Influence of Sn Content, Nanostructural Morphology, and Synthesis Temperature on the Electrochemical Active Area of Ni-Sn/C Nanocomposite: Verification of Methanol and Urea Electrooxidation
Abstract
:1. Introduction
2. Results Discussion
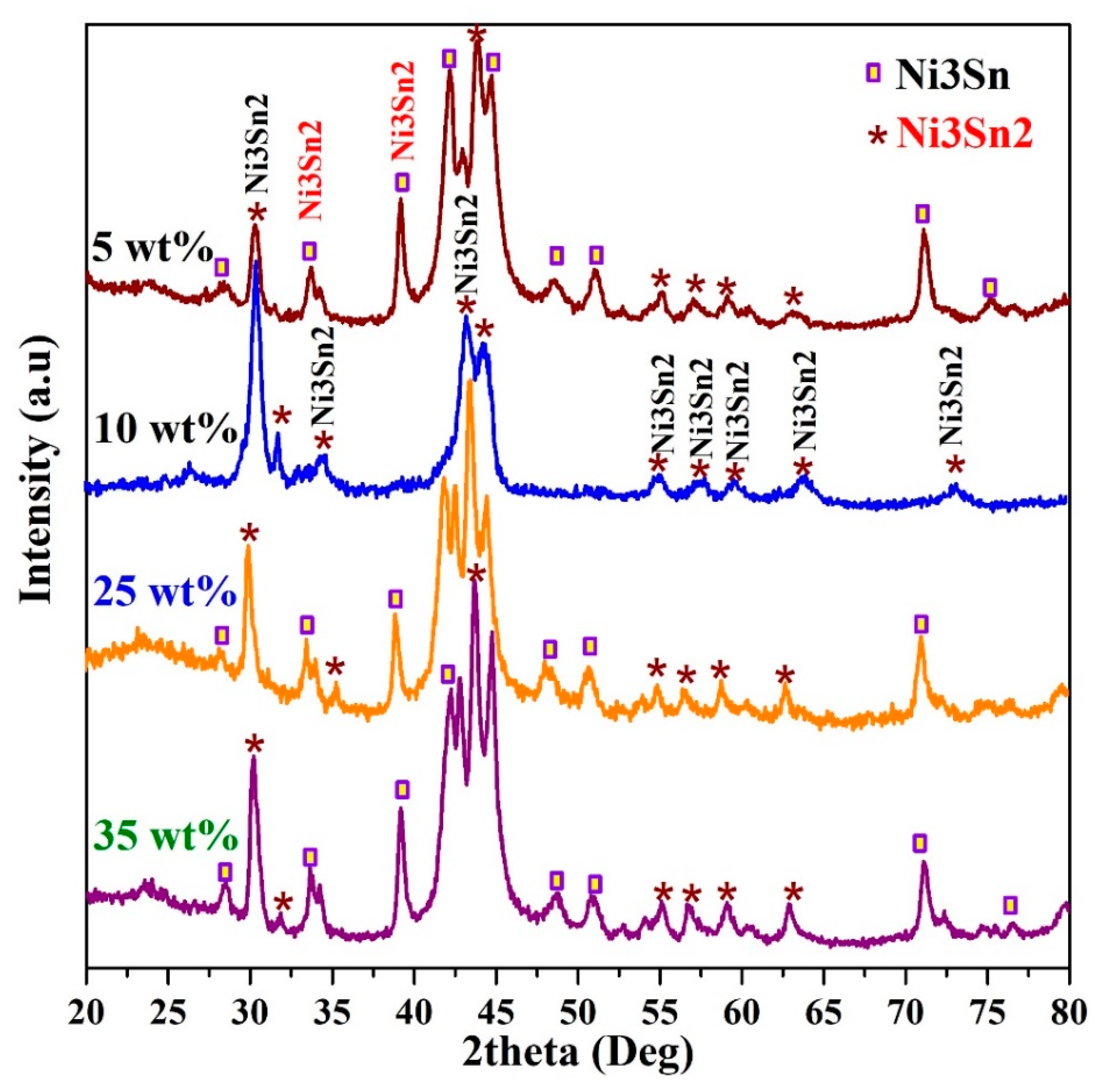
2.1. Crystalline Structure, Surface Morphology, and Composition of the Prepared Materials
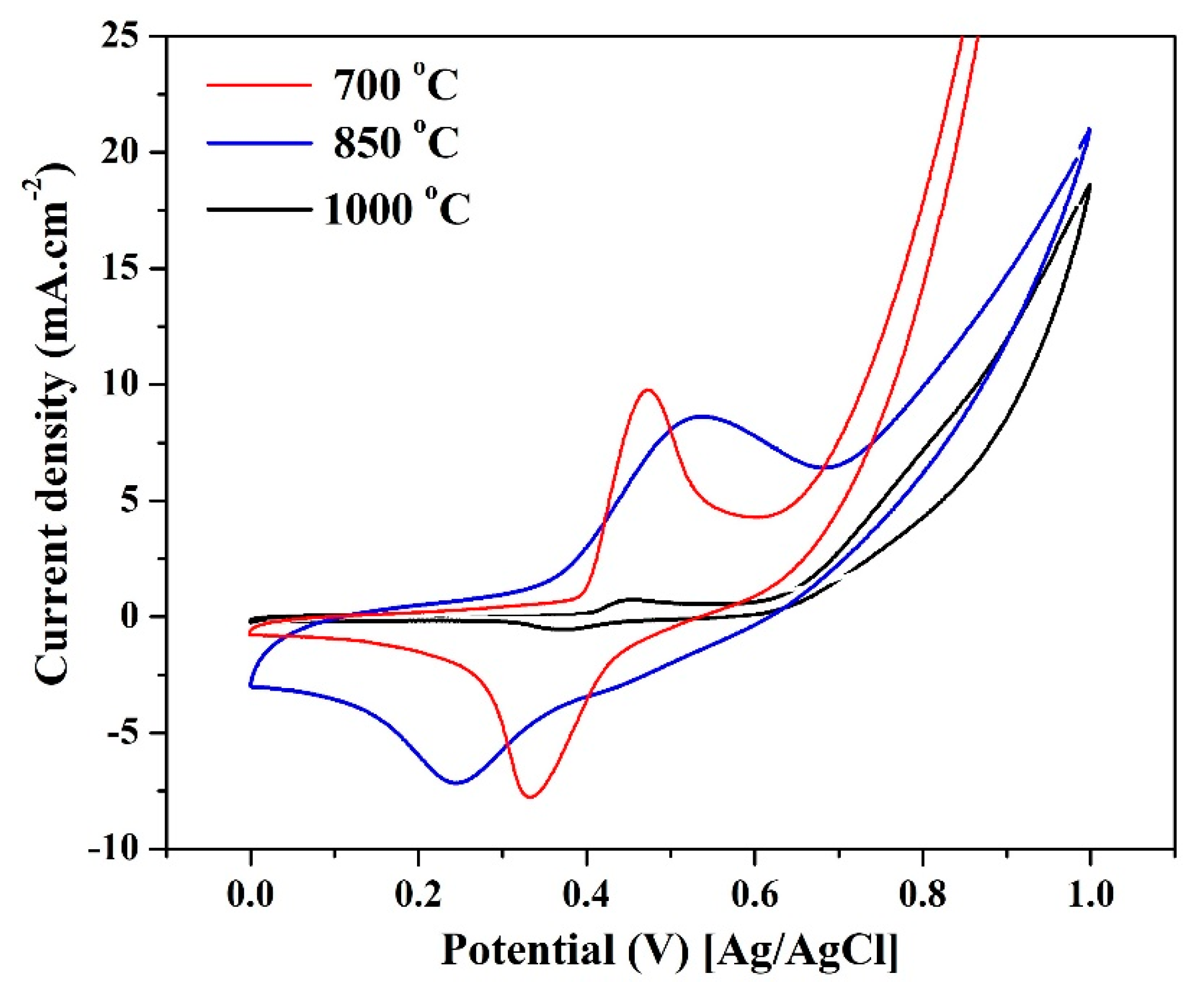
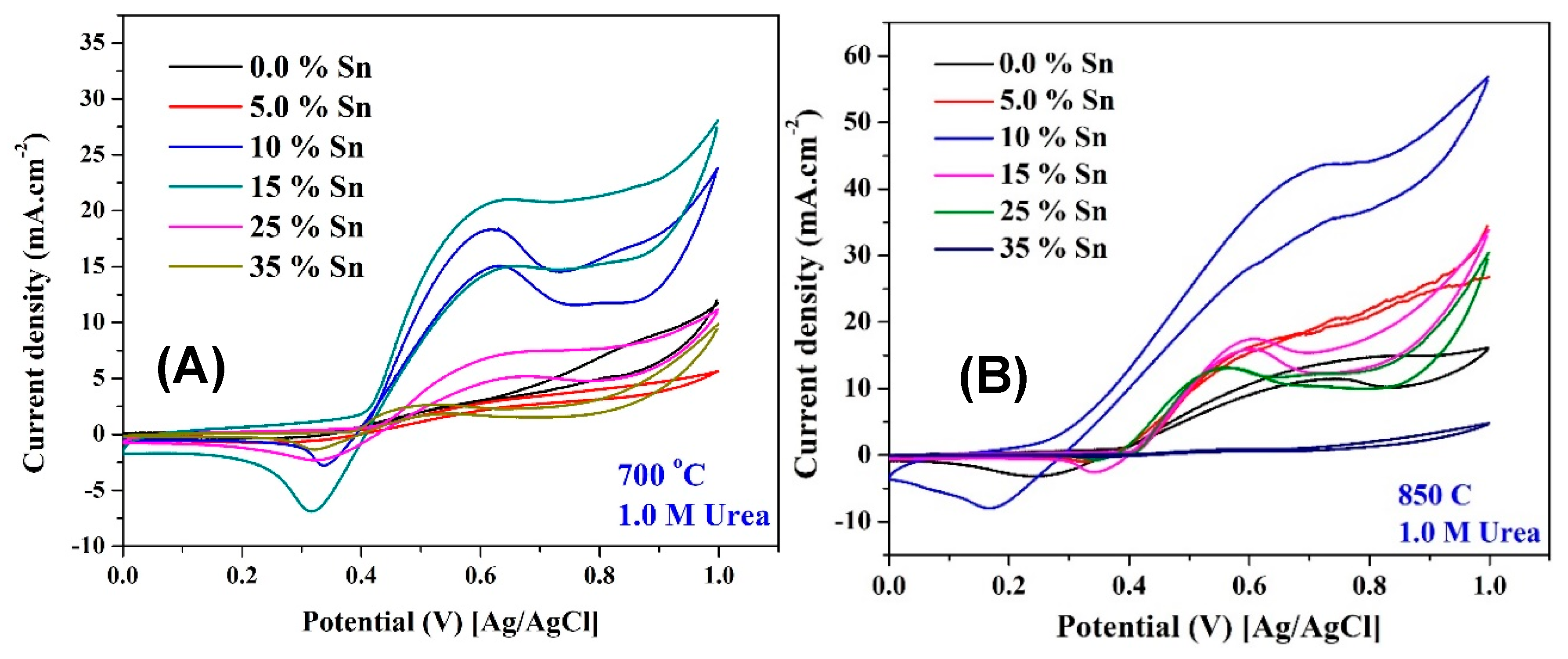
2.2. Electrochemical Performance
ESA Investigation
3. Materials and Methods
3.1. Preparation of the Nanofibers and Nanoparticles
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Badwal, S.; Giddey, S.; Munnings, C.; Kulkarni, A. Review of progress in high temperature solid oxide fuel cells. ChemInform 2015, 46. [Google Scholar] [CrossRef]
- Stambouli, A.B. Fuel cells: The expectations for an environmental-friendly and sustainable source of energy. Renew. Sustain. Energy Rev. 2011, 15, 4507–4520. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrog. Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrog. Energy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.; Abdelkareem, M.A.; Yousef, A.; Al-Deyab, S.S.; El-Newehy, M.; Kim, H.Y. Cadmium-doped cobalt/carbon nanoparticles as novel nonprecious electrocatalyst for methanol oxidation. Int. J. Hydrog. Energy 2013, 38, 3387–3394. [Google Scholar] [CrossRef]
- Barakat, N.A.; El-Newehy, M.H.; Yasin, A.S.; Ghouri, Z.K.; Al-Deyab, S.S. Ni&Mn nanoparticles-decorated carbon nanofibers as effective electrocatalyst for urea oxidation. Appl. Catal. A Gen. 2016, 510, 180–188. [Google Scholar]
- Barakat, N.A.; Motlak, M.; Elzatahry, A.A.; Khalil, K.A.; Abdelghani, E.A. NixCo1 − x alloy nanoparticle-doped carbon nanofibers as effective non-precious catalyst for ethanol oxidation. Int. J. Hydrog. Energy 2014, 39, 305–316. [Google Scholar] [CrossRef]
- Demirbas, A. Biomethanol production from organic waste materials. Energy Sources Part A 2008, 30, 565–572. [Google Scholar] [CrossRef]
- Amigun, B.; Gorgens, J.; Knoetze, H. Biomethanol production from gasification of non-woody plant in South Africa: Optimum scale and economic performance. Energy Policy 2010, 38, 312–322. [Google Scholar] [CrossRef]
