Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
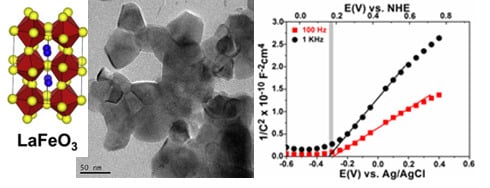
2.1. Structural and Optical Characterization of LaFeO3
2.2. Photocatalytic Properties
3. Experimental
3.1. Materials
3.2. Synthesis of LaFeO3 by the Citric Acid Assisted Sol-gel Method
3.3. Characterization
3.4. Photocatalytic Degradation Activity and Hydrogen Evolution Measurements
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.M.; Martin, T.S.; Choi, W.; Bahnemann, W.D. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Hu, C.; Hu, X.; Guo, J.; Qu, J. Efficient Destruction of Pathogenic Bacteria with NiO/SrBi2O4 under Visible Light Irradiation. Environ. Sci. Technol. 2006, 40, 5508–5513. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Lightkap, V.I.; Goodwin, K.; Matsumura, M.; Kamat, V.P. To What Extent Do Graphene Scaffolds Improve the Photovoltaic and Photocatalytic Response of TiO2 Nanostructured Films? J. Phys. Chem. Lett. 2010, 1, 2222–2227. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, T.; Zhang, J.; Tian, B.; Chen, F.; He, D. Preparation of Fe3+ doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef]
- Xu, J.J.; Ao, H.Y.; Fu, G.D.; Yuan, W.S. Synthesis of Gd-doped TiO2 nanoparticles under mild condition and their photocatalytic activity. Colloid Surf. A 2009, 334, 107–111. [Google Scholar] [CrossRef]
- Dodd, A.; Mckinley, A.; Tsuzuki, T.; Sauners, M. Optical and photocatalytic properties of nanoparticulate (TiO2)x (ZnO)1−x powders. J. Alloys Compd. 2010, 489, L17–L21. [Google Scholar] [CrossRef]
- Dai, K.; Peng, Y.T.; Ke, N.D.; Wei, Q.B. Photocatalytic hydrogen generation using a nanocomposite of multi-walled carbon nanotubes and TiO2 nanoparticles under visible light irradiation. Nanotechnology 2009, 20, 125603. [Google Scholar] [CrossRef]
- Chai, B.; Peng, Y.T.; Zeng, P.; Mao, J. Synthesis of floriated In2S3 decorated with TiO2 nanoparticles for efficient photocatalytic hydrogen production under visible light. J. Mater. Chem. 2011, 21, 14587–14593. [Google Scholar] [CrossRef]
- Laokiat, L.; Khemthong, P.; Grisdanurak, N.; Sreearunothai, P.; Pattanasiriwisawa, W.; Klysubun, W. Photocatalytic degradation of benzene, toluene, ethylbenzene, and xylene (BTEX) using transition metal-doped titanium dioxide immobilized on fiberglass cloth. Korean J. Chem. Eng. 2012, 29, 377–383. [Google Scholar] [CrossRef]
- Taffa, H.D.; Dillert, R.; Ulpe, C.A.; Bauerfeind, L.C.K.; Bredow, T.; Bahnemann, W.D.; Wark, M. Photoelectrochemical and theoretical investigations of spinel type ferrites (MxFe3-xO4) for water splitting: A mini-review. J. Photon. Energy 2016, 7, 12009. [Google Scholar] [CrossRef]
- Su, H.M.; He, C.; Sharma, K.V.; Abou Asi, M.; Xia, D.; Li, Z.X.; Deng, Q.H.; Xiong, Y. Mesoporous zinc ferrite: Synthesis, characterization, and photocatalytic activity with H2O2/visible light. J. Hazard. Mater. 2012, 211–212, 95–103. [Google Scholar] [CrossRef]
- Cao, W.S.; Zhu, J.Y.; Cheng, F.G.; Huang, H.Y. ZnFe2O4 nanoparticles: Microwave-hydrothermal ionic liquid synthesis and photocatalytic property over phenol. J. Hazard. Mater. 2009, 171, 431–435. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, K.V.; Li, Z.X. Synthesis and photocatalytic activity of ferrites under visible light. A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Nakanishi, T.; Masuda, Y.; Koumoto, K. Site-Selective Deposition of Magnetite Particulate Thin Films on Patterned Self-assembled Monolayers. Chem. Mater. 2004, 16, 3484–3488. [Google Scholar] [CrossRef]
