. [Google Scholar] [CrossRef]
- Rutkowska, M. Catalytic Decomposition of N2O Using a New Generation of Functionalized Microporous and Mesoporous Inorganic Materials. Wiadomości Chemiczne 2015, 69, 297–315. [Google Scholar]
- Piumetti, M.; Hussain, M.; Fino, D.; Russo, N. Mesoporous silica supported Rh catalysts for high concentration N2O decomposition. Appl. Catal. B Environ. 2015, 165, 158–168. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.J.; Hong, S.C. Effect of CeO2 addition to Rh/Al2O3 catalyst on N2O decomposition. Chem. Eng. J. 2011, 169, 173–179. [Google Scholar] [CrossRef]
- Hussain, M.; Fino, D.; Russo, N. Development of modified KIT-6 and SBA-15-spherical supported Rh catalysts for N2O abatement: From powder to monolith supported catalysts. Chem. Eng. J. 2014, 238, 198–205. [Google Scholar] [CrossRef]
- Wu, Y.; Cordier, C.; Berrier, E.; Nuns, N.; Dujardin, C.; Granger, P. Surface reconstructions of LaCo1−xFexO3 at high temperature during N2O decomposition in realistic exhaust gas composition: Impact on the catalytic properties. Appl. Catal. B Environ. 2013, 140–141, 151–163. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Isupova, L.A.; Sadovskaya, E.M.; Prosvirin, I.P.; Gerasimov, E.Y.; Yakovleva, I.S. Effect of surface decoration with LaSrFeO4 on oxygen mobility and catalytic activity of La0.4Sr0.6FeO3−δ in high-temperature N2O decomposition, methane combustion and ammonia oxidation. Appl. Catal. A Gen. 2013, 457, 42–51. [Google Scholar] [CrossRef]
- Kumar, S.; Vinu, A.; Subrt, J.; Bakardjieva, S.; Rayalu, S.; Teraoka, Y.; Labhsetwar, N. Catalytic N2O decomposition on Pr0.8Ba0.2MnO3 type perovskite catalyst for industrial emission control. Catal. Today 2012, 198, 125–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhu, Y.; Zhang, T. Stabilization mechanism and crystallographic sites of Ru in Fe-promoted barium hexaaluminate under high-temperature condition for N2O decomposition. Appl. Catal. B Environ. 2013, 129, 382–393. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhu, Y.; Hou, B.; Yang, X.; Liu, X.; Wang, J.; Li, J.; Zhang, T. Characterization of Fe substitution into La-hexaaluminate systems and the effect on N2O catalytic decomposition. J. Phys. Chem. C 2014, 118, 1999–2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhu, Y.; Liu, X.; Zhang, T. Thermal evolution crystal structure and Fe crystallographic sites in LaFexAl12-xO19 hexaaluminates. J. Phys. Chem. C 2014, 118, 10792–10804. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Santiago, M. Metal-substituted hexaaluminates for high-temperature N2O abatement. Chem. Commun. 2007, 2, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Stelmachowski, P.; Maniak, G.; Kaczmarczyk, J.; Zasada, F.; Piskorz, W.; Kotarba, A.; Sojka, Z. Mg and Al substituted cobalt spinels as catalysts for low temperature deN2O-Evidence for octahedral cobalt active sites. Appl. Catal. B Environ. 2014, 146, 105–111. [Google Scholar] [CrossRef]
- Grzybek, G.; Stelmachowski, P.; Gudyka, S.; Duch, J.; Ćmil, K.; Kotarba, A.; Sojka, Z. Insights into the twofold role of Cs doping on deN2O activity of cobalt spinel catalyst-towards rational optimization of the precursor and loading. Appl. Catal. B Environ. 2015, 168–169, 509–514. [Google Scholar] [CrossRef]
- Zasada, F.; Piskorz, W.; Janas, J.; Gryboś, J.; Indyka, P.; Sojka, Z. Reactive Oxygen Species on the (100) Facet of Cobalt Spinel Nanocatalyst and their Relevance in 16O2/18O2 Isotopic Exchange, deN2O, and deCH4 Processes-A Theoretical and Experimental Account. ACS Catal. 2015, 5, 6879–6892. [Google Scholar] [CrossRef]