- Shamsul, N.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Momeni, M.M. UV-cleaning properties of Pt nanoparticle-decorated titania nanotubes in the electro-oxidation of methanol: An anti-poisoning and refreshable electrode. Electrochim. Acta 2012, 70, 1–9. [Google Scholar] [CrossRef]
- Mehmood, A.; Scibioh, M.A.; Prabhuram, J.; An, M.-G.; Ha, H.Y. A review on durability issues and restoration techniques in long-term operations of direct methanol fuel cells. J. Power Sources 2015, 297, 224–241. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Allagui, A.; Tsujiguchi, T.; Nakagawa, N. Effect of the Ratio Carbon Nanofiber/Carbon Black in the Anodic Microporous Layer on the Performance of Passive Direct Methanol Fuel Cell. J. Electrochem. Soc. 2016, 163, F1011–F1016. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Nickel and cobalt bimetallic hydroxide catalysts for urea electro-oxidation. Electrochim. Acta 2012, 61, 25–30. [Google Scholar] [CrossRef]
- King, R.L.; Botte, G.G. Investigation of multi-metal catalysts for stable hydrogen production via urea electrolysis. J. Power Sources 2011, 196, 9579–9584. [Google Scholar] [CrossRef]
- Wang, D.; Yan, W.; Vijapur, S.H.; Botte, G.G. Electrochemically reduced graphene oxide–nickel nanocomposites for urea electrolysis. Electrochim. Acta 2013, 89, 732–736. [Google Scholar] [CrossRef]
- Barakat, N.A.; Amen, M.T.; Al-Mubaddel, F.S.; Karim, M.R.; Alrashed, M. NiSn nanoparticle-incorporated carbon nanofibers as efficient electrocatalysts for urea oxidation and working anodes in direct urea fuel cells. J. Adv. Res. 2019, 16, 43–53. [Google Scholar] [CrossRef]
- Ferdowsi, G.S. Ni nanoparticle modified graphite electrode for methanol electrocatalytic oxidation in alkaline media. J. Nanostruct. Chem. 2015, 5, 17–23. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P. Nickel nanocatalysts supported on sulfonated polyaniline: Potential toward methanol oxidation and as anode materials for DMFCs. J. Mater. Chem. A 2015, 3, 11349–11357. [Google Scholar] [CrossRef]
- Barakat, N.A.; Yassin, M.A.; Al-Mubaddel, F.S.; Amen, M.T. New electrooxidation characteristic for Ni-based electrodes for wide application in methanol fuel cells. Appl. Catal. A Gen. 2018, 555, 148–154. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Kim, K.-H.; Han, J.-N. Analysis of stresses generated during hydrogen extraction from and injection into Ni(OH)2/NiOOH film electrode. J. Power Sources 2000, 91, 92–98. [Google Scholar] [CrossRef]