- Juan, X.W.; Yun, H.S.; Yan, H.T.; Hua, Q.Y. Photocatalytic Degradation of Water-Soluble Azo Dyes by LaFeO3 and YFeO3. Adv. Mater. Res. 2012, 465, 37–43. [Google Scholar]
- Hou, L.; Sun, G.; Liu, K.; Li, Y.; Gao, F. Preparation, characterization and investigation of catalytic activity of Li-doped LaFeO3 nanoparticles. J. Sol-Gel Sci. Technol. 2006, 40, 9–14. [Google Scholar] [CrossRef]
- Tang, P.; Fu, M.; Chen, H.; Cao, F. Synthesis of Nanocrystalline LaFeO3 by Precipitation and its Visible-Light Photocatalytic Activity. Mater. Sci. Forum 2011, 694, 150–154. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Masteralo, R.V.; Ponpandian, N. Photocatalytic degradation of organic dyes under visible light irradiation by floral-like LaFeO3 nanostructures comprised of nanosheet petals. New J. Chem. 2014, 38, 5480–5490. [Google Scholar] [CrossRef]
- Su, H.; Jing, L.; Shi, K.; Yao, C.; Fu, H. Synthesis of large surface area LaFeO3 nanoparticles by SBA-16 template method as high active visible photocatalysts. J. Nanopart. Res. 2010, 12, 967–974. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, H.; Li, M.; Zhang, L.; Zhang, Y. Markedly enhancing the visible-light photocatalytic activity of LaFeO3 by post-treatment in molten salt. React. Kinet. Catal. Lett. 2009, 97, 269–274. [Google Scholar] [CrossRef]
- Tijare, N.S.; Joshi, V.M.; Padole, S.P.; Manguklar, A.P.; Rayalu, S.S.; Labhsetwar, K.N. Photocatalytic hydrogen generation through water splitting on nano-crystalline LaFeO3 perovskite. Int. J. Hydrog. Energy 2012, 37, 10451–10456. [Google Scholar] [CrossRef]
- Wu, H.; Hu, R.; Zhou, T.; Li, C.; Meng, W.; Yang, J. A novel efficient boron-doped LaFeO3 photocatalyst with large specific surface area for phenol degradation under simulated sunlight. CrystEngComm 2015, 17, 3859–3865. [Google Scholar] [CrossRef]
- Ju, L.; Chen, Z.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Sol-Gel Synthesis and Photo-Fenton-Like Catalytic Activity of EuFeO3 Nanoparticles. J. Am. Ceram. Soc. 2011, 94, 3418–3424. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Ganesh, I.; Mangalaraj, D.; Viswanathan, C.; Balamurugan, A. Controlled synthesis of perovskite LaFeO3 microsphere composed of nanoparticles via self-assembly process and their associated photocatalytic activity. Chem. Eng. J. 2012, 209, 420–428. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, L. Porous structure dependent photoreactivity of graphitic carbon nitride under visible light. J. Mater. Chem. 2012, 22, 1160–1166. [Google Scholar] [CrossRef]
- Li, K.; Wang, D.; Wu, F.; Xie, T.; Li, T. Surface electronic states and photovoltage gas-sensitive characters of nanocrystalline LaFeO3. Mater. Chem. Phys. 2000, 64, 269–272. [Google Scholar] [CrossRef]
- Marschall, R.; Soldat, J.; Wark, M. Enhanced photocatalytic hydrogen generation from barium tantalate composites. Photochem. Photobiol. Sci. 2013, 12, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhou, J.; Yue, Z.; Gui, Z.; Li, L. Auto-combustion synthesis of nanocrystalline LaFeO3. Mater. Chem. Phys. 2002, 78, 25–29. [Google Scholar] [CrossRef]