- Amrousse, R.; Chang, P.-J.; Choklati, A.; Friche, A.; Rai, M.; Bachar, A.; Follet-Houttemane, C.; Hori, K. Catalytic decomposition of N2O over Ni and Mg-magnetite catalysts. Catal. Sci. Technol. 2013, 3, 2288. [Google Scholar] [CrossRef]
- Zou, W.; Xie, P.; Hua, W.; Wang, Y.; Kong, D.; Yue, Y.; Ma, Z.; Yang, W.; Gao, Z. Catalytic decomposition of N2O over Cu-ZSM-5 nanosheets. J. Mol. Catal. A Chem. 2014, 394, 83–88. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; He, C.; Ma, C.; Cheng, J.; Liu, Z.; Hao, Z. Decomposition of nitrous oxide over Co-zeolite catalysts: role of zeolite structure and active site. Catal. Sci. Technol. 2012, 2, 1249–1258. [Google Scholar] [CrossRef]
- Rutkowska, M.; Piwowarska, Z.; Micek, E.; Chmielarz, L. Hierarchical Fe-, Cu- and Co-Beta zeolites obtained by mesotemplate-free method. Part I: Synthesis and catalytic activity in N2O decomposition. Microporous Mesoporous Mater. 2015, 209, 54–65. [Google Scholar] [CrossRef]
- Xie, P.; Luo, Y.; Ma, Z.; Wang, L.; Huang, C.; Yue, Y.; Hua, W.; Gao, Z. CoZSM-11 catalysts for N2O decomposition: Effect of preparation methods and nature of active sites. Appl. Catal. B Environ. 2015, 170–171, 34–42. [Google Scholar] [CrossRef]
- Grzybek, G.; Stelmachowski, P.; Gudyka, S.; Indyka, P.; Sojka, Z.; Guillén-Hurtado, N.; Rico-Pérez, V.; Bueno-López, A.; Kotarba, A. Strong dispersion effect of cobalt spinel active phase spread over ceria for catalytic N2O decomposition: The role of the interface periphery. Appl. Catal. B Environ. 2016, 180, 622–629. [Google Scholar] [CrossRef]
- Chromčáková, Ž.; Obalová, L.; Kovanda, F.; Legut, D.; Titov, A.; Ritz, M.; Fridrichová, D.; Michalik, S.; Kuśtrowski, P.; Jirátová, K. Effect of precursor synthesis on catalytic activity of Co3O4 in N2O decomposition. Catal. Today 2015, 257, 18–25. [Google Scholar] [CrossRef]
- Franken, T.; Palkovits, R. Investigation of potassium doped mixed spinels CuxCo3-xO4 as catalysts for an efficient N2O decomposition in real reaction conditions. Appl. Catal. B Environ. 2015, 176–177, 298–305. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Erjavec, B.; Djinović, P.; Pintar, A. Ordered mesoporous CuO-CeO2 mixed oxides as an effective catalyst for N2O decomposition. Chem. Eng. J. 2014, 254, 153–162. [Google Scholar] [CrossRef]
- Xue, L.; He, H.; Liu, C.; Zhang, C.; Zhang, B. Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth Metals on Cobalt - Cerium Composite Oxide Catalysts for N2O Decomposition. Environ. Sci. Technol. 2009, 43, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, C.; Chen, B.; Liu, H. CuO-CeO2 mixed oxide catalyst for the catalytic decomposition of N2O in the presence of oxygen. Catal. Today 2017, 297, 78–83. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. N2O catalytic decomposition over various spinel-type oxides. Catal. Today 2007, 119, 228–232. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B Environ. 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Yang, J.; Lukashuk, L.; Akbarzadeh, J.; Stöger-Pollach, M.; Peterlik, H.; Föttinger, K.; Rupprechter, G.; Schubert, U. Different Synthesis Protocols for Co3O4-CeO2 Catalysts-Part 1: Influence on the Morphology on the Nanoscale. Chem. Eur. J. 2015, 21, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Li, L.; Li, J.; Tian, H.; Hao, Z. A study on N2O catalytic decomposition over Co/MgO catalysts. J. Hazard. Mater. 2009, 163, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-Y.; Meng, M.; Li, X.; Li, X.-G.; Zha, Y.-Q.; Hu, T.-D.; Xie, Y.-N.; Zhang, J. Mesoporous Co3O4–CeO2 and Pd/Co3O4–CeO2 catalysts: Synthesis, characterization and mechanistic study of their catalytic properties for low-temperature CO oxidation. J. Catal. 2008, 254, 310–324. [Google Scholar] [CrossRef]