- Klaus, S.; Cai, Y.; Louie, M.W.; Trotochaud, L.; Bell, A.T. Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity. J. Phys. Chem. C 2015, 119, 7243–7254. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. Preparation of nickel nanowire arrays electrode for urea electro-oxidation in alkaline medium. J. Power Sources 2015, 278, 562–568. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, D.; Guo, F.; Cheng, K.; Wang, G.; Cao, D. Highly porous nickel@ carbon sponge as a novel type of three-dimensional anode with low cost for high catalytic performance of urea electro-oxidation in alkaline medium. J. Power Sources 2015, 283, 408–415. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Moustafa, H.M.; Nassar, M.M.; Abdelkareem, M.A.; Mahmoud, M.S.; Almajid, A.A.; Khalil, K.A. Distinct influence for carbon nano-morphology on the activity and optimum metal loading of Ni/C composite used for ethanol oxidation. Electrochim. Acta 2015, 182, 143–155. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Liu, X.; Zhu, X. Three-dimensional porous graphene supported Ni nanoparticles with enhanced catalytic performance for Methanol electrooxidation. Int. J. Hydrog. Energy 2017, 42, 11206–11214. [Google Scholar] [CrossRef]
- Danaee, I.; Jafarian, M.; Mirzapoor, A.; Gobal, F.; Mahjani, M.G. Electrooxidation of methanol on NiMn alloy modified graphite electrode. Electrochim. Acta 2010, 55, 2093–2100. [Google Scholar] [CrossRef]
- Deng, Z.; Yi, Q.; Zhang, Y.; Nie, H. NiCo/C-N/CNT composite catalysts for electro-catalytic oxidation of methanol and ethanol. J. Electroanal. Chem. 2017, 803, 95–103. [Google Scholar] [CrossRef]
- Tarrús, X.; Montiel, M.; Vallés, E.; Gómez, E. Electrocatalytic oxidation of methanol on CoNi electrodeposited materials. Int. J. Hydrog. Energy 2014, 39, 6705–6713. [Google Scholar] [CrossRef]
- Gu, Y.; Luo, J.; Liu, Y.; Yang, H.; Ouyang, R.; Miao, Y. Synthesis of Bimetallic Ni–Cr Nano-Oxides as Catalysts for Methanol Oxidation in NaOH Solution. J. Nanosci. Nanotechnol. 2015, 15, 3743–3749. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Cheng, D. Facile Synthesis of Cu/NiCu Electrocatalysts Integrating Alloy, Core–Shell, and One-Dimensional Structures for Efficient Methanol Oxidation Reaction. ACS Appl. Mater. Interfaces 2017, 9, 19843–19851. [Google Scholar] [CrossRef]