- Rida, K.; Benabbas, A.; Bouremmad, F.; Pena, A.M.; Sastre, E.; Martinez, A. Effect of calcination temperature on the structural characteristics and catalytic activity for propene combustion of sol-gel derived lanthanum chromite perovskite. Appl. Catal. A 2007, 327, 173–179. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Hebalkar, Y.N.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activity. RSC Adv. 2013, 3, 7549–7561. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman spectroscopic study on TiO2. I. Phase transformation at the surface and in the bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Wu, W.; Liang, S.; Shen, L.; Ding, Z.; Zheng, H.; Su, W.; Wu, L. Preparation, characterization and enhanced visible light photocatalytic activities of polyaniline/Bi3NbO7 nanocomposites. J. Alloys Compd. 2012, 520, 213–219. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Ismael, M.; Elhaddad, E.; Taffa, H.D.; Wark, M. Synthesis of Phase Pure Hexagonal YFeO3 Perovskite as Efficient Visible Light Active Photocatalyst. Catalysts 2017, 7, 326. [Google Scholar] [CrossRef]
- Parida, M.K.; Reddy, H.K.; Martha, S.; Das, P.D.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol-gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrog. Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D. Enhanced Photocatalytic Hydrogen Production from Glucose on Rh-Doped LaFeO3. Chem. Eng. Trans. 2017, 60, 235–240. [Google Scholar]
- Xu, K.; Feng, J. Superior photocatalytic performance of LaFeO3/gC3N4 heterojunction nanocomposites under visible light irradiation. RSC Adv. 2017, 7, 45369–45376. [Google Scholar] [CrossRef]
- Yang, H.; Yan, J.; Lu, Z.; Cheng, X.; Tang, Y. Photocatalytic activity evaluation of tetragonal CuFe2O4 nanoparticles for the H2 evolution under visible light irradiation. J. Alloys Compd. 2009, 476, 715–719. [Google Scholar] [CrossRef]
- Rekhila, G.; Bessekhouad, Y.; Trari, M. Visible light hydrogen production on the novel ferrite NiFe2O4. Int. J. Hydrog. Energy 2013, 38, 6335–6343. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Yang, S.; Wu, H.; Wu, Y.; Wu, Y.; Pan, J.; Xiong, X. In situ construction of a SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- He, M.Y.; Cai, J.; Zhang, H.L.; Wang, X.X.; Lin, J.H.; Teng, T.B.; Zhao, H.L.; Weng, Z.W.; Wan, L.H.; Fan, M. Comparing Two New Composite Photocatalysts, t-LaVO4/g-C3N4 and m-LaVO4/g-C3N4, for Their Structures and Performances. Ind. Eng. Chem. Res. 2014, 53, 5905–5915. [Google Scholar] [CrossRef]
- Yu, T.H.; Quan, X.; Chen, S.; Zhao, M.H.; Zhang, B.Y. TiO2–carbon nanotube heterojunction arrays with a controllable thickness of TiO2 layer and their first application in photocatalysis. J. Photochem. Photobiol. A Chem. 2008, 200, 301–306. [Google Scholar] [CrossRef]
- Lim, J.; Monllor-Satocaa, D.; Jang, S.J.; Lee, S.; Choi, W. Visible light photocatalysis of fullerol-complexed TiO2 enhanced by Nb doping. Appl. Catal. B Environ. 2014, 152–153, 233–240. [Google Scholar] [CrossRef]
- Bi, P.Y.; Quyang, X.S.; Cao, Y.J.; Ye, H.J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys. Chem. Chem. Phys. 2011, 13, 10071–10075. [Google Scholar] [CrossRef]
- Hong, S.; Lee, S.; Jang, S.J.; Lee, S.J. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Li, X.; Ye, J. Photocatalytic Degradation of Rhodamine B over Pb3Nb4O13/Fumed SiO2 Composite under Visible Light Irradiation. J. Phys. Chem. C 2007, 111, 13109–13116. [Google Scholar] [CrossRef]