- Wang, H.; Ye, J.L.; Liu, Y.; Li, Y.D.; Qin, Y.N. Steam reforming of ethanol over Co3O4/CeO2 catalysts prepared by different methods. Catal. Today 2007, 129, 305–312. [Google Scholar] [CrossRef]
- Qiu, N.; Zhang, J.; Wu, Z. Peculiar surface-interface properties of nanocrystalline ceria-cobalt oxides with enhanced oxygen storage capacity. Phys. Chem. Chem. Phys. 2014, 16, 22659–22664. [Google Scholar] [CrossRef] [PubMed]
- Vinod, C.P. Surface science as a tool for probing nanocatalysis phenomena. Catal. Today 2010, 154, 113–117. [Google Scholar] [CrossRef]
- Hu, P.; Huang, Z.; Amghouz, Z.; Makkee, M.; Xu, F.; Kapteijn, F.; Dikhtiarenko, A.; Chen, Y.; Gu, X.; Tang, X. Electronic Metal-Support Interactions in Single-Atom Catalysts. Angew. Chem. 2014, 126, 3486–3489. [Google Scholar] [CrossRef]
- Elias, J.S.; Risch, M.; Giordano, L.; Mansour, A.N.; Shao-Horn, Y. Structure, Bonding, and Catalytic Activity of Monodisperse, Transition-Metal-Substituted CeO2 Nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Fornasiero, P.; Gorte, R.J. Opportunities for tailoring catalytic properties through metal-support interactions. Catal. Lett. 2012, 142, 1043–1048. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Sánchez, P.; Kyriakou, V.; Zafeiratos, S.; Marnellos, G.E.; Konsolakis, M.; Dorado, F. Effect of support nature on the cobalt-catalyzed CO2 hydrogenation. J. CO2 Util. 2017, 21, 562–571. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Mahammadunnisa, S.K.; Akanksha, T.; Krushnamurty, K.; Subrahmanyam, C. Catalytic decomposition of N2O over CeO2 supported Co3O4 catalysts. J. Chem. Sci. 2016, 128, 1795–1804. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Heng, L.; Ni, J.; Lin, J.; Jiang, L. Effect of ceria morphology on the catalytic activity of Co/CeO2 catalyst for ammonia synthesis. Catal. Commun. 2017, 101, 15–19. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinović, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 Materials: Effect of the Exposed Ceria Surfaces on Catalytic Activity in N2O Decomposition Reaction. ACS Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Andrade-Martínez, J.; Ortega-Zarzosa, G.; Gómez-Cortés, A.; Rodríguez-González, V. N2O catalytic reduction over different porous SiO2 materials functionalized with copper. Powder Technol. 2015, 274, 305–312. [Google Scholar] [CrossRef]
- Mei, J.; Ke, Y.; Yu, Z.; Hu, X.; Qu, Z.; Yan, N. Morphology-dependent properties of Co3O4/CeO2 catalysts for low temperature dibromomethane (CH2Br2) oxidation. Chem. Eng. J. 2017, 320, 124–134. [Google Scholar] [CrossRef]
- Hu, F.; Peng, Y.; Chen, J.; Liu, S.; Song, H.; Li, J. Low content of CoOx supported on nanocrystalline CeO2 for toluene combustion: The importance of interfaces between active sites and supports. Appl. Catal. B Environ. 2019, 240, 329–336. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Allwar, A.; Md Noor, A.; Nawi, M. Textural Characteristics of Activated Carbons Prepared from Oil Palm Shells Activated with ZnCl2 and Pyrolysis Under Nitrogen and Carbon Dioxide. J. Phys. Sci. 2008, 19, 93–104. [Google Scholar]
- Kumar, S.; Sharma, C. Synthesis, characterization and catalytic wet air oxidation property of mesoporous Ce1-xFexO2 mixed oxides. Mater. Chem. Phys. 2015, 155, 223–231. [Google Scholar]