- He, H.; Xiao, P.; Zhou, M.; Zhang, Y.; Lou, Q.; Dong, X. Boosting catalytic activity with a p–n junction: Ni/TiO2 nanotube arrays composite catalyst for methanol oxidation. Int. J. Hydrog. Energy 2012, 37, 4967–4973. [Google Scholar] [CrossRef]
- Guo, Y.G.; Hu, J.S.; Zhang, H.M.; Liang, H.P.; Wan, L.J.; Bai, C.L. Tin/Platinum Bimetallic Nanotube Array and its Electrocatalytic Activity for Methanol Oxidation. Adv. Mater. 2005, 17, 746–750. [Google Scholar] [CrossRef]
- Aricò, A.S.; Antonucci, V.; Giordano, N.; Shukla, A.K.; Ravikumar, M.K.; Roy, A.; Barman, S.R.; Sarma, D.D. Methanol oxidation on carbon-supported platinum-tin electrodes in sulfuric acid. J. Power Sources 1994, 50, 295–309. [Google Scholar] [CrossRef]
- Veizaga, N.S.; Rodriguez, V.I.; Rocha, T.A.; Bruno, M.; Scelza, O.A.; de Miguel, S.R.; Gonzalez, E.R. Promoting Effect of Tin in Platinum Electrocatalysts for Direct Methanol Fuel Cells (DMFC). J. Electrochem. Soc. 2015, 162, F243–F249. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Chai, Y.; Zhang, S. Carbon nanotubes/tin oxide nanocomposite-supported Pt catalysts for methanol electro-oxidation. J. Colloid Interface Sci. 2015, 450, 74–81. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Liu, Y.; Feng, B.; Li, L.; Pan, H.; Kellogg, W.; Higgins, D.; Wu, G. Sn-doped TiO2 modified carbon to support Pt anode catalysts for direct methanol fuel cells. J. Power Sources 2015, 286, 354–361. [Google Scholar] [CrossRef]
- Yi, Q.F.; Huang, W.; Yu, W.Q.; Li, L.; Liu, X.P. Fabrication of Novel Titanium-supported Ni-Sn Catalysts for Methanol Electro-oxidation. Chin. J. Chem. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Barakat, N.A.; Al-Mubaddel, F.S.; Karim, M.R.; Alrashed, M.; Kim, H.Y. Influence of Sn content on the electrocatalytic activity of NiSn alloy nanoparticles-incorporated carbon nanofibers toward methanol oxidation. Int. J. Hydrog. Energy 2018, 43, 21333–21344. [Google Scholar] [CrossRef]
- Li, J.; Luo, Z.; Zuo, Y.; Liu, J.; Zhang, T.; Tang, P.; Arbiol, J.; Llorca, J.; Cabot, A. NiSn bimetallic nanoparticles as stable electrocatalysts for methanol oxidation reaction. Appl. Catal. B 2018, 234, 10–18. [Google Scholar] [CrossRef]