- Merka, O.; Yarovyi, V.; Bahnemann, D.W.; Wark, M. pH-Control of the Photocatalytic Degradation Mechanism of Rhodamine B over Pb3Nb4O13. J. Phys. Chem. C 2011, 115, 8014–8023. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Lin, H.; Nong, Q.; Wu, Y.; Wu, T.; He, Y. Synthesis and characterization of ZrO2/g-C3N4 composite with enhanced visible-light photoactivity for rhodamine degradation. RSC Adv. 2014, 4, 40029–40035. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z- what we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S. Chem. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem. Eng. J. 2012, 209, 386–393. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Cheng, B. Effect of Crystallization Methods on Morphology and Photocatalytic Activity of Anodized TiO2 Nanotube Array Films. J. Phys. Chem. C 2010, 114, 19378–19385. [Google Scholar] [CrossRef]
- He, M.Y.; Cai, J.; Li, T.T.; Wu, Y.; Lin, J.H.; Zhao, H.L.; Luo, F.M. Efficient degradation of RhB over GdVO4/g-C3N4 composites under visible-light irradiation. Chem. Eng. J. 2013, 215–216, 721–730. [Google Scholar] [CrossRef]
- Yang, X.; Cui, H.; Li, Y.; Qin, J.; Zhang, R.; Tang, H. Fabrication of Ag3PO4-Graphene Composites with Highly Efficient and Stable Visible Light Photocatalytic Performance. ACS Catal. 2013, 3, 363–369. [Google Scholar] [CrossRef]
- Wang, F.D.; Kako, T.; Ye, H.J. Efficient Photocatalytic Decomposition of Acetaldehyde over a Solid-Solution Perovskite (Ag0.75Sr0.25)(Nb0.75Ti0.25)O3 under Visible-Light Irradiation. J. Am. Chem. Soc. 2008, 130, 2724–2725. [Google Scholar] [CrossRef]
- Kormali, P.; Triantis, T.; Dimotikali, D.; Hiskia, A.; Papaconstantinou, E. On the photooxidative behavior of TiO2 and PW12O403−: OH radicals versus holes. Appl. Catal. B 2006, 68, 139–146. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Zhang, D.-W. A Simple Strategy for the preparation of g-C3N4/SnO2 Nanocomposite Photocatalysts. Sci. Adv. Mater. 2014, 6, 1091–1098. [Google Scholar] [CrossRef]
- Pirzada, M.B.; Kunchala, K.R.P.; Naidu, S.B. Synthesis of LaFeO3/Ag2CO3 Nanocomposites for Photocatalytic Degradation of Rhodamine B and p-Chlorophenol under Natural Sunlight. ACS Omega 2019, 4, 2618–2629. [Google Scholar] [CrossRef]
- Hu, R.; Li, C.; Wang, X.; Sun, Y.; Jia, H.; Su, H.; Zhang, Y. Photocatalytic activities of LaFeO3 and La2FeTiO6 in p-chlorophenol degradation under visible light. Catal. Commun. 2012, 29, 35–39. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismael, M.; Wark, M. Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Catalysts 2019, 9, 342. https://doi.org/10.3390/catal9040342
Ismael M, Wark M. Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Catalysts. 2019; 9(4):342. https://doi.org/10.3390/catal9040342
Chicago/Turabian StyleIsmael, Mohammed, and Michael Wark. 2019. "Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation" Catalysts 9, no. 4: 342. https://doi.org/10.3390/catal9040342
APA StyleIsmael, M., & Wark, M. (2019). Perovskite-type LaFeO3: Photoelectrochemical Properties and Photocatalytic Degradation of Organic Pollutants Under Visible Light Irradiation. Catalysts, 9(4), 342. https://doi.org/10.3390/catal9040342