- Tan, L.; Tao, Q.; Gao, H.; Li, J.; Jia, D.; Yang, M. Preparation and catalytic performance of mesoporous ceria-base composites CuO/CeO2, Fe2O3/CeO2 and La2O3/CeO2. J. Porous Mater. 2017, 24, 795–803. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zarinkamar, M. Synthesis of nano-sized ceria (CeO2) particles via a cerium hydroxy carbonate precursor and the effect of reaction temperature on particle morphology. J. Ultrafine Grained Nanostructured Mater. 2015, 48, 5–10. [Google Scholar]
- Sharma, V.; Eberhardt, K.M.; Sharma, R.; Adams, J.B.; Crozier, P.A. A spray drying system for synthesis of rare-earth doped cerium oxide nanoparticles. Chem. Phys. Lett. 2010, 495, 280–286. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Wang, J.; Xu, C.; Duan, A.; Jiang, G.; Yang, Q. The highly active catalysts of nanometric CeO2-supported cobalt oxides for soot combustion. Appl. Catal. B Environ. 2008, 84, 185–195. [Google Scholar] [CrossRef]
- Yu, S.W.; Huang, H.H.; Tang, C.W.; Wang, C.B. The effect of accessible oxygen over Co3O4-CeO2 catalysts on the steam reforming of ethanol. Int. J. Hydrogen Energy 2014, 39, 20700–20711. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid Interface Sci. 2017, 496, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mock, S.A.; Sharp, S.E.; Stoner, T.R.; Radetic, M.J.; Zell, E.T.; Wang, R. CeO2 nanorods-supported transition metal catalysts for CO oxidation. J. Colloid Interface Sci. 2016, 466, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Zheng, K.; Zhang, H.; Zhao, Y. Effect of precipitants on the catalytic activity of Co–Ce composite oxide for N2O catalytic decomposition. Reac. Kinet. Mech. Cat. 2018, 123, 707–721. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H. Mesoporous Co-CeO2 catalyst prepared by colloidal solution combustion method for reverse water-gas shift reaction. Catal. Today 2018, 316, 155–161. [Google Scholar] [CrossRef]
- Kumar Megarajan, S.; Rayalu, S.; Teraoka, Y.; Labhsetwar, N. High NO oxidation catalytic activity on non-noble metal based cobalt-ceria catalyst for diesel soot oxidation. J. Mol. Catal. A Chem. 2014, 385, 112–118. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Cui, Y.; Dai, W.-L. Support morphology and crystal plane effect of Cu/CeO2 nanomaterial on the physicochemical and catalytic properties for carbonate hydrogenation. Catal. Sci. Technol. 2016, 6, 7752–7762. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Q.; Wang, X.; Ma, K.; Bai, X.; Tan, S.; Tian, Y.; Ding, T.; Zheng, L.; Zhang, J.; et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals. Appl. Surf. Sci. 2017, 422, 932–943. [Google Scholar] [CrossRef]
- Dou, J.; Tang, Y.; Nie, L.; Andolina, C.M.; Zhang, X.; House, S.; Li, Y.; Yang, J.; Tao, F.F. Complete Oxidation of Methane on Co3O4/CeO2 Nanocomposite: A Synergic Effect. Catal. Today 2018, 311, 48–55. [Google Scholar] [CrossRef]
- Konsolakis, M.; Sgourakis, M.; Carabineiro, S.A.C. Surface and redox properties of cobalt-ceria binary oxides: On the effect of Co content and pretreatment conditions. Appl. Surf. Sci. 2015, 341, 48–54. [Google Scholar] [CrossRef]
- Ma, J.; Rodriguez, N.M.; Vannice, M.A.; Baker, R.T.K. Nitrous oxide decomposition and reduction over copper catalysts supported on various types of carbonaceous materials. Top. Catal. 2000, 10, 27–38. [Google Scholar] [CrossRef]
- You, Y.; Chang, H.; Ma, L.; Guo, L.; Qin, X.; Li, J.; Li, J. Enhancement of N2O decomposition performance by N2O pretreatment over Ce-Co-O catalyst. Chem. Eng. J. 2018, 347, 184–192. [Google Scholar] [CrossRef]
- Iwanek, E.; Krawczyk, K.; Petryk, J.; Sobczak, J.W.; Kaszkur, Z. Direct nitrous oxide decomposition with CoOx-CeO2 catalysts. Appl. Catal. B Environ. 2011, 106, 416–422. [Google Scholar] [CrossRef]