- Barakat, N.A.; Abdelkareem, M.A.; El-Newehy, M.; Kim, H.Y. Influence of the nanofibrous morphology on the catalytic activity of NiO nanostructures: An effective impact toward methanol electrooxidation. Nanoscale Res. Lett. 2013, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.L.; Desai, T.A. Aligned Arrays of Biodegradable Poly(ε-caprolactone) Nanowires and Nanofibers by Template Synthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar] [CrossRef]
- Chen, S. Self-Assembly of Perylene Imide Molecules into 1D Nanostructures: Methods, Morphologies, and Applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef]
- Sehaqui, H.; Mushi, N.E.; Morimune, S.; Salajkova, M.; Nishino, T.; Berglund, L.A. Cellulose Nanofiber Orientation in Nanopaper and Nanocomposites by Cold Drawing. ACS Appl. Mater. Interfaces 2012, 4, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M.; Abdelkareem, M.A.; Shin, G.; Kim, H.Y. Pd-doped Co nanofibers immobilized on a chemically stable metallic bipolar plate as novel strategy for direct formic acid fuel cells. Int. J. Hydrog. Energy 2013, 38, 7438–7447. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.M.; Abdelkareem, M.A.; Al-Deyab, S.S.; Kim, H.Y. Influence of Nitrogen doping on the Catalytic Activity of Ni-incorporated Carbon Nanofibers for Alkaline Direct Methanol Fuel Cells. Electrochim. Acta 2014, 142, 228–239. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Tian, J.; Wang, F.; Zhan, L. Methanol electro-oxidation on Ni@ Pd core-shell nanoparticles supported on multi-walled carbon nanotubes in alkaline media. Int. J. Hydrog. Energy 2010, 35, 3249–3257. [Google Scholar] [CrossRef]
- Asgari, M.; Maragheh, M.G.; Davarkhah, R.; Lohrasbi, E.; Golikand, A.N. Electrocatalytic oxidation of methanol on the nickel–cobalt modified glassy carbon electrode in alkaline medium. Electrochim. Acta 2012, 59, 284–289. [Google Scholar] [CrossRef]
- Zhong, J.-P.; Fan, Y.-J.; Wang, H.; Wang, R.-X.; Fan, L.-L.; Shen, X.-C.; Shi, Z.-J. Highly active Pt nanoparticles on nickel phthalocyanine functionalized graphene nanosheets for methanol electrooxidation. Electrochim. Acta 2013, 113, 653–660. [Google Scholar] [CrossRef]
- Yu, M.; Chen, J.; Liu, J.; Li, S.; Ma, Y.; Zhang, J.; An, J. Mesoporous NiCo2O4 nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation. Electrochim. Acta 2015, 151, 99–108. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.; Amna, T.; Unnithan, A.R.; Al-Deyab, S.S.; Kim, H.Y. Influence of CdO-doping on the photoluminescence properties of ZnO nanofibers: Effective visible light photocatalyst for waste water treatment. J. Lumin. 2012, 132, 1668–1677. [Google Scholar] [CrossRef]
- Nash, P.; Nash, A. The Ni−Sn (Nickel-Tin) system. Bull. Alloy Phase Diagr. 1985, 6, 350–359. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.M.; El-Newehy, M.; Kim, H.Y. Chemically stable electrospun NiCu nanorods@carbon nanofibers for highly efficient dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2012, 37, 17715–17723. [Google Scholar] [CrossRef]
- Rahim, A.; Hameed, R.A.; Khalil, M. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Hahn, F.; Beden, B.; Croissant, M.; Lamy, C. In situ UV visible reflectance spectroscopic investigation of the nickel electrode-alkaline solution interface. Electrochim. Acta 1986, 31, 335–342. [Google Scholar] [CrossRef]
- Vuković, M. Voltammetry and anodic stability of a hydrous oxide film on a nickel electrode in alkaline solution. J. Appl. Electrochem. 1994, 24, 878–882. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chem. Interfacial Electrochem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Enea, O. Molecular structure effects in electrocatalysis--II. Oxidation of d-glucose and of linear polyols on Ni electrodes. Electrochim. Acta 1990, 35, 375–378. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Electrochemical decomposition of urea with Ni-based catalysts. Appl. Catal. B Environ. 2012, 127, 221–226. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wang, J.N.; Wang, X.X. Synthesis and application of Pt nanocrystals with controlled crystallographic planes. J. Phys. Chem. C 2009, 113, 18115–18120. [Google Scholar] [CrossRef]
- Wang, D.; Yan, W.; Vijapur, S.H.; Botte, G.G. Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons. J. Power Sources 2012, 217, 498–502. [Google Scholar] [CrossRef]
- Machado, S.A.; Avaca, L. The hydrogen evolution reaction on nickel surfaces stabilized by H-absorption. Electrochim. Acta 1994, 39, 1385–1391. [Google Scholar] [CrossRef]