- Piskorz, W.; Zasada, F.; Stelmachowski, P.; Kotarba, A.; Sojka, Z. Decomposition of N2O over the surface of cobalt spinel: A DFT account of reactivity experiments. Catal. Today 2008, 137, 418–422. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Soliman, S.A.; Abdellah, S.E. Pure and Ni-substituted Co3O4 spinel catalysts for direct N2O decomposition. Chin. J. Catal. 2014, 35, 1105–1112. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Maniak, G.; Kotarba, A.; Sojka, Z. Strong electronic promotion of Co3O4 towards N2O decomposition by surface alkali dopants. Catal. Commun. 2009, 10, 1062–1065. [Google Scholar] [CrossRef]
- Savereide, L.; Nauert, S.L.; Roberts, C.A.; Notestein, J.M. The effect of support morphology on CoOX/CeO2 catalysts for the reduction of NO by CO. J. Catal. 2018, 366, 150–158. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barthos, R.; Hegyessy, A.; Klébert, S.; Valyon, J. Vanadium dispersion and catalytic activity of Pd/VOx/SBA-15 catalysts in the Wacker oxidation of ethylene. Microporous Mesoporous Mater. 2015, 207, 1–8. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef] [PubMed]





| Sample | BET Analysis | XRD Analysis | |||
|---|---|---|---|---|---|
| BET Surface Area (m2 g−1) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Crystallite Size (nm), DXRD 1 | ||
| CeO2 | Co3O4 | ||||
| CeO2-NC | 37 | 0.26 | 27.4 | 27 ± 1 | - |
| CeO2-NR | 79 | 0.48 | 24.2 | 15 ± 1 | - |
| CeO2-NP | 88 | 0.17 | 7.9 | 11 ± 1 | - |
| Co/CeO2-NC | 28 | 0.15 | 22.6 | 24 ± 1 | 19 ± 1 |
| Co/CeO2-NR | 72 | 0.31 | 17.4 | 14 ± 1 | 16 ± 1 |
| Co/CeO2-NP | 71 | 0.17 | 9.8 | 11 ± 1 | 15 ± 1 |
| Sample | H2 Consumption (mmol H2 g−1) a | Os/Ob Ratio | Peak Temperature (°C) | |||
|---|---|---|---|---|---|---|
| Os Peak | Ob Peak | Total | Os Peak | Ob Peak | ||
| CeO2-NP | 0.48 | 0.51 | 0.99 | 0.94 | 555 | 804 |
| CeO2-NR | 0.59 | 0.52 | 1.11 | 1.13 | 545 | 788 |
| CeO2-NC | 0.41 | 0.58 | 0.99 | 0.71 | 589 | 809 |
| Peaks a+b | CeO2 Peak | Total | Peak a | Peak b | ||
| Co/CeO2-NP | 2.40 | 0.61 | 3.01 | 333 | 388 | |
| Co/CeO2-NR | 2.37 | 0.62 | 2.99 | 318 | 388 | |
| Co/CeO2-NC | 2.05 | 0.32 | 2.37 | 335 | 405 | |
| Sample | Co2+/Co3+ | Ce3+ (%) | OII/OI |
|---|---|---|---|
| CeO2-NC | - | 23.3 | 0.50 |
| CeO2-NR | - | 24.3 | 0.47 |
| CeO2-NP | - | 25.3 | 0.49 |
| Co/CeO2-NC | 1.06 | 26.1 | 0.51 |
| Co/CeO2-NR | 1.32 | 28.5 | 0.60 |
| Co/CeO2-NP | 0.94 | 26.7 | 0.53 |
| Sample | N2O Conversion (%) | Specific Activity | ||||
|---|---|---|---|---|---|---|
| O2 Absence | O2 Presence | O2 Absence | O2 Presence | |||
| r (nmol g−1 s−1) | r (nmol m−2 s−1) | r (nmol g−1 s−1) | r (nmol m−2 s−1) | |||
| Co/CeO2-NC | 16.2 | 8.6 | 166 | 5.9 | 88 | 3.1 |
| Co/CeO2-NP | 20.2 | 10.7 | 207 | 2.9 | 109 | 1.5 |
| Co/CeO2-NR | 25 | 14 | 256 | 3.6 | 143 | 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lykaki, M.; Papista, E.; Kaklidis, N.; Carabineiro, S.A.C.; Konsolakis, M. Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides. Catalysts 2019, 9, 233. https://doi.org/10.3390/catal9030233
Lykaki M, Papista E, Kaklidis N, Carabineiro SAC, Konsolakis M. Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides. Catalysts. 2019; 9(3):233. https://doi.org/10.3390/catal9030233
Chicago/Turabian StyleLykaki, Maria, Eleni Papista, Nikolaos Kaklidis, Sόnia A. C. Carabineiro, and Michalis Konsolakis. 2019. "Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides" Catalysts 9, no. 3: 233. https://doi.org/10.3390/catal9030233
APA StyleLykaki, M., Papista, E., Kaklidis, N., Carabineiro, S. A. C., & Konsolakis, M. (2019). Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides. Catalysts, 9(3), 233. https://doi.org/10.3390/catal9030233