- Brown, I.; Sotiropoulos, S. Preparation and characterization of microporous Ni coatings as hydrogen evolving cathodes. J. Appl. Electrochem. 2000, 30, 107–111. [Google Scholar] [CrossRef]









| SnCl2 in the Initial Solution (%) | NiSn Alloys Content | Elemental Compostion | |||||
|---|---|---|---|---|---|---|---|
| Ni3Sn2 (%) | Ni3Sn (%) | Ni (in NiSn Alloys) (%) | Ni (Free) (%) | Sn (%) | Snevap (%) | C (%) | |
| 5 | 42 | 58 | 5.457 | 28.082 | 4.510 | 0 | 61.952 |
| 10 | 100 | 0 | 6.544 | 24.380 | 8.779 | 0 | 60.297 |
| 25 | 59 | 41 | 23.399 | 0.459 | 20.31 | 0 | 55.823 |
| 35 | 54 | 46 | 19.703 | 0 | 16.890 | 10.215 | 53.192 |
| Synthesis Temp. °C | Sn Precusor Content (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 25 | 35 | ||
| Methanol | 700 | 375 | 415 | 412 | 405 | 423 | 425 |
| 850 | 395 | 403 | 360 | 415 | 445 | 415 | |
| Urea | 700 | 408 | 412 | 405 | 401 | 415 | 425 |
| 850 | 410 | 412 | 195 | 405 | 406 | 408 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, N.A.M.; Ali Abdelkareem, M.; Abdelghani, E.A.M. Influence of Sn Content, Nanostructural Morphology, and Synthesis Temperature on the Electrochemical Active Area of Ni-Sn/C Nanocomposite: Verification of Methanol and Urea Electrooxidation. Catalysts 2019, 9, 330. https://doi.org/10.3390/catal9040330
Barakat NAM, Ali Abdelkareem M, Abdelghani EAM. Influence of Sn Content, Nanostructural Morphology, and Synthesis Temperature on the Electrochemical Active Area of Ni-Sn/C Nanocomposite: Verification of Methanol and Urea Electrooxidation. Catalysts. 2019; 9(4):330. https://doi.org/10.3390/catal9040330
Chicago/Turabian StyleBarakat, Nasser A. M., Mohammad Ali Abdelkareem, and Emad A. M. Abdelghani. 2019. "Influence of Sn Content, Nanostructural Morphology, and Synthesis Temperature on the Electrochemical Active Area of Ni-Sn/C Nanocomposite: Verification of Methanol and Urea Electrooxidation" Catalysts 9, no. 4: 330. https://doi.org/10.3390/catal9040330
APA StyleBarakat, N. A. M., Ali Abdelkareem, M., & Abdelghani, E. A. M. (2019). Influence of Sn Content, Nanostructural Morphology, and Synthesis Temperature on the Electrochemical Active Area of Ni-Sn/C Nanocomposite: Verification of Methanol and Urea Electrooxidation. Catalysts, 9(4), 330. https://doi.org/10.3390/catal9040330